Recombinant Human Matriptase/ST14 Catalytic Domain, CF Summary
Product Specifications
Gly596-Val855, with an N-terminal Met and 6-His tag
The protein was purified, auto-activated and further purified.
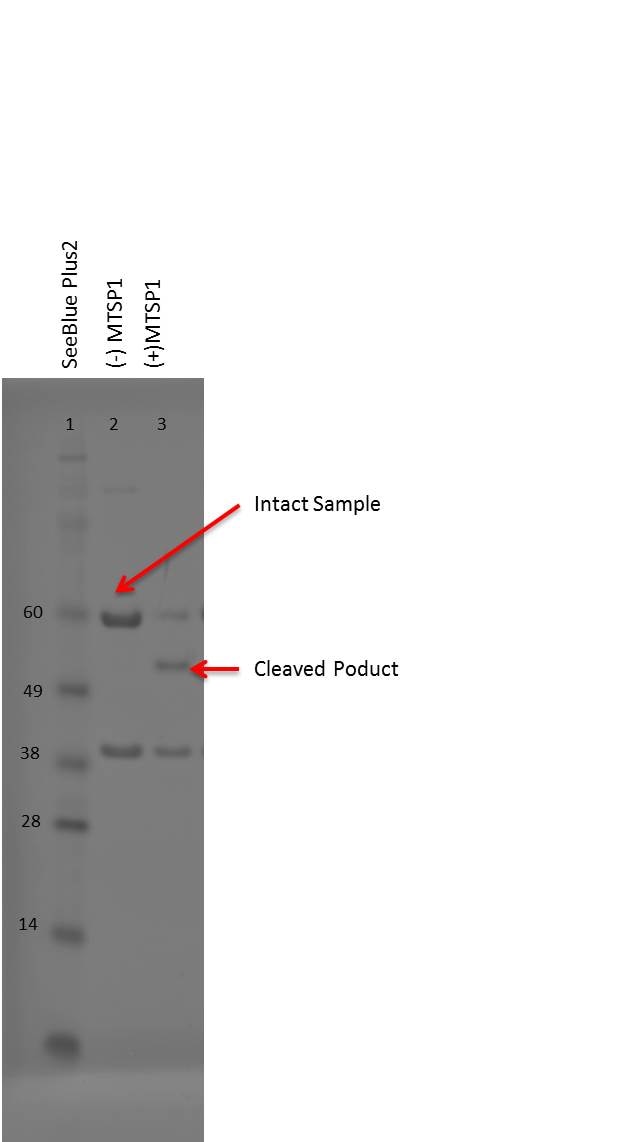
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
3946-SE
| Formulation | Supplied as a 0.2 μm filtered solution in Tris-HCl and Glycerol. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Assay Procedure
- Assay Buffer: 50 mM Tris, 50 mM NaCl, 0.01% (v/v) Tween® 20, pH 9.0
- Recombinant Human Matriptase/ST14 Catalytic Domain (rhMatriptase) (Catalog # 3946-SE)
- Substrate: BOC-Gln-Ala-Arg-AMC (Catalog # ES014), 10 mM stock in DMSO
- F16 Black Maxisorp Plate (Nunc Cat. # 475515)
- Fluorescent Plate Reader (Model: SpectraMax Gemini EM by Molecular Devices) or equivalent
- Dilute rhMatriptase to 0.1 µg/mL in Assay Buffer.
- Dilute Substrate to 50 µM in Assay Buffer.
- Load 50 µL of 0.1 µg/mL rhMatriptase into a plate, and start the reaction by adding 50 µL of 50 µM substrate. Include a Substrate Blank containing 50 µL of Assay Buffer and 50 µL of Substrate.
- Read at excitation and emission wavelengths of 380 nm and 460 nm (top read), respectively, in kinetic mode for 5 minutes.
- Calculate specific activity:
|
Specific Activity (pmol/min/µg) = |
Adjusted Vmax* (RFU/min) x Conversion Factor** (pmol/RFU) |
| amount of enzyme (µg) |
*Adjusted for Substrate Blank
**Derived using calibration standard 7-Amino, 4-Methyl Coumarin (AMC) (Sigma, Cat. # A-9891).
- rhMatriptase: 0.005 µg
- Substrate: 25 µM
Reconstitution Calculator
Background: Matriptase/ST14
Human matriptase, encoded by the ST14 (suppression of tumorogenicity 14) gene, is also known as tumor associated differentially expressed gene 15 protein/TADG‑15), epithin, and membrane‑type serine protease 1/MT‑SP1 (1). Predicted to have a significant role in tumor biology, matriptase may be a novel target for anti‑cancer therapy (2). However, expressed in most human epithelia, matriptase is also important in several physiological processes (1). For example, it activates prostasin to initiate a protease cascade that is essential for epidermal differentiation (3), and it converts a single‑chain IGFBP-rp1 into the two‑chain form (4).
Matriptase is a type II transmembrane serine protease with a complex modular structure (1). The 855 amino acid (aa) sequence of human matriptase consists of a cytoplasmic tail (aa 1‑55), a transmembrane domain (aa 56‑76), and an extracellular portion (aa 77‑855). The latter contains the following domains: SEA (aa 86‑201), two CUBs (aa 214‑334 and 340‑447), four LDLRAs (aa 452‑486, 487‑523, 524‑560, and 566‑603), and a serine protease (aa 615‑855). The physiological activation of the single‑chain zymogen requires the cleavage at the SEA domain within the ER or Golgi, association with HAI-1, which facilitates the transport of the protease to the cell surface, and auto‑cleavage at QAR-V(615)VGG (1). The activated matriptase is inhibited by HAI-1, and the resulting HAI-1 complex can be shed from the cell surface (1). R&D Systems recombinant human (rh) ST14 corresponds to the catalytic domain, and is inhibited effectively by rhHAI-1 and rhHAI-2A (Catalog # 1048‑PI and 1106‑PI).
- List, K. et al. (2006) Mol. Med. 12:1.
- Uhland, K. (2006) Cell. Mol. Life Sci. 63:2968.
- Netzel-Arnett, S. et al. (2006) J. Biol. Chem. 281:32941.
- Ahmed, S. et al. (2006) FEBS J. 273:615.
Citations for Recombinant Human Matriptase/ST14 Catalytic Domain, CF
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
15
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Engineering and Structural Insights of a Novel BBI-like Protease Inhibitor Livisin from the Frog Skin Secretion
Authors: J Yang, C Tong, J Qi, X Liao, X Li, X Zhang, M Zhou, L Wang, C Ma, X Xi, T Chen, Y Gao, D Wu
Toxins, 2022-04-12;14(4):.
Species: Odorrana livida
Sample Types: Peptide
Applications: Bioassay -
The activation fragment of PAR2 is elevated in serum from patients with rheumatoid arthritis and reduced in response to anti-IL6R treatment
Authors: S Kalogera, Y He, AC Bay-Jensen, T Gantzel, S Sun, T Manon-Jens, MA Karsdal, CS Thudium
Scientific Reports, 2021-12-20;11(1):24285.
Species: Human
Sample Types: Recombinant Protein
Applications: Bioassay -
Novel Ex Vivo Zymography Approach for Assessment of Protease Activity in Tissues with Activatable Antibodies
Authors: B Howng, MB Winter, C LePage, I Popova, M Krimm, O Vasiljeva
Pharmaceutics, 2021-09-02;13(9):.
Species: Human
Sample Types: Peptides
Applications: Bioassay -
Dexamethasone-Loaded Nanostructured Lipid Carriers for the Treatment of Dry Eye Disease
Authors: S Kumari, M Dandamudi, S Rani, E Behaeghel, G Behl, D Kent, NJ O'Reilly, O O'Donovan, P McLoughlin, L Fitzhenry
Pharmaceutics, 2021-06-18;13(6):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
PAR2 Activation on Human Kidney Tubular Epithelial Cells Induces Tissue Factor Synthesis, That Enhances Blood Clotting
Authors: A Iyer, TLR Humphries, EP Owens, KN Zhao, PP Masci, DW Johnson, D Nikolic-Pa, GC Gobe, DP Fairlie, DA Vesey
Frontiers in Physiology, 2021-03-10;12(0):615428.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Protease-activation using anti-idiotypic masks enables tumor specificity of a folate receptor 1-T cell bispecific antibody
Authors: M Geiger, KG Stubenrauc, J Sam, WF Richter, G Jordan, J Eckmann, C Hage, V Nicolini, A Freimoser-, M Ritter, ME Lauer, H Stahlberg, P Ringler, J Patel, E Sullivan, S Grau-Richa, S Endres, S Kobold, P Umaña, P Brünker, C Klein
Nat Commun, 2020-06-24;11(1):3196.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Matriptase cleaves the amyloid-beta peptide 1-42 at Arg-5, Lys-16, and Lys-28
Authors: LM Chen, KX Chai
BMC Res Notes, 2019-01-03;12(1):5.
Applications: Enzyme Assay -
Blocking the proteolytic activity of zymogen matriptase�with antibody-based inhibitors
Authors: T Tamberg, Z Hong, D Schepper, S Skovbjerg, DM Dupont, L Vitved, CR Schar, K Skjoedt, LK Vogel, JK Jensen
J. Biol. Chem., 2018-11-08;0(0):.
Applications: Enzyme Assay -
A peptide-based approach to evaluate the adaptability of influenza A virus to humans based on its hemagglutinin proteolytic cleavage site
Authors: MR Straus, GR Whittaker
PLoS ONE, 2017-03-30;12(3):e0174827.
Species: Virus - Influenza A
Sample Types: Peptide
Applications: Enzyme Assay -
A Selective Irreversible Inhibitor of Furin Does Not Prevent Pseudomonas Aeruginosa Exotoxin A-Induced Airway Epithelial Cytotoxicity
PLoS ONE, 2016-07-26;11(7):e0159868.
Species: Human
Sample Types: Protein
Applications: Enzyme Assay -
The HIV-1 gp41 ectodomain is cleaved by matriptase to produce a chemotactic peptide that acts through FPR2.
Authors: Wood M, Cole A, Eade C, Chen L, Chai K, Cole A
Immunology, 2014-07-01;142(3):474-83.
Species: Human
Sample Types: Peptide
Applications: Bioassay -
High-affinity cyclic peptide matriptase inhibitors.
Authors: Quimbar, Pedro, Malik, Uru, Sommerhoff, Christia, Kaas, Quentin, Chan, Lai Y, Huang, Yen-Hua, Grundhuber, Maresa, Dunse, Kerry, Craik, David J, Anderson, Marilyn, Daly, Norelle
J Biol Chem, 2013-04-02;288(19):13885-96.
Applications: Enzyme Assay -
Matriptase initiates activation of epidermal pro-kallikrein and disease onset in a mouse model of Netherton syndrome.
Authors: Sales KU, Masedunskas A, Bey AL
Nat. Genet., 2010-07-25;42(8):676-83.
Species: Human
Sample Types: Recombinant Protein
Applications: Bioassay -
Proteolytic cleavage of human acid-sensing ion channel 1 by the serine protease matriptase.
Authors: Clark EB, Jovov B, Rooj AK
J. Biol. Chem., 2010-07-02;285(35):27130-43.
Species: Xenopus
Sample Types: Whole Cells
Applications: Enzyme Assay -
Prostasin expression is regulated by airway surface liquid volume and is increased in cystic fibrosis.
Authors: Myerburg MM, McKenna EE, Luke CJ, Frizzell RA, Kleyman TR, Pilewski JM
Am. J. Physiol. Lung Cell Mol. Physiol., 2008-02-29;294(5):L932-41.
Species: Human
Sample Types: Peptide
Applications: Bioassay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human Matriptase/ST14 Catalytic Domain, CF
Average Rating: 5 (Based on 1 Review)
Have you used Recombinant Human Matriptase/ST14 Catalytic Domain, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: