Human/Mouse TNF-alpha Antibody Summary
Leu80-Leu235
Accession # P06804
Applications
Mouse TNF-alpha Sandwich Immunoassay
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human TNF‑ alpha by Western Blot. Western blot shows conditioned media from CHO Chinese hamster ovary cell line either mock transfected or transfected with human TNF- alpha. PVDF membrane was probed with 2 µg/mL of Goat Anti-Human/Mouse TNF-a Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-410-NA) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF017). Specific bands were detected for TNF-a at approximately 14 and 17 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of Mouse TNF‑ alpha by Western Blot. Western blot shows lysates of RAW 264.7 mouse monocyte/macrophage cell line untreated (-) or treated (+) with 10 µg/mL LPS for 4 hours. PVDF membrane was probed with 2 µg/mL of Goat Anti-Human/Mouse TNF-a Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-410-NA) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF017). Specific bands were detected for TNF-a at approximately 14 and 17 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
TNF‑ alpha in RAW 264.7 Mouse Cell Line. TNF-a was detected in immersion fixed RAW 264.7 mouse monocyte/ macrophage cell line treated with LPS using Human/Mouse TNF-a Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-410-NA) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; NL001) and counterstained with DAPI (blue). View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
TNF‑ alpha in Mouse T Cells. TNF‑ alpha was detected in immersion fixed activated mouse T Cells using 15 µg/mL Human/Mouse TNF‑ alpha Antigen Affinity-purified Polyclonal Antibody (Catalog # AF‑410‑NA) for 3 hours at room temperature. Cells were stained (red) and counterstained (green). View our protocol for Fluorescent ICC Staining of Non-adherent Cells.
 View Larger
View Larger
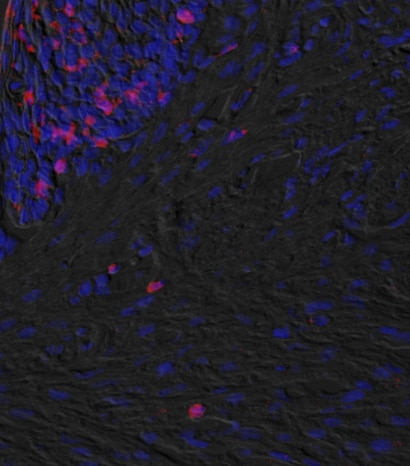
TNF-alpha in Mouse Spleen Tissue. TNF‑ alpha was detected in immersion fixed paraffin-embedded sections of mouse spleen tissue using Goat Anti-Human/Mouse TNF‑ alpha Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-410-NA) at 3 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (VC004). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to lymphocytes. Staining was performed using our IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
Cytotoxicity Induced by TNF‑ alpha and Neutralization by Mouse TNF‑ alpha Antibody. Recombinant Mouse TNF-a (410-MT) induces cytotoxicity in the the L-929 mouse fibroblast cell line in a dose-dependent manner (orange line). Cytotoxicity elicited by Recombinant Mouse TNF-a (0.1 ng/mL) is neutralized (green line) by increasing concentrations of Human/Mouse TNF-a Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-410-NA). The ND50 is typically 1.5-10 ng/mL in the presence of the metabolic inhibitor actinomycin D.
 View Larger
View Larger
Detection of TNF‑ alpha in Human Spleen. TNF‑ alpha was detected in immersion fixed paraffin-embedded sections of Human Spleen using Goat Anti-Human/Mouse TNF‑ alpha Antigen Affinity-purified Polyclonal Antibody (Catalog # AF-410-NA) at 5 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC004). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using VisUCyte Antigen Retrieval Reagent-Basic (Catalog # VCTS021). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cytoplasm in lymphocytes. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
Detection of Mouse TNF-alpha by Western Blot WNT-5A induces a proinflammatory transformation in mouse microglia. (A, B) iNOS, COX-2 and TNF alpha were detected by immunoblotting in lysates from mouse primary microglia after WNT-5A stimulation (ctrl, 300 ng/ml, 6 hours). (B’) shows TNF alpha levels in the supernatant of primary microglia under ctrl conditions and upon WNT-5A stimulation (ctrl, 300 ng/ml, 24 hours; n = 4). At least three experiments are summarized in the bar graphs. Data are normalized to ctrl. Error bars give s.e.m. (C) Microglial proliferation was assessed by an MTT assay monitoring mitochondrial activity, which is proportional to cell number [see Additional file 1: Figure S5]. Stimulation with WNT-5A (300 ng/ml, 24 hours) increased MTT, which was blocked by PTX (100 ng/ml, overnight) or the MEK1/2 (10 μM) inhibitor, SL327. (D). Experiments were done in triplicate and data from three independent experiments are shown. *, P < 0.01; ***, P < 0.001: Error bars show s.e.m.. (E) Cell tracker (red)-stained primary microglia were seeded on top of a collagen matrix in 35 mm glass bottom dishes. One day after ctrl or 300 ng/ml WNT-5A stimulation, invasion was observed by confocal microscopy and Z-stacking using a Zeiss LSM710 microscope and subsequent analysis with the Bitplane Imaris software. The size of the collagen cube shown is 1,000 (Z) x 1,400 (Y) x 1,400 (X) μm. Three invasion experiments in the absence and presence of the MEK1/2 inhibitor SL327 were quantified. Data are presented in a bar graph (F). *, P < 0.05; error bars show s.e.m.. (G) cDNA of ctrl stimulated (−) and WNT-5A stimulated (+) primary microglia was analyzed by QPCR for expression of inflammatory genes. At least three independent experiments are summarized. Gene expression is normalized to the housekeeping gene GAPDH and expressed as arbitrary units (2-delta ct). *, P < 0.05; **, P < 0.01. COX-2, cyclooxygenase 2; iNOS, inducible nitric oxide synthase; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; n, number; s.e.m., standard error of the mean. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/22647544), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: TNF-alpha
Tumor necrosis factor alpha (TNF-alpha, TNF- alpha, TNFA ), also known as Cachectin and TNFSF2, is the prototypic ligand of the TNF superfamily. It is a pleiotropic molecule that plays a central role in inflammation, immune system development, apoptosis, and lipid metabolism. TNF-alpha is produced by several lymphoid cells as well as by astrocytes, endothelial cells, and smooth muscle cells. Mouse TNF-alpha consists of a 35 amino acid (aa) cytoplasmic domain, a 21 aa transmembrane segment, and a 179 aa extracellular domain (ECD). Within the ECD, mouse TNF-alpha shares 94% aa sequence identity with rat and 70%-77% with bovine, canine, cotton rat, equine, feline, human, porcine, and rhesus TNF-alpha. TNF-alpha is produced by a wide variety of immune, epithelial, endothelial, and tumor cells. TNF-alpha is assembled intracellularly to form a noncovalently linked homotrimer which is expressed on the cell surface. Cell surface TNF-alpha can induce the lysis of neighboring tumor cells and virus infected cells, and it can generate its own downstream cell signaling following ligation by soluble TNFR I. Shedding of membrane bound TNF-alpha by TACE/ADAM17 releases the bioactive cytokine, a 55 kDa molecular weight soluble trimer of the TNF-alpha extracellular domain. TNF-alpha binds the ubiquitous 55-60 kDa TNF RI and the hematopoietic cell-restricted 80 kDa TNF RII, both of which are also expressed as homotrimers present on virtually all cell types. Both type I and type II receptors bind TNF-alpha with comparable affinity, although only TNF RI contains a cytoplasmic death domain which triggers the activation of apoptosis. Soluble forms of both types of receptors are released and can neutralize the biological activity of TNF-alpha.
Product Datasheets
Citations for Human/Mouse TNF-alpha Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
113
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Effects of CD4+ T lymphocytes from ovariectomized mice on bone marrow mesenchymal stem cell proliferation and osteogenic differentiation
Authors: Bing-Yi Shao, Lan Wang, Yang Yu, Liang Chen, Ning Gan, Wen-Ming Huang
Experimental and Therapeutic Medicine
-
Murine macrophages or their secretome delivered in alginate dressings enhance impaired wound healing in diabetic mice
Authors: Georgios Theocharidis, Sahar Rahmani, Sangmin Lee, Zhuqing Li, Antonio Lobao, Konstantinos Kounas et al.
Biomaterials
-
Beneficial effects of the traditional medicine Igongsan and its constituent ergosterol on dextran sulfate sodium-induced colitis in mice
Authors: SU-JIN KIM, HYUN-JI SHIN, GEUN-HYUK LEE, DAE-SEUNG KIM, HYE-LIN KIM, JINBONG PARK et al.
Molecular Medicine Reports
-
Smac mimetics and oncolytic viruses synergize in driving anticancer T-cell responses through complementary mechanisms
Authors: Dae-Sun Kim, Himika Dastidar, Chunfen Zhang, Franz J. Zemp, Keith Lau, Matthias Ernst et al.
Nature Communications
-
The Chinese Medicine Wu-Tou Decoction Relieves Neuropathic Pain by Inhibiting Hippocampal Microglia Activation
Authors: Chunyan Zhu, Qionghong Xu, Zhiyun Mao, Na Lin
Scientific Reports
-
Tumor necrosis factor enhances the sleep-like state and electrical stimulation induces a wake-like state in co-cultures of neurons and glia
Authors: Kathryn A. Jewett, Ping Taishi, Parijat Sengupta, Sandip Roy, Christopher J. Davis, James M. Krueger
European Journal of Neuroscience
-
Extracellularly Delivered Single-Stranded Viral RNA Causes Neurodegeneration Dependent on TLR7
Authors: Sabrina M. Lehmann, Karen Rosenberger, Christina Krüger, Piet Habbel, Katja Derkow, David Kaul et al.
The Journal of Immunology
-
ADAM17 Boosts Cholesterol Efflux and Downstream Effects of High-Density Lipoprotein on Inflammatory Pathways in Macrophages
Authors: Vishal Kothari, Jingjing Tang, Yi He, Farah Kramer, Jenny E. Kanter, Karin E. Bornfeldt
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Nucleoside Reverse Transcriptase Inhibitors (NRTIs) Induce Pathological Pain through Wnt5a-Mediated Neuroinflammation in Aging Mice
Authors: Subo Yuan, Yuqiang Shi, Kaiwen Guo, Shao-Jun Tang
Journal of Neuroimmune Pharmacology
-
Transfer of CD11c+ lamina propria mononuclear phagocytes from post-infectious irritable bowel syndrome causes mucosal barrier dysfunction and visceral hypersensitivity in recipient mice
Authors: Ya-Jun Ren, Lei Zhang, Tao Bai, Hong-Lu Yu, Ying Li, Wei Qian et al.
International Journal of Molecular Medicine
-
Deregulated JAK3 mediates growth advantage and hemophagocytosis in extranodal nasal-type natural killer/T-cell lymphoma
Authors: Adrien Picod, Suella Martino, Pascale Cervera, Gregory Manuceau, Marc Arca, Monica Wittner et al.
Haematologica
-
Alleviation of LPS-Induced Inflammation and Septic Shock by Lactiplantibacillus plantarum K8 Lysates
Authors: Gayoung Kim, Kyeong-Hun Choi, Hangeun Kim, Dae-Kyun Chung
International Journal of Molecular Sciences
-
Myeloid-derived suppressor cells promote B-cell production of IgA in a TNFR2-dependent manner.
Authors: Xu X, Meng Q, Erben U et al.
Cell. Mol. Immunol.
-
Suboptimal T-cell Therapy Drives a Tumor Cell Mutator Phenotype That Promotes Escape from First-Line Treatment
Authors: Laura Evgin, Amanda L. Huff, Timothy Kottke, Jill Thompson, Amy M. Molan, Christopher B. Driscoll et al.
Cancer Immunology Research
-
Tumor necrosis factor alpha derived from classically activated “M1” macrophages reduces interstitial cell of Cajal numbers
Authors: Seth T. Eisenman, Simon J. Gibbons, Pieter-Jan Verhulst, Gianluca Cipriani, Dieter Saur, Gianrico Farrugia
Neurogastroenterology & Motility
-
Transformation of primary murine peritoneal mast cells by constitutive KIT activation is accompanied by loss of Cdkn2a/Arf expression
Authors: Sandro Capellmann, Roland Sonntag, Herdit Schüler, Steffen K. Meurer, Lin Gan, Marlies Kauffmann et al.
Frontiers in Immunology
-
The antiallodynic action of pregabalin in neuropathic pain is independent from the opioid system
Authors: Mélanie Kremer, Ipek Yalcin, Laurent Nexon, Xavier Wurtz, Rhian Alice Ceredig, Dorothée Daniel et al.
Mol Pain
-
Covalently grafted human serum albumin coating mitigates the foreign body response against silicone implants in mice
Authors: Zhou, X;Hao, H;Chen, Y;Cao, W;Zhu, Z;Ni, Y;Liu, Z;Jia, F;Wang, Y;Ji, J;Peng Zhang, ;
Bioactive materials
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Fanca deficiency is associated with alterations in osteoclastogenesis that are rescued by TNF?
Authors: Oppezzo, A;Monney, L;Kilian, H;Slimani, L;Maczkowiak-Chartois, F;Rosselli, F;
Cell & bioscience
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Effect of rottlerin on astrocyte phenotype polarization after trimethyltin insult in the dentate gyrus of mice
Authors: Y Hwang, HC Kim, EJ Shin
Journal of Neuroinflammation, 2022-06-11;19(1):142.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Molecular mechanism of the TGF?beta/Smad7 signaling pathway in ulcerative colitis
Authors: B Bai, H Li, L Han, Y Mei, C Hu, Q Mei, J Xu, X Liu
Molecular Medicine Reports, 2022-02-09;25(4):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Single cell transcriptomic landscape of diabetic foot ulcers
Authors: G Theocharid, BE Thomas, D Sarkar, HL Mumme, WJR Pilcher, B Dwivedi, T Sandoval-S, RF Sîrbulescu, A Kafanas, I Mezghani, P Wang, A Lobao, IS Vlachos, B Dash, HC Hsia, V Horsley, SS Bhasin, A Veves, M Bhasin
Nature Communications, 2022-01-10;13(1):181.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
M1 macrophages impair tight junctions between endothelial cells after spinal cord injury
Authors: Y Luo, F Yao, X Hu, Y Li, Y Chen, Z Li, Z Zhu, S Yu, D Tian, L Cheng, M Zheng, J Jing
Brain research bulletin, 2022-01-04;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Lipopolysaccharide- TLR-4 Axis regulates Osteoclastogenesis independent of RANKL/RANK signaling
Authors: MS AlQranei, LT Senbanjo, H Aljohani, T Hamza, MA Chellaiah
BMC immunology, 2021-03-25;22(1):23.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Cornus officinalis var. koreana Kitam polyphenol extract decreases pro-inflammatory markers in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages by reducing Akt phosphorylation
Authors: RS Najjar, NS Akhavan, S Pourafshar, BH Arjmandi, RG Feresin
Journal of ethnopharmacology, 2020-12-24;0(0):113734.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Minocycline reverses IL-17A/TRAF3IP2-mediated p38 MAPK/NF-kappaB/iNOS/NO-dependent cardiomyocyte contractile depression and death
Authors: T Yoshida, NA Das, AJ Carpenter, R Izadpanah, SA Kumar, S Gautam, SB Bender, U Siebenlist, B Chandrasek
Cell. Signal., 2020-06-15;73(0):109690.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Cells
Applications: Neutralization, Western Blot -
Adipocytes promote tumor progression and induce PD-L1 expression via TNF-&alpha/IL-6 signaling
Authors: Z Li, C Zhang, JX Du, J Zhao, MT Shi, MW Jin, H Liu
Cancer Cell Int., 2020-05-20;20(0):179.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Endothelial autophagy deficiency induces IL6 - dependent endothelial mesenchymal transition and organ fibrosis
Authors: Y Takagaki, SM Lee, Z Dongqing, M Kitada, K Kanasaki, D Koya
Autophagy, 2020-01-22;0(0):1-10.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Paclitaxel-activated astrocytes produce mechanical allodynia in mice by releasing tumor necrosis factor-alpha and stromal-derived cell factor 1
Authors: X Liu, R Tonello, Y Ling, YJ Gao, T Berta
J Neuroinflammation, 2019-11-10;16(1):209.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-alpha/IL-33/ST2L signaling promotes gastric cancer metastasis
Authors: Q Zhou, X Wu, X Wang, Z Yu, T Pan, Z Li, X Chang, Z Jin, J Li, Z Zhu, B Liu, L Su
Oncogene, 2019-10-28;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
CCL28 promotes locomotor recovery after spinal cord injury via recruiting regulatory T cells
Authors: P Wang, X Qi, G Xu, J Liu, J Guo, X Li, X Ma, H Sun
Aging (Albany NY), 2019-09-26;11(18):7402-7415.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Macrophage miR-34a Is a Key Regulator of Cholesterol Efflux and Atherosclerosis
Authors: Y Xu, Y Xu, Y Zhu, H Sun, C Juguilon, F Li, D Fan, L Yin, Y Zhang
Mol. Ther., 2019-09-12;0(0):.
Species: Human, Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Role of Proinflammatory Cytokines in Feedback Modulation of Circadian Clock Gene Rhythms by Saturated Fatty Acids
Authors: SM Kim, N Neuendorff, DJ Earnest
Sci Rep, 2019-06-20;9(1):8909.
Species: Mouse
Sample Types: Transduced Whole Cells
Applications: Neutralization -
Aspergillus�fumigatus enhances human NK cell activity by regulating M1 macrophage polarization
Authors: X Zhang, D He, S Gao, Y Wei, L Wang
Mol Med Rep, 2019-06-06;20(2):1241-1249.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Inhibition of tumour necrosis factor alpha in the R6/2 mouse model of Huntington's disease by etanercept treatment
Authors: J Pido-Lopez, B Tanudjojo, S Farag, MK Bondulich, R Andre, SJ Tabrizi, GP Bates
Sci Rep, 2019-05-10;9(1):7202.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
MicroRNA-29b Enhances Osteoclast Survival by Targeting BCL-2-Modifying Factor after Lipopolysaccharide Stimulation
Authors: OJ Sul, M Rajasekara, HJ Park, JH Suh, HS Choi
Oxid Med Cell Longev, 2019-04-10;2019(0):6018180.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Neuropathology in intrauterine growth restricted newborn piglets is associated with glial activation and proinflammatory status in the brain
Authors: JA Wixey, KM Lee, SM Miller, K Goasdoue, PB Colditz, S Tracey Bjo, KK Chand
J Neuroinflammation, 2019-01-08;16(1):5.
Species: Porcine
Sample Types: Whole Tissue
Applications: IHC-P -
The Traditional Chinese Medicine MLC901 inhibits inflammation processes after focal cerebral ischemia
Authors: C Widmann, C Gandin, A Petit-Pait, M Lazdunski, C Heurteaux
Sci Rep, 2018-12-24;8(1):18062.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Protective Effects of Anti-IL17 on Acute Lung Injury Induced by LPS in Mice
Authors: RF Righetti, TM Dos Santos, LDN Camargo, LRCRB Aristótele, S Fukuzaki, FCR de Souza, FPR Santana, MVR de Agrela, MM Cruz, MIC Alonso-Val, IS Genaro, BM Saraiva-Ro, EA Leick, MA Martins, CM Prado, IFLC Tibério
Front Pharmacol, 2018-10-04;9(0):1021.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline
Authors: M Kremer, I Yalcin, Y Goumon, X Wurtz, L Nexon, D Daniel, S Megat, RA Ceredig, C Ernst, G Turecki, V Chavant, JF Theroux, A Lacaud, LE Joganah, V Lelievre, D Massotte, PE Lutz, R Gilsbach, E Salvat, M Barrot
J. Neurosci., 2018-09-24;0(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Kaempferol targeting on the fibroblast growth factor receptor 3-ribosomal S6 kinase 2 signaling axis prevents the development of rheumatoid arthritis
Authors: CJ Lee, SJ Moon, JH Jeong, S Lee, MH Lee, SM Yoo, HS Lee, HC Kang, JY Lee, WS Lee, HJ Lee, EK Kim, JY Jhun, ML Cho, JK Min, YY Cho
Cell Death Dis, 2018-03-14;9(3):401.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development (Capture) -
AKT1 distinctively suppresses MyD88-depenedent and TRIF-dependent Toll-like receptor signaling in a kinase activity-independent manner
Authors: K Zenke, M Muroi, KI Tanamoto
Cell. Signal., 2017-12-11;43(0):32-39.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Subversion of NK-cell and TNF? Immune Surveillance Drives Tumor Recurrence
Authors: T Kottke, L Evgin, KG Shim, D Rommelfang, N Boisgeraul, S Zaidi, RM Diaz, J Thompson, E Ilett, M Coffey, P Selby, H Pandha, K Harrington, A Melcher, R Vile
Cancer Immunol Res, 2017-10-15;5(11):1029-1045.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Interferon-gamma regulates inflammatory cell death by targeting necroptosis in experimental autoimmune arthritis
Authors: SH Lee, JY Kwon, SY Kim, K Jung, ML Cho
Sci Rep, 2017-08-31;7(1):10133.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development (Capture) -
Gender difference in NASH susceptibility: Roles of hepatocyte Ikk? and Sult1e1
Authors: N Matsushita, MT Hassanein, M Martinez-C, R Lazaro, SW French, W Xie, K Lai, M Karin, H Tsukamoto
PLoS ONE, 2017-08-10;12(8):e0181052.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Anti-inflammatory Effects of the Natural Compounds Cortex Phellodendri and Humulus japonicus on Pelvic Inflammatory Disease in Mice
Authors: Y Oh, YS Kwon, BD Jung
Int J Med Sci, 2017-07-18;14(8):729-734.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: ELISA Development (Capture) -
A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity
Authors: KJ Chung, A Chatzigeor, M Economopou, R Garcia-Mar, VI Alexaki, I Mitroulis, M Nati, J Gebler, T Ziemssen, SE Goelz, J Phieler, JH Lim, KP Karalis, T Papayannop, M Blüher, G Hajishenga, T Chavakis
Nat. Immunol., 2017-04-17;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Deficiency of PTP1B Attenuates Hypothalamic Inflammation via Activation of the JAK2-STAT3 Pathway in Microglia
Authors: T Tsunekawa, R Banno, A Mizoguchi, M Sugiyama, T Tominaga, T Onoue, D Hagiwara, Y Ito, S Iwama, M Goto, H Suga, Y Sugimura, H Arima
EBioMedicine, 2017-01-09;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-? and cognitive function in APP/PS1 mice
Authors: C Dempsey
Brain Behav. Immun, 2016-12-18;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development (Capture) -
Chronic blockade of the AT2 receptor with PD123319 impairs insulin signaling in C57BL/6 mice
Authors: M C Muñoz
Peptides, 2016-12-12;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Oryza sativa (Rice) Hull Extract Inhibits Lipopolysaccharide-Induced Inflammatory Response in RAW264.7 Macrophages by Suppressing Extracellular Signal-regulated Kinase, c-Jun N-terminal Kinase, and Nuclear Factor-?B Activation
Authors: Yoonsook Kim
Pharmacogn Mag, 2016-10-01;12(48):295-301.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development (Capture) -
Tumor-infiltrating monocytes/macrophages promote tumor invasion and migration by upregulating S100A8 and S100A9 expression in cancer cells
Authors: SY Lim, AE Yuzhalin, AN Gordon-Wee, RJ Muschel
Oncogene, 2016-04-18;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: Neutralization -
FcgammaRIIb inhibits immune complex-induced VEGF-A production and intranodal lymphangiogenesis.
Authors: Clatworthy M, Harford S, Mathews R, Smith K
Proc Natl Acad Sci U S A, 2014-12-04;111(50):17971-6.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
No effect of ablation of surfactant protein-D on acute cerebral infarction in mice.
Authors: Lambertsen K, Ostergaard K, Clausen B, Hansen S, Stenvang J, Thorsen S, Meldgaard M, Kristensen B, Hansen P, Sorensen G, Finsen B
J Neuroinflammation, 2014-07-19;11(0):123.
Species: Mouse
Sample Types: Plasma
Applications: Functional Assay -
Cytokine conditioning enhances systemic delivery and therapy of an oncolytic virus.
Authors: Ilett E, Kottke T, Donnelly O, Thompson J, Willmon C, Diaz R, Zaidi S, Coffey M, Selby P, Harrington K, Pandha H, Melcher A, Vile R
Mol Ther, 2014-06-24;22(10):1851-63.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Glimepiride reduces CD14 expression and cytokine secretion from macrophages.
Authors: Ingham V, Williams A, Bate C
J Neuroinflammation, 2014-06-21;11(0):115.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development (Capture) -
Pathophysiology of lung injury induced by common bile duct ligation in mice.
Authors: Shikata F, Sakaue T, Nakashiro K, Okazaki M, Kurata M, Okamura T, Okura M, Ryugo M, Nakamura Y, Yasugi T, Higashiyama S, Izutani H
PLoS ONE, 2014-04-14;9(4):e94550.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity.
Authors: Ramkhelawon B, Hennessy E, Menager M, Ray T, Sheedy F, Hutchison S, Wanschel A, Oldebeken S, Geoffrion M, Spiro W, Miller G, McPherson R, Rayner K, Moore K
Nat Med, 2014-03-02;20(4):377-84.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Mice lacking C1q are protected from high fat diet-induced hepatic insulin resistance and impaired glucose homeostasis.
Authors: Hillian, Antoinet, McMullen, Megan R, Sebastian, Becky M, Roychowdhury, Sanjoy, Kashyap, Sangeeta, Schauer, Philip R, Kirwan, John P, Feldstein, Ariel E, Nagy, Laura E
J Biol Chem, 2013-06-20;288(31):22565-75.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Regulator of calcineurin 1 suppresses inflammation during respiratory tract infections.
Authors: Junkins R, MacNeil A, Wu Z, McCormick C, Lin T
J Immunol, 2013-04-15;190(10):5178-86.
Species: Mouse
Sample Types: BALF
Applications: ELISA Development (Capture) -
Wingless-type mammary tumor virus integration site family, member 5A (Wnt5a) regulates human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein 120 (gp120)-induced expression of pro-inflammatory cytokines via the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and c-Jun N-terminal kinase (JNK) signaling pathways.
Authors: Li B, Shi Y, Shu J, Gao J, Wu P, Tang S
J Biol Chem, 2013-03-28;288(19):13610-9.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Defects in the peripheral taste structure and function in the MRL/lpr mouse model of autoimmune disease.
Authors: Kim A, Feng P, Ohkuri T, Sauers D, Cohn ZJ, Chai J, Nelson T, Bachmanov AA, Huang L, Wang H
PLoS ONE, 2012-04-19;7(4):e35588.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Response patterns of cytokines/chemokines in two murine strains after irradiation.
Authors: Zhang M, Yin L, Zhang K, Sun W, Yang S, Zhang B, Salzman P, Wang W, Liu C, Vidyasagar S, Zhang L, Ju S, Okunieff P, Zhang L
Cytokine, 2012-01-25;58(2):169-77.
Species: Mouse
Sample Types: Plasma
Applications: Luminex Development -
IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells.
Authors: Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, Vardam TD, Weis EL, Passanese J, Wang WC, Gollnick SO, Dewhirst MW, Rose-John S, Repasky EA, Baumann H, Evans SS
J. Clin. Invest., 2011-09-19;121(10):3846-59.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Resistance of human alveolar macrophages to Bacillus anthracis lethal toxin.
Authors: Wu W, Mehta H, Chakrabarty K, Booth JL, Duggan ES, Patel KB, Ballard JD, Coggeshall KM, Metcalf JP
J. Immunol., 2009-10-07;183(9):5799-806.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Attenuation of AD-like neuropathology by harnessing peripheral immune cells: local elevation of IL-10 and MMP-9.
Authors: Koronyo-Hamaoui M, Ko MK, Koronyo Y, Azoulay D, Seksenyan A, Kunis G, Pham M, Bakhsheshian J, Rogeri P, Black KL, Farkas DL, Schwartz M
J. Neurochem., 2009-09-24;111(6):1409-24.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice.
Authors: Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM
J. Clin. Invest., 2009-09-14;119(10):2954-64.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Intra- versus extracellular effects of microglia-derived cysteine proteases in a conditioned medium transfer model.
Authors: Wendt W, Schulten R, Stichel CC, Lubbert H
J. Neurochem., 2009-07-17;110(6):1931-41.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Increased cytokine production in IL-18 receptor alpha-deficient cells is associated with dysregulation of suppressors of cytokine signaling (SOCS).
Authors: Nold-Petry CA, Nold MF, Nielsen JW, Bustamante A, Zepp JA, Storm KA, Hong JW, Kim SH, Dinarello CA
J. Biol. Chem., 2009-07-10;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Cooperative phagocytes: resident microglia and bone marrow immigrants remove dead photoreceptors in retinal lesions.
Authors: Joly S, Francke M, Ulbricht E, Beck S, Seeliger M, Hirrlinger P, Hirrlinger J, Lang KS, Zinkernagel M, Odermatt B, Samardzija M, Reichenbach A, Grimm C, Reme CE
Am. J. Pathol., 2009-05-12;174(6):2310-23.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
ST2 deficient mice display a normal host defense against pulmonary infection with Mycobacterium tuberculosis.
Authors: Wieland CW, van der Windt GJ, Florquin S, McKenzie AN, van der Poll T
Microbes Infect., 2009-03-13;11(4):524-30.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Rod-Shaped monocytes patrol the brain vasculature and give rise to perivascular macrophages under the influence of proinflammatory cytokines and angiopoietin-2.
Authors: Audoy-Remus J, Richard JF, Soulet D, Zhou H, Kubes P, Vallieres L
J. Neurosci., 2008-10-08;28(41):10187-99.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Hypoxia enhances lysosomal TNF-alpha degradation in mouse peritoneal macrophages.
Authors: Lahat N, Rahat MA, Kinarty A, Weiss-Cerem L, Pinchevski S, Bitterman H
Am. J. Physiol., Cell Physiol., 2008-04-23;295(1):C2-12.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
CT20126, a novel immunosuppressant, prevents collagen-induced arthritis through the down-regulation of inflammatory gene expression by inhibiting NF-kappaB activation.
Authors: Lee SJ, Nam WD, Na HJ, Cho YL, Ha KS, Hwang JY, Lee H, Kim SO, Kwon YG, Kim YM
Biochem. Pharmacol., 2008-04-18;76(1):79-90.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
CD14 contributes to pulmonary inflammation and mortality during murine tuberculosis.
Authors: Wieland CW, van der Windt GJ, Wiersinga WJ, Florquin S, van der Poll T
Immunology, 2008-04-03;125(2):272-9.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: ELISA Development -
Tumor-specific Th17-polarized cells eradicate large established melanoma.
Authors: Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP
Blood, 2008-03-19;112(2):362-73.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Lipoteichoic acid isolated from Lactobacillus plantarum inhibits lipopolysaccharide-induced TNF-alpha production in THP-1 cells and endotoxin shock in mice.
Authors: Kim HG, Kim NR, Gim MG, Lee JM, Lee SY, Ko MY, Kim JY, Han SH, Chung DK
J. Immunol., 2008-02-15;180(4):2553-61.
Species: Mouse
Sample Types: Serum
Applications: ELISA Development -
Antipsoriatic effects of avarol-3'-thiosalicylate are mediated by inhibition of TNF-alpha generation and NF-kappaB activation in mouse skin.
Authors: Amigo M, Paya M, De Rosa S, Terencio MC
Br. J. Pharmacol., 2007-07-16;152(3):353-65.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
TNF-alpha and IL-1beta mediate inflammatory hypernociception in mice triggered by B1 but not B2 kinin receptor.
Authors: Cunha TM, Verri WA, Jr, Fukada SY, Guerrero AT, Santodomingo-Garzon T, Poole S, Parada CA, Ferreira SH, Cunha FQ
Eur. J. Pharmacol., 2007-07-13;573(1):221-9.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: ELISA Development -
Overexpression of suppressor of cytokine signaling-5 in T cells augments innate immunity during septic peritonitis.
Authors: Watanabe H, Kubo M, Numata K, Takagi K, Mizuta H, Okada S, Ito T, Matsukawa A
J. Immunol., 2006-12-15;177(12):8650-7.
Species: Mouse
Sample Types: Peritoneal Lavage Fluid
Applications: ELISA Development -
Antiinflammatory effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on zymosan-induced arthritis in mice.
Authors: Penido C, Conte FP, Chagas MS, Rodrigues CA, Pereira JF, Henriques MG
Inflamm. Res., 2006-11-01;55(11):457-64.
Species: Mouse
Sample Types: Synovial Fluid
Applications: ELISA Development -
Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors.
Authors: Hajjar AM, Harvey MD, Shaffer SA, Goodlett DR, Sjostedt A, Edebro H, Forsman M, Bystrom M, Pelletier M, Wilson CB, Miller SI, Skerrett SJ, Ernst RK
Infect. Immun., 2006-09-18;74(12):6730-8.
Species: Mouse
Sample Types: BALF
Applications: ELISA Development -
Neutrophil recruitment in immunized mice depends on MIP-2 inducing the sequential release of MIP-1alpha, TNF-alpha and LTB(4).
Authors: Ramos CD, Fernandes KS, Canetti C, Teixeira MM, Silva JS, Cunha FQ
Eur. J. Immunol., 2006-08-01;36(8):2025-34.
Species: Mouse
Sample Types: Tissue Secretion
Applications: ELISA Development -
Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality.
Authors: Osuchowski MF, Welch K, Siddiqui J, Remick DG
J. Immunol., 2006-08-01;177(3):1967-74.
Species: Mouse
Sample Types: Plasma
Applications: ELISA Development -
Glatiramer acetate fights against Alzheimer's disease by inducing dendritic-like microglia expressing insulin-like growth factor 1.
Authors: Butovsky O, Koronyo-Hamaoui M, Kunis G, Ophir E, Landa G, Cohen H, Schwartz M
Proc. Natl. Acad. Sci. U.S.A., 2006-07-24;103(31):11784-9.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
T lineage differentiation from human embryonic stem cells.
Authors: Galic Z, Kitchen SG, Kacena A, Subramanian A, Burke B, Cortado R, Zack JA
Proc. Natl. Acad. Sci. U.S.A., 2006-07-14;103(31):11742-7.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Adenoviral-mediated overexpression of SOCS3 enhances IgG immune complex-induced acute lung injury.
Authors: Gao H, Hoesel LM, Guo RF, Rancilio NJ, Sarma JV, Ward PA
J. Immunol., 2006-07-01;177(1):612-20.
Species: Mouse
Sample Types: BALF
Applications: ELISA Development -
Protective effects of neurotrophic factors on tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis of murine adrenal chromaffin cell line tsAM5D.
Authors: Murata T, Tsuboi M, Hikita K, Kaneda N
J. Biol. Chem., 2006-06-13;281(32):22503-16.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Glechoma hederacea inhibits inflammatory mediator release in IFN-gamma and LPS-stimulated mouse peritoneal macrophages.
Authors: An HJ, Jeong HJ, Um JY, Kim HM, Hong SH
J Ethnopharmacol, 2006-03-10;106(3):418-24.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells.
Authors: Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M
Mol. Cell. Neurosci., 2005-11-16;31(1):149-60.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Tumor necrosis factor-like weak inducer of apoptosis increases the permeability of the neurovascular unit through nuclear factor-kappa B pathway activation.
Authors: Polavarapu R, Gongora MC, Winkles JA, Yepes M
J. Neurosci., 2005-11-02;25(44):10094-100.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
The sex steroid precursor DHEA accelerates cutaneous wound healing via the estrogen receptors.
Authors: Mills SJ, Ashworth JJ, Gilliver SC, Hardman MJ, Ashcroft GS
J. Invest. Dermatol., 2005-11-01;125(5):1053-62.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC-P, Western Blot -
Sequential ELISA to profile multiple cytokines from small volumes.
Authors: Osuchowski MF, Siddiqui J, Copeland S, Remick DG
J. Immunol. Methods, 2005-07-01;302(1):172-81.
Species: Mouse
Sample Types: Plasma
Applications: ELISA Development -
Antibody-bound beta-amyloid precursor protein stimulates the production of tumor necrosis factor-alpha and monocyte chemoattractant protein-1 by cortical neurons.
Authors: Gonzalez de Aguilar JL, Dupuis L, Larmet Y
Neurobiol. Dis., 2005-06-01;19(1):129-41.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Neutralization, Western Blot -
Selective macrophage suppression during sepsis.
Authors: Ellaban E, Bolgos G, Remick D
Cell. Immunol., 2005-02-26;231(1):103-11.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Transcriptional profiling of lipopolysaccharide-induced acute lung injury.
Authors: Jeyaseelan S, Chu HW, Young SK, Worthen GS
Infect. Immun., 2004-12-01;72(12):7247-56.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: ELISA Development -
Thyrocytes isolated from autoimmune-diseased thyroids secrete soluble tumor necrosis factor-R1 that is related to their elevated protein kinase C activity.
Authors: LaBue M, Colburn KK, Green LM
Thyroid, 2004-04-01;14(4):249-62.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: Immunoprecipitation, Western Blot -
Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice.
Authors: Tezel G, Yang X, Yang J, Wax MB
Brain Res., 2004-01-23;996(2):202-12.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Elevated levels of tumor necrosis factor alpha (TNF-alpha) in human immunodeficiency virus type 1-transgenic mice: prevention of death by antibody to TNF-alpha.
Authors: De SK, Devadas K, Notkins AL
J. Virol., 2002-11-01;76(22):11710-4.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Modulation of the Sigma-1 Receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis.
Authors: Dorian A. Rosen, Scott M. Seki, Anthony Fernández-Castañeda, Rebecca M. Beiter, Jacob D. Eccles, Judith A. Woodfolk et al.
Science Translational Medicine
-
Blocking the ZZ domain of sequestosome1/p62 suppresses myeloma growth and osteoclast formation in vitro and induces dramatic bone formation in myeloma-bearing bones in vivo
Authors: Jumpei Teramachi, Rebecca Silbermann, Peng Yang, Wei Zhao, Khalid S. Mohammad, Jianxia Guo et al.
Leukemia
-
Direct protection of cultured neurons from ischemia-like injury by minocycline
Authors: Wendy C. Huang, Yanli Qiao, Lijun Xu, Rachid Kacimi, Xiaoyun Sun, Rona G. Giffard et al.
Anatomy & Cell Biology
-
B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation
Authors: W Sun, N Meednu, A Rosenberg, J Rangel-Mor, V Wang, J Glanzman, T Owen, X Zhou, H Zhang, BF Boyce, JH Anolik, L Xing
Nat Commun, 2018-12-03;9(1):5127.
-
Expansion and Activation Kinetics of Immune Cells during Early Phase of GVHD in Mouse Model Based on Chemotherapy Conditioning
Authors: Behnam Sadeghi, Suleiman Al-Hashmi, Zuzana Hassan, Bjorn Rozell, Hernan Concha, Carin Lundmark et al.
Clinical and Developmental Immunology
-
Neuroprotective effects of SMTP-44D in mice stroke model in relation to neurovascular unit and trophic coupling
Authors: Xiaowen Shi, Yasuyuki Ohta, Jingwei Shang, Ryuta Morihara, Yumiko Nakano, Yusuke Fukui et al.
Journal of Neuroscience Research
-
LRP1 expression in microglia is protective during CNS autoimmunity
Authors: Tzu-Ying Chuang, Yong Guo, Scott M. Seki, Abagail M. Rosen, David M. Johanson, James W. Mandell et al.
Acta Neuropathologica Communications
-
Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation
Authors: Carina Halleskog, Jacomijn Petronella Dijksterhuis, Michaela Brita Christina Kilander, Javier Becerril-Ortega, Juan Carlos Villaescusa, Eva Lindgren et al.
Journal of Neuroinflammation
-
Somatotopic Organization and Intensity Dependence in Driving Distinct NPY-Expressing Sympathetic Pathways by Electroacupuncture
Authors: Shenbin Liu, Zhi-Fu Wang, Yang-Shuai Su, Russell S. Ray, Xiang-Hong Jing, Yan-Qing Wang et al.
Neuron
-
Cytokine signaling by grafted neuroectodermal stem cells rescues motoneurons destined to die
Authors: Krisztián Pajer, Georg A. Feichtinger, Gábor Márton, Sonja Sabitzer, Dieter Klein, Heinz Redl et al.
Experimental Neurology
-
Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1 beta
Authors: Jung-Eun Kim, Thuy Xuan Phan, Vu Hong Nguyen, Hong-Van Dinh-Vu, Jin Hai Zheng, Misun Yun et al.
Theranostics
-
Induction of microglial toll-like receptor 4 by prothrombin kringle-2: a potential pathogenic mechanism in Parkinson’s disease
Authors: Won-Ho Shin, Min-Tae Jeon, Eunju Leem, So-Yoon Won, Kyoung Hoon Jeong, Sang-Joon Park et al.
Scientific Reports
-
SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-kappa B
Authors: Christopher J. Neufeldt, Berati Cerikan, Mirko Cortese, Jamie Frankish, Ji-Young Lee, Agnieszka Plociennikowska et al.
Communications Biology
-
Eosinophil persistence in vivo and sustained viability ex vivo in response to respiratory challenge with fungal allergens
Authors: Wendy E. Geslewitz, Caroline M. Percopo, Helene F. Rosenberg
Clinical & Experimental Allergy
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse TNF-alpha Antibody
Average Rating: 4.4 (Based on 5 Reviews)
Have you used Human/Mouse TNF-alpha Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: