Human TGF-beta 1 Protein Summary
Product Specifications
The human platelets used for the isolation of this product were certified by the supplier to be HIV-1 and HBsAg negative at the time of shipment. Human blood products should always be treated in accordance with universal handling precautions.
Customers also Viewed
Product Datasheets
100-B/CF (carrier free)
Discontinued Product
100-B
| Formulation | Lyophilized from a 0.2 μm filtered solution in Acetonitrile and TFA with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 10 μg/mL in sterile 4 mM HCl containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
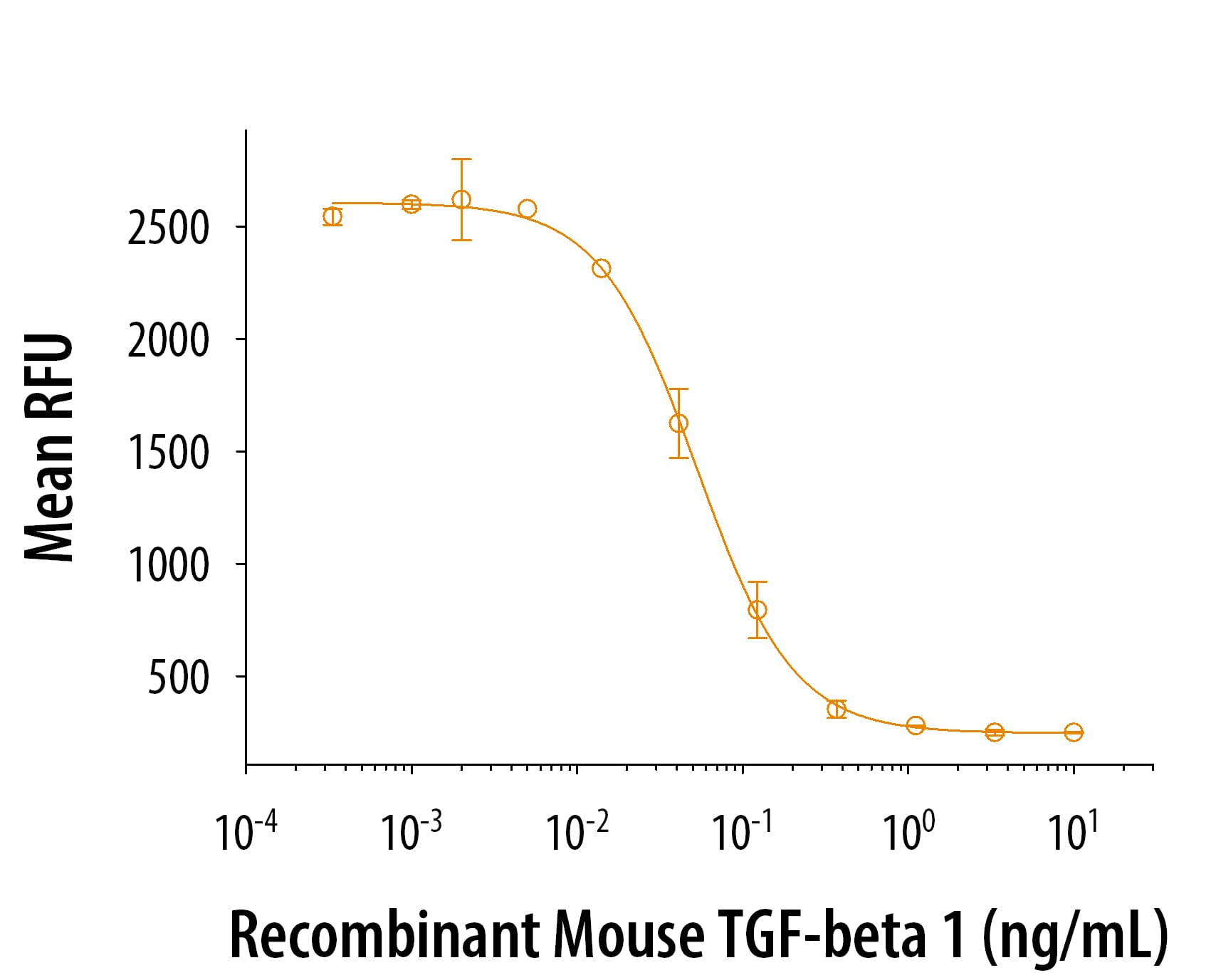
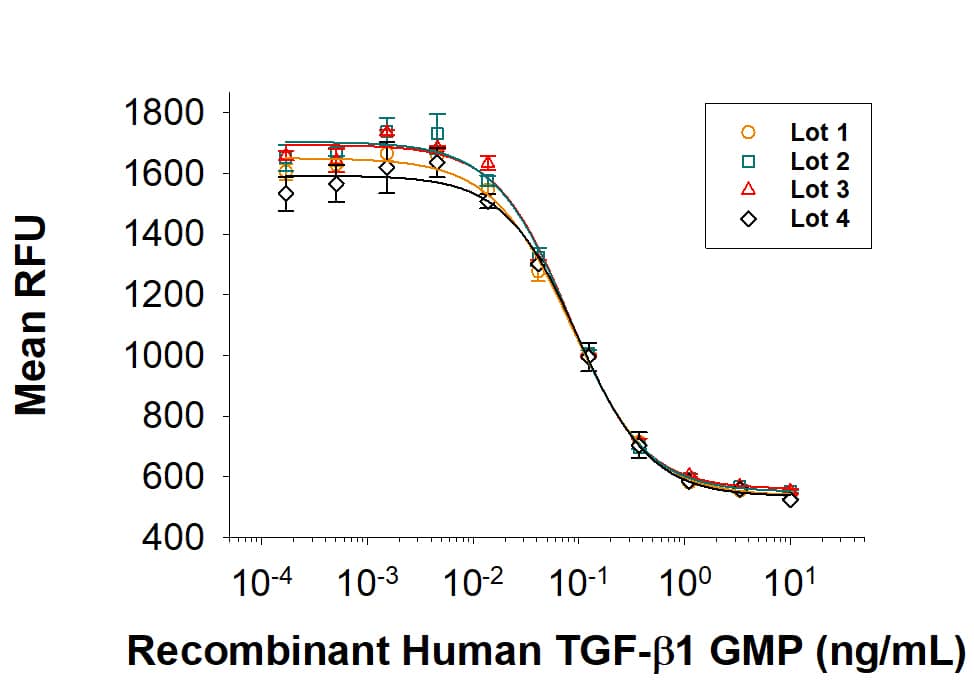
Human TGF-beta 1 (Catalog # 100-B) inhibits Recombinant Mouse IL-4 (Catalog # 404-ML) induced cell proliferation in the HT-2 mouse T cell line. The ED50 for this effect is 0.04-0.2 ng/mL.
 View Larger
View Larger
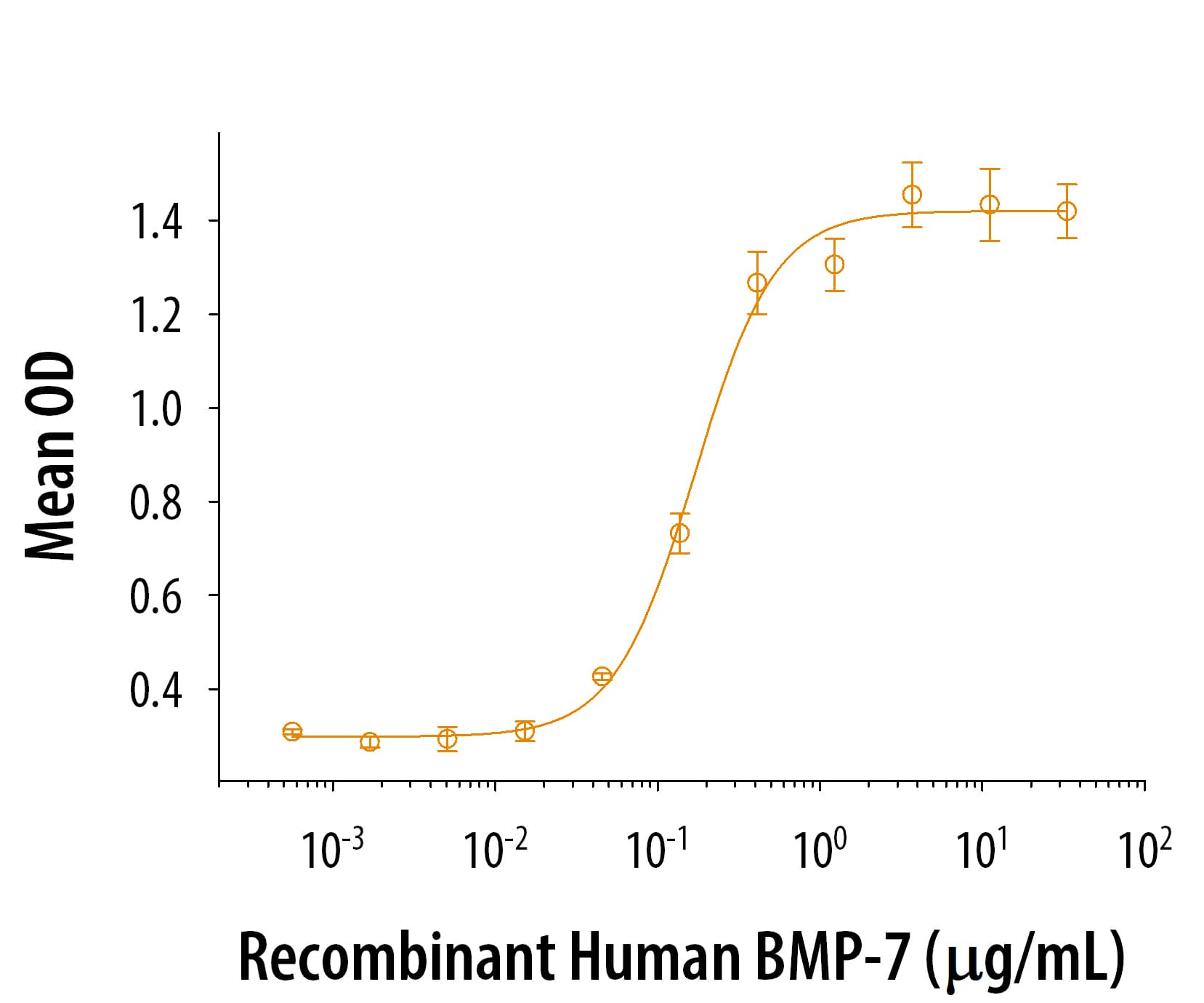
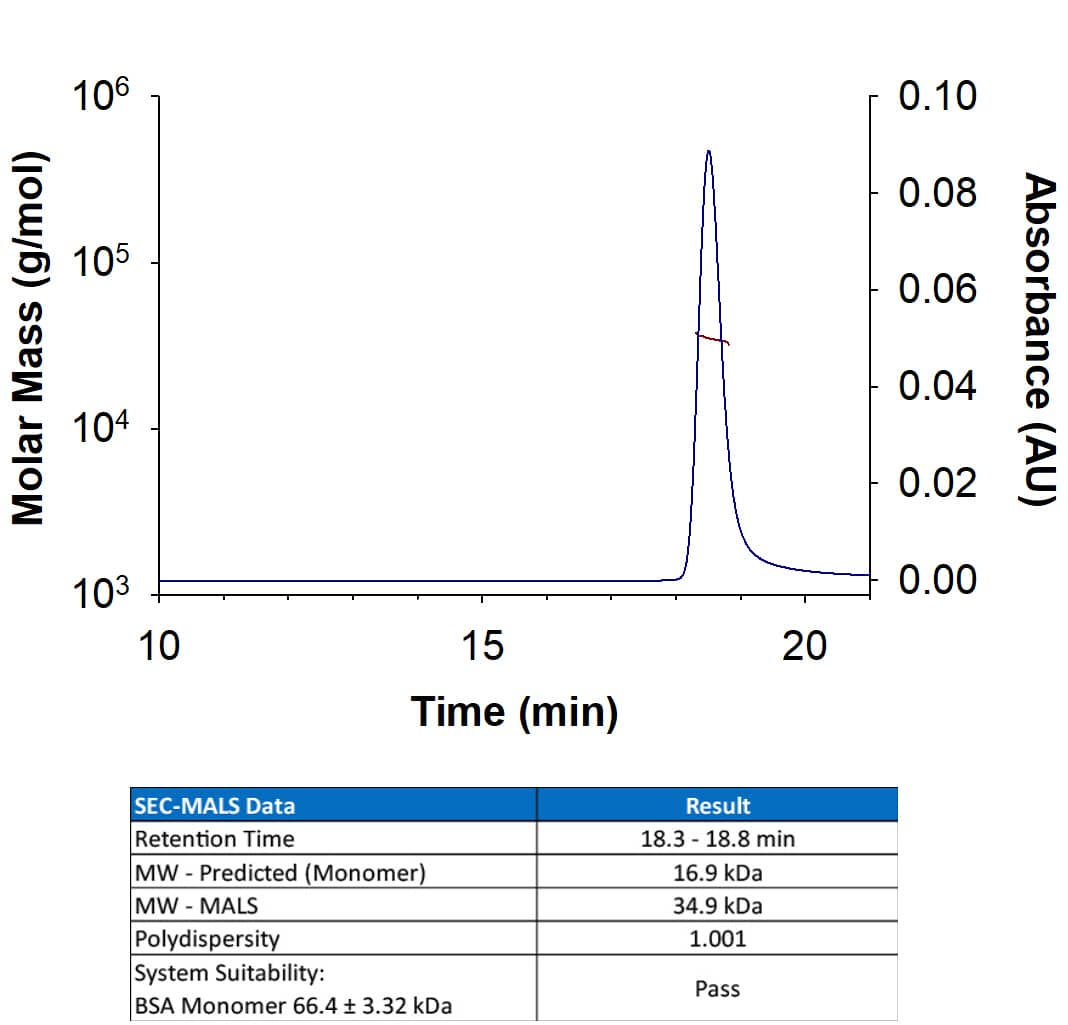
1 μg/lane of Recombinant Human TGF-beta 1 was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by silver staining, showing single bands at 12 kDa and 24 kDa, respectively.
Background: TGF-beta 1
TGF-beta 1 (transforming growth factor beta 1) is one of three closely related mammalian members of the large TGF-beta superfamily that share a characteristic cysteine knot structure (1-7). TGF-beta 1, -2 and -3 are highly pleiotropic cytokines that are proposed to act as cellular switches that regulate processes such as immune function, proliferation and epithelial-mesenchymal transition (1-4). Each TGF-beta isoform has some non-redundant functions; for TGF-beta 1, mice with targeted deletion show defects in hematopoiesis and endothelial differentiation, and die of overwhelming inflammation (2). Human TGF-beta 1 cDNA encodes a 390 amino acid (aa) precursor that contains a 29 aa signal peptide and a 361 aa proprotein (8). A furin-like convertase processes the proprotein to generate an N-terminal 249 aa latency-associated peptide (LAP) and a C-terminal 112 aa mature TGF- beta 1 (8, 9). Disulfide-linked homodimers of LAP and TGF-beta 1 remain non-covalently associated after secretion, forming the small latent TGF-beta 1 complex (8-10). Covalent linkage of LAP to one of three latent TGF-beta binding proteins (LTBPs) creates a large latent complex that may interact with the extracellular matrix (9, 10). TGF-beta is activated from latency by pathways that include actions of the protease plasmin, matrix metalloproteases, thrombospondin 1 and a subset of integrins (10). Mature human TGF-beta 1 shares 100% aa identity with pig, dog and cow TGF-beta 1, and 99% aa identity with mouse, rat and horse TGF-beta 1. It demonstrates cross-species activity (1). TGF-beta 1 signaling begins with high-affinity binding to a type II ser/thr kinase receptor termed TGF-beta RII. This receptor then phosphorylates and activates a second ser/thr kinase receptor, TGF-beta RI (also called activin receptor-like kinase (ALK) -5), or alternatively,
ALK‑1.This complex phosphorylates and activates Smad proteins that regulate transcription (3, 11, 12). Contributions of the accessory receptors betaglycan (also known as TGF-beta RIII) and endoglin, or use of Smad-independent signaling pathways, allow for disparate actions observed in response to TGF-beta in different contexts (11).
- Derynck, R. and K. Miyazono (2008) “TGF-beta and the TGF-beta Family” in The TGF-beta Family, Derynck, R. and K. Miyazono eds., Cold Spring Harbor Laboratory Press, p. 29.
- Dunker, N. & K. Krieglstein, 2000, Eur. J. Biochem. 267:6982.
- Wahl, S.M. (2006) Immunol. Rev. 213:213.
- Chang, H. et al. (2002) Endocr. Rev. 23:787.
- Lin, J.S. et al. (2006) Reproduction 132:179.
- Hinck, A.P. et al. (1996) Biochemistry 35:8517.
- Mittl, P.R.E. et al. (1996) Protein Sci. 5:1261.
- Derynck, R. et al. (1985) Nature 316:701.
- Miyazono, K. et al. (1988) J. Biol. Chem. 263:6407.
- Oklu, R. and R. Hesketh (2000) Biochem. J. 352:601.
- de Caestecker, M. et al. (2004) Cytokine Growth Factor Rev. 15:1.
- Zuniga, J.E. et al. (2005) J. Mol. Biol. 354:1052.
Citations for Human TGF-beta 1 Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
43
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Inhibiting stromal Class I HDACs curbs pancreatic cancer progression
Authors: Liang, G;Oh, TG;Hah, N;Tiriac, H;Shi, Y;Truitt, ML;Antal, CE;Atkins, AR;Li, Y;Fraser, C;Ng, S;Pinto, AFM;Nelson, DC;Estepa, G;Bashi, S;Banayo, E;Dai, Y;Liddle, C;Yu, RT;Hunter, T;Engle, DD;Han, H;Von Hoff, DD;Downes, M;Evans, RM;
Nature communications
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Inhibiting Stromal Class I HDACs Curbs Pancreatic Cancer Progression
Authors: Liang, G;Oh, TG;Hah, N;Tiriac, H;Shi, Y;Truitt, ML;Antal, CE;Atkins, AR;Li, Y;Fraser, C;Ng, S;Pinto, AFM;Nelson, DC;Estepa, G;Bashi, S;Banayo, E;Dai, Y;Liddle, C;Yu, RT;Hunter, T;Engle, DD;Han, H;Von Hoff, DD;Downes, M;Evans, RM;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
UDP-glucose dehydrogenase (UGDH) activity is suppressed by peroxide and promoted by PDGF in fibroblast-like synoviocytes: Evidence of a redox control mechanism
Authors: R Chandrasek, C Mathieu, R Sheth, AP Cheng, D Fong, R McCormack, H El-Gabalaw, S Alishetty, M Paige, CD Hoemann
PLoS ONE, 2022-09-15;17(9):e0274420.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A disease-driver population within interstitial cells of human calcific aortic valves identified via single-cell and proteomic profiling
Authors: JL Decano, Y Iwamoto, S Goto, JY Lee, JT Matamalas, A Halu, M Blaser, LH Lee, B Pieper, S Chelvanamb, J Silva-Nico, F Bartoli-Le, H Higashi, H Shibata, P Vyas, J Wang, E Gostjeva, SC Body, SA Singh, M Aikawa, E Aikawa
Cell Reports, 2022-04-12;39(2):110685.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
LPA1 antagonist BMS-986020 changes collagen dynamics and exerts antifibrotic effects in vitro and in patients with idiopathic pulmonary fibrosis
Authors: BE Decato, DJ Leeming, JMB Sand, A Fischer, S Du, SM Palmer, M Karsdal, Y Luo, A Minnich
Respiratory Research, 2022-03-18;23(1):61.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
E4 engages uPAR and enolase-1 and activates urokinase to exert antifibrotic effects
Authors: S Sharma, T Watanabe, T Nishimoto, T Takihara, L Mlakar, XX Nguyen, M Sanderson, Y Su, RA Chambers, C Feghali-Bo
JCI Insight, 2021-12-22;6(24):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
PP2A Catalytic Subunit alpha promotes fibroblast activation and kidney fibrosis via ERK pathway
Authors: Q Lu, M Tan, Q Hou, M Wang, C Dai
Cellular Signalling, 2021-11-13;90(0):110187.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Red-COLA1: a human fibroblast reporter cell line for type I collagen transcription
Authors: HH Wong, SH Seet, CC Bascom, RJ Isfort, F Bard
Sci Rep, 2020-11-12;10(1):19723.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Prolonged Scar-in-a-Jar: an in vitro screening tool for anti-fibrotic therapies using biomarkers of extracellular matrix synthesis
Authors: SR Rønnow, RQ Dabbagh, F Genovese, CB Nanthakuma, VJ Barrett, RB Good, S Brockbank, S Cruwys, H Jessen, GL Sorensen, MA Karsdal, DJ Leeming, JMB Sand
Respir. Res., 2020-05-07;21(1):108.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Involvement of GPx4-Regulated Lipid Peroxidation in Idiopathic Pulmonary Fibrosis Pathogenesis
Authors: K Tsubouchi, J Araya, M Yoshida, T Sakamoto, T Koumura, S Minagawa, H Hara, Y Hosaka, A Ichikawa, N Saito, T Kadota, Y Kurita, K Kobayashi, S Ito, Y Fujita, H Utsumi, M Hashimoto, H Wakui, T Numata, Y Kaneko, S Mori, H Asano, H Matsudaira, T Ohtsuka, K Nakayama, Y Nakanishi, H Imai, K Kuwano
J. Immunol., 2019-09-18;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
IL-23R Signaling Plays No Role in Myocardial Infarction
Authors: E Engelowski, NF Modares, S Gorressen, P Bouvain, D Semmler, C Alter, Z Ding, U Flögel, J Schrader, H Xu, PA Lang, J Fischer, DM Floss, J Scheller
Sci Rep, 2018-11-20;8(1):17078.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Activation of Ras in the Vascular Endothelium Induces Brain Vascular Malformations and Hemorrhagic Stroke
Authors: QF Li, B Decker-Roc, A Bajaj, K Pumiglia
Cell Rep, 2018-09-11;24(11):2869-2882.
Species: Human
Sample Types: Cell Culture Treatment
Applications: Bioassay -
Protein kinase C alpha drives fibroblast activation and kidney fibrosis by stimulating autophagic flux
Authors: X Xue, J Ren, X Sun, Y Gui, Y Feng, B Shu, W Wei, Q Lu, Y Liang, W He, J Yang, C Dai
J. Biol. Chem., 2018-05-23;0(0):.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Aloperine Protects Mice against DSS-Induced Colitis by PP2A-Mediated PI3K/Akt/mTOR Signaling Suppression
Authors: X Fu, F Sun, F Wang, J Zhang, B Zheng, J Zhong, T Yue, X Zheng, JF Xu, CY Wang
Mediators Inflamm., 2017-09-19;2017(0):5706152.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Omega-3 Polyunsaturated Fatty Acids Attenuate Fibroblast Activation and Kidney Fibrosis Involving MTORC2 Signaling Suppression
Authors: Z Zeng, H Yang, Y Wang, J Ren, Y Dai, C Dai
Sci Rep, 2017-04-10;7(0):46146.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Quercetin Inhibits Fibroblast Activation and Kidney Fibrosis Involving the Suppression of Mammalian Target of Rapamycin and ?-catenin Signaling
Authors: J Ren, J Li, X Liu, Y Feng, Y Gui, J Yang, W He, C Dai
Sci Rep, 2016-04-07;6(0):23968.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Rictor/mTORC2 signaling mediates TGFbeta1-induced fibroblast activation and kidney fibrosis.
Authors: Li J, Ren J, Liu X, Jiang L, He W, Yuan W, Yang J, Dai C
Kidney Int, 2015-05-13;88(3):515-27.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Recruitment of Matrix Metalloproteinase-9 (MMP-9) to the Fibroblast Cell Surface by Lysyl Hydroxylase 3 (LH3) Triggers Transforming Growth Factor-beta (TGF-beta) Activation and Fibroblast Differentiation.
Authors: Dayer C, Stamenkovic I
J Biol Chem, 2015-03-30;290(22):13763-78.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Autocrine CSF1R signaling mediates switching between invasion and proliferation downstream of TGFbeta in claudin-low breast tumor cells.
Authors: Patsialou A, Wang Y, Pignatelli J, Chen X, Entenberg D, Oktay M, Condeelis J
Oncogene, 2014-08-04;34(21):2721-31.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Opposing roles for Smad2 and Smad3 in peritoneal fibrosis in vivo and in vitro.
Authors: Duan W, Yu X, Huang X, Yu J, Lan H
Am J Pathol, 2014-06-10;184(8):2275-84.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Autophagy fosters myofibroblast differentiation through MTORC2 activation and downstream upregulation of CTGF.
Authors: Bernard M, Dieude M, Yang B, Hamelin K, Underwood K, Hebert M
Autophagy, 2014-01-01;10(12):2193-207.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Induction of high temperature requirement A1, a serine protease, by TGF-beta1 in articular chondrocytes of mouse models of OA.
Authors: Xu L, Golshirazian I, Asbury B, Li Y
2013-10-18;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Rheb/mTORC1 signaling promotes kidney fibroblast activation and fibrosis.
Authors: Jiang L, Xu L, Mao J, Li J, Fang L, Zhou Y, Liu W, He W, Zhao A, Yang J, Dai C
J Am Soc Nephrol, 2013-05-09;24(7):1114-26.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth.
Authors: Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat J, Kanellopoulos J, Martin F, Rebe C, Apetoh L, Ghiringhelli F
Nat Med, 2012-12-02;19(1):57-64.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Transforming growth factor beta induces sensory neuronal hyperexcitability, and contributes to pancreatic pain and hyperalgesia in rats with chronic pancreatitis.
Authors: Zhu Y, Colak T, Shenoy M, Liu L, Mehta K, Pai R, Zou B, Xie X, Pasricha P
Mol Pain, 2012-09-11;8(0):65.
Species: Rat
Sample Types: In Vivo
Applications: Bioassay -
TGF-beta signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation.
Authors: Zhang N, Bevan MJ
Nat. Immunol., 2012-05-27;13(7):667-73.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Severe dermatitis with loss of epidermal Langerhans cells in human and mouse zinc deficiency.
Authors: Kawamura T, Ogawa Y, Nakamura Y
J. Clin. Invest., 2012-01-03;122(2):722-32.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A role for IL-27p28 as an antagonist of gp130-mediated signaling.
Authors: Stumhofer JS, Tait ED, Quinn WJ, Hosken N, Spudy B, Goenka R, Fielding CA, O'Hara AC, Chen Y, Jones ML, Saris CJ, Rose-John S, Cua DJ, Jones SA, Elloso MM, Grotzinger J, Cancro MP, Levin SD, Hunter CA
Nat. Immunol., 2010-11-07;11(12):1119-26.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
B and T lymphocyte attenuator suppresses IL-21 production from follicular Th cells and subsequent humoral immune responses.
Authors: Kashiwakuma D, Suto A, Hiramatsu Y
J. Immunol., 2010-07-26;185(5):2730-6.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
VEGFR2 is selectively expressed by FOXP3high CD4+ Treg.
Authors: Suzuki H, Onishi H, Wada J, Yamasaki A, Tanaka H, Nakano K, Morisaki T, Katano M
Eur. J. Immunol., 2010-01-01;40(1):197-203.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Gram-positive bacteria enhance HIV-1 susceptibility in Langerhans cells, but not in dendritic cells, via Toll-like receptor activation.
Authors: Ogawa Y, Kawamura T, Kimura T, Ito M, Blauvelt A, Shimada S
Blood, 2009-03-11;113(21):5157-66.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Pax3 activation promotes the differentiation of mesenchymal stem cells toward the myogenic lineage.
Authors: Gang EJ, Bosnakovski D, Simsek T, To K, Perlingeiro RC
Exp. Cell Res., 2008-03-05;314(8):1721-33.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Activin A is an anticatabolic autocrine cytokine in articular cartilage whose production is controlled by fibroblast growth factor 2 and NF-kappaB.
Authors: Alexander S, Watt F, Sawaji Y, Hermansson M, Saklatvala J
Arthritis Rheum., 2007-11-01;56(11):3715-25.
Species: Human, Porcine
Sample Types: Whole Cells
Applications: Bioassay -
Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells.
Authors: Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Yokota SJ, Bankert RB
J. Immunol., 2007-05-01;178(9):5552-62.
Species: Human
Sample Types:
Applications: Bioassay -
Increase in transforming growth factor-beta in the brain during infection is related to fever, not depression of spontaneous motor activity.
Authors: Matsumura S, Shibakusa T, Fujikawa T, Yamada H, Inoue K, Fushiki T
Neuroscience, 2006-12-06;144(3):1133-40.
Species: Rat
Sample Types: In Vivo, Serum
Applications: Bioassay, In Vivo -
Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells.
Authors: David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S
Blood, 2006-10-26;109(5):1953-61.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm.
Authors: Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM
Development, 2006-08-30;133(19):3787-96.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
trans fatty acids and systemic inflammation in heart failure.
Authors: Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC
Am. J. Clin. Nutr., 2004-12-01;80(6):1521-5.
Applications: ELISA (Standard) -
Interferon-gamma inhibits transforming growth factor-beta production in human airway epithelial cells by targeting Smads.
Authors: Wen FQ, Liu X, Kobayashi T, Abe S, Fang Q, Kohyama T, Ertl R, Terasaki Y, Manouilova L, Rennard SI
Am. J. Respir. Cell Mol. Biol., 2004-01-12;30(6):816-22.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Dual regulation of proliferation and growth arrest in prostatic stromal cells by transforming growth factor-beta1.
Authors: Zhou W, Park I, Pins M, Kozlowski JM, Jovanovic B, Zhang J, Lee C, Ilio K
Endocrinology, 2003-07-24;144(10):4280-4.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Transforming growth factor-beta -Smad signaling pathway cooperates with NF-kappa B to mediate nontypeable Haemophilus influenzae-induced MUC2 mucin transcription.
Authors: Jono H, Shuto T, Xu H, Kai H, Lim DJ, Gum JR, Kim YS, Yamaoka S, Feng XH, Li JD
J. Biol. Chem., 2002-09-16;277(47):45547-57.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Transforming growth factor beta(1) selectively inhibits the cyclic AMP-dependent proliferation of primary thyroid epithelial cells by preventing the association of cyclin D3-cdk4 with nuclear p27(kip1).
Authors: Depoortere F, Pirson I, Bartek J, Dumont JE, Roger PP
Mol. Biol. Cell, 2000-03-01;11(3):1061-76.
Species: Canine
Sample Types: Whole Cells
Applications: Bioassay -
Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF.
Authors: Fadok, V A, Bratton, D L, Konowal, A, Freed, P W, Westcott, J Y, Henson, P M
J Clin Invest, 1998-02-15;101(4):890-8.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Human TGF-beta 1 Protein
Average Rating: 5 (Based on 4 Reviews)
Have you used Human TGF-beta 1 Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Reason for Rating: Excellent response in cell tissue studies at the NM level concentration