Human CXCL13/BLC/BCA-1 Quantikine ELISA Kit Summary
Sample Values
Serum/Plasma/Saliva - Samples from apparently healthy volunteers were evaluated for the presence of human BLC/BCA-1 in this assay. No medical histories were available for the donors used in this study.| Sample Type | Mean (pg/mL) | Range (pg/mL) | Standard Deviation (pg/mL) |
| Serum* (n=35) | 81.9 | 39.4-252 | 43.5 |
| EDTA plasma (n=35) | 62.5 | 29.4-246 | 42.7 |
| Heparin plasma (n=35) | 63.5 | 21.7-240 | 44.1 |
| Sample Type | Mean of Detectable (pg/mL) | % Detectable | Range (pg/mL) |
| Saliva (n=10) | 67.4 | 70 | ND-284 |
| Condition | Day 1 (pg/mL) | Day 5 (pg/mL) |
| Unstimulated | ND | ND |
| Stimulated | 11 | 640 |
Customers also Viewed
Product Summary
Precision
Cell Culture Supernates, Serum, EDTA Plasma, Heparin Plasma, Saliva
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 50.2 | 136 | 294 | 54 | 138 | 285 |
| Standard Deviation | 2.2 | 4.5 | 8 | 5.1 | 13.2 | 24.8 |
| CV% | 4.3 | 3.3 | 2.7 | 9.4 | 9.6 | 8.7 |
Recovery
The recovery of natural BLC/BCA-1 spiked to levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=4) | 98 | 87-110 |
| EDTA Plasma (n=4) | 99 | 92-107 |
| Heparin Plasma (n=4) | 101 | 92-115 |
| Serum (n=4) | 99 | 86-108 |
Linearity
Scientific Data
 View Larger
View Larger
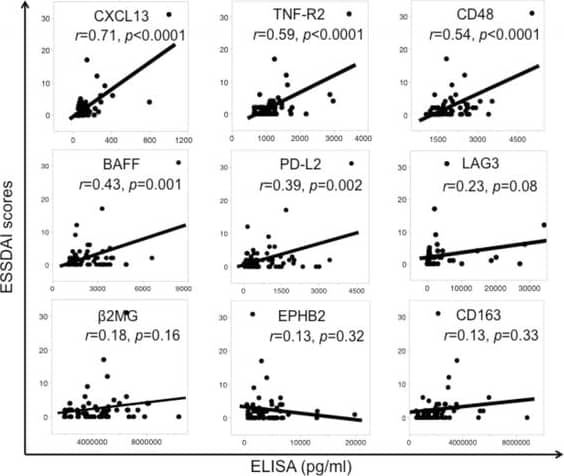
Human CXCL13/BLC/BCA-1 Quantikine ELISA Kit Correlation between European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index (ESSDAI) scores and concentrations of nine screened serum proteins analyzed by ELISA in the validation cohort of patients with primary Sjögren’s syndrome (n = 58). The nine proteins were CXCL13, TNF receptor 2 (TNF-R2), CD48, B-cell activating factor (BAFF), programmed cell death protein 1 ligand 2 (PD-L2), lymphocyte activation gene-3 (LAG-3), beta 2-microglobulin ( beta 2MG), ephrin type-B receptor 2 (EPHB2), and CD163. Pearson’s correlation test was used. The correlation coefficients (r) and P values are shown in the table. P <0.05 was considered significant Image collected and cropped by CiteAb from the following open publication (https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-016-1006-1), licensed under a CC-BY license. Not internally tested by R&D Systems.
Product Datasheets
Preparation and Storage
Background: CXCL13/BLC/BCA-1
CXCL13/BLC/BCA-1 is a constitutively expressed chemokine that plays an important role in B and T cell homing. It is expressed by salivary gland epithelium, dendritic cells, osteoclasts, and peritoneal macrophages. It can form homodimers or heterodimers with FGF basic, and it signals through CXCR3 or CXCR5. CXCL13 induces the migration of naïve B cells and a subset of memory T cells to lymphoid tissue. It also promotes B1 cell migration into the omentum and peritoneum. In the fetus, CXCL13 attracts CD4+ CD3- IL-7 R alpha+ hematopoietic cells to sites of future Peyer’s patch development.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 µL of Assay Diluent to each well.
- Add 50 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes.
- Add 200 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours.
- Aspirate and wash 4 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Human CXCL13/BLC/BCA-1 Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
49
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Follicular helper- and peripheral helper-like T cells drive autoimmune disease in human immune system mice
Authors: Vecchione, A;Khosravi-Maharlooei, M;Danzl, N;Li, HW;Nauman, G;Madley, R;Waffarn, E;Winchester, R;Ruiz, A;Ding, X;Fousteri, G;Sykes, M;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Plasma
-
CXCL13 in Cerebrospinal Fluid: Clinical Value in a Large Cross-Sectional Study
Authors: Erhart, DK;Klose, V;Schäper, T;Tumani, H;Senel, M;
International journal of molecular sciences
Species: Human
Sample Types: CSF, Serum
-
Intrathecal Th17-driven inflammation is associated with prolonged post-treatment convalescence for patients with Lyme neuroborreliosis
Authors: Gyllemark, P;Sjöwall, J;Forsberg, P;Ernerudh, J;Henningsson, AJ;
Scientific reports
Species: Human
Sample Types: CSF
-
Safety and immunogenicity following co-administration of Yellow fever vaccine with Tick-borne encephalitis or Japanese encephalitis vaccines: Results from an open label, non-randomized clinical trial
Authors: JT Sandberg, M Löfling, R Varnait?, J Emgård, N Al-Tawil, L Lindquist, S Gredmark-R, J Klingström, K Loré, K Blom, HG Ljunggren
PloS Neglected Tropical Diseases, 2023-02-09;17(2):e0010616.
Species: Human
Sample Types: Serum
-
CXCL13 is a predictive biomarker in idiopathic multicentric Castleman disease
Authors: SK Pierson, L Katz, R Williams, M Mumau, M Gonzalez, S Guzman, A Rubenstein, AB Oromendia, P Beineke, A Fosså, F van Rhee, DC Fajgenbaum
Nature Communications, 2022-11-24;13(1):7236.
Species: Human
Sample Types: Serum
-
Escherichia coli-specific CXCL13-producing TFH are associated with clinical efficacy of neoadjuvant PD-1 blockade against muscle-invasive bladder cancer
Authors: AG Goubet, L Lordello, C Alves Cost, I Peguillet, M Gazzano, MD Mbogning-F, C Thelemaque, C Lebacle, C Thibault, F Audenet, G Pignot, G Gravis, C Helissey, L Campedel, M Roupret, E Xylinas, I Ouzaid, A Dubuisson, M Mazzenga, C Flament, P Ly, V Marty, N Signolle, A Sauvat, T Sbarrato, M Filahi, C Davin, G Haddad, J Bou Khalil, C Bleriot, FX Danlos, G Dunsmore, K Mulder, A Silvin, T Raoult, B Archambaud, S Belhechmi, I Gomperts B, N Cayet, M Moya-Nilge, A Mallet, R Daillere, E Rouleau, C Radulescu, Y Allory, J Fieschi, M Rouanne, F Ginhoux, G Le Teuff, L Derosa, A Marabelle, J VAN Dorp, N VAN Dijk, MS van der He, B Besse, F Andre, M Merad, G Kroemer, JY Scoazec, L Zitvogel, Y Loriot
Cancer Discovery, 2022-10-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Dynamics of Inflammatory and Neurodegenerative Biomarkers after Autologous Hematopoietic Stem Cell Transplantation in Multiple Sclerosis
Authors: J Ruder, G Dinner, A Maceski, E Berenjeno-, AM Müller, I Jelcic, J Kuhle, R Martin
International Journal of Molecular Sciences, 2022-09-19;23(18):.
Species: Human
Sample Types: Serum
-
CXCL13-producing CD4+ T cells accumulate in early phase of tertiary lymphoid structures in ovarian cancer
Authors: M Ukita, J Hamanishi, H Yoshitomi, K Yamanoi, S Takamatsu, A Ueda, H Suzuki, Y Hosoe, Y Furutake, M Taki, K Abiko, K Yamaguchi, H Nakai, T Baba, N Matsumura, A Yoshizawa, H Ueno, M Mandai
JCI Insight, 2022-06-22;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Clinical value of urinary cytokines/chemokines as prognostic markers in patients with crescentic glomerulonephritis
Authors: J Jeon, J Park, HJ Boo, KE Yang, CJ Lee, JE Lee, K Kim, GY Kwon, W Huh, DJ Kim, YG Kim, HR Jang
Oncogene, 2022-06-17;12(1):10221.
Species: Human
Sample Types: Serum
-
EBV/HHV-6A dUTPases contribute to myalgic encephalomyelitis/chronic fatigue syndrome pathophysiology by enhancing TFH cell differentiation and extrafollicular activities
Authors: BS Cox, K Alharshawi, I Mena-Palom, WP Lafuse, ME Ariza
JCI Insight, 2022-06-08;7(11):.
Species: Human
Sample Types: Serum
-
Cerebrospinal Fluid Biomarkers in Relation to MRZ Reaction Status in Primary Progressive Multiple Sclerosis
Authors: T Robinson, A Abdelhak, T Bose, E Meinl, M Otto, UK Zettl, R Dersch, H Tumani, S Rauer, A Huss
Cells, 2020-11-25;9(12):.
Species: Human
Sample Types: CSF
-
CXCL13 plasma levels function as a biomarker for disease activity in patients with chronic lymphocytic leukemia
Authors: M Sivina, L Xiao, E Kim, A Vaca, SS Chen, MJ Keating, A Ferrajoli, Z Estrov, N Jain, WG Wierda, X Huang, N Chiorazzi, JA Burger
Leukemia, 2020-10-21;0(0):.
Species: Human
Sample Types: Plasma
-
Mediating effect of soluble B-cell activation immune markers on the association between anthropometric and lifestyle factors and lymphoma development
Authors: F Saberi Hos, PM Kolijn, D Casabonne, A Nieters, M Solans, S Naudin, P Ferrari, JD Mckay, E Weiderpass, V Perduca, C Besson, FR Mancini, G Masala, V Krogh, F Ricceri, JM Huerta, D Petrova, N Sala, A Trichopoul, A Karakatsan, C La Vecchia, R Kaaks, F Canzian, D Aune, H Boeing, MB Schulze, A Perez-Corn, AW Langerak, VHJ van der Ve, R Vermeulen
Sci Rep, 2020-08-14;10(1):13814.
Species: Human
Sample Types: Serum
-
Serum and CSF cytokine levels mirror different neuroimmunological mechanisms in patients with LGI1 and Caspr2 encephalitis
Authors: P Körtvelyes, A Goihl, K Guttek, B Schraven, H Prüss, D Reinhold
Cytokine, 2020-08-12;135(0):155226.
Species: Human
Sample Types: Serum
-
Ustekinumab inhibits T follicular helper cell differentiation in patients with Crohn's disease
Authors: AM Globig, NP Sommer, K Wild, J Schardey, K Zoldan, AK Thomann, LA Schulte, R Schreiner, W Reindl, J Klaus, CM Schempp, M Hofmann, R Thimme, T Boettler, P Hasselblat
Cell Mol Gastroenterol Hepatol, 2020-07-15;0(0):.
Species: Human
Sample Types: Plasma
-
Association between the cytokine storm, immune cell dynamics, and viral replicative capacity in hyperacute HIV infection
Authors: DM Muema, NA Akilimali, OC Ndumnego, SS Rasehlo, R Durgiah, DBA Ojwach, N Ismail, M Dong, A Moodley, KL Dong, ZM Ndhlovu, JM Mabuka, BD Walker, JK Mann, T Ndung'u
BMC Med, 2020-03-25;18(1):81.
Species: Human
Sample Types: Plasma
-
Activated circulating T follicular helper cells and skewing of T follicular helper 2�cells are down-regulated by treatment including an inhaled corticosteroid in patients with allergic asthma
Authors: S Miyajima, K Shigehara, R Kamekura, H Takaki, H Yabe, I Ikegami, Y Asai, H Nishikiori, H Chiba, E Uno, H Takahashi, S Ichimiya
Allergol Int, 2019-10-22;0(0):.
Species: Human
Sample Types: Plasma
-
Altered differentiation is central to HIV-specific CD4+ T cell dysfunction in progressive disease
Authors: A Morou, E Brunet-Rat, M Dubé, R Charlebois, E Mercier, S Darko, N Brassard, K Nganou-Mak, S Arumugam, G Gendron-Le, L Yang, J Niessl, AE Baxter, JM Billingsle, PA Rajakumar, F Lefebvre, RP Johnson, C Tremblay, JP Routy, RT Wyatt, A Finzi, DC Douek, DE Kaufmann
Nat. Immunol., 2019-07-15;20(8):1059-1070.
Species: Human
Sample Types: Cell Culture Supernates
-
Group 3 innate lymphoid cells mediate early protective immunity against tuberculosis
Authors: A Ardain, R Domingo-Go, S Das, SW Kazer, NC Howard, A Singh, M Ahmed, S Nhamoyebon, J Rangel-Mor, P Ogongo, L Lu, D Ramsuran, M de la Luz, T K Ulland, M Darby, E Park, F Karim, L Melocchi, R Madansein, KJ Dullabh, M Dunlap, N Marin-Agud, T Ebihara, T Ndung'u, D Kaushal, AS Pym, JK Kolls, A Steyn, J Zúñiga, W Horsnell, WM Yokoyama, AK Shalek, HN Kløverpris, M Colonna, A Leslie, SA Khader
Nature, 2019-06-05;570(7762):528-532.
Species: Human
Sample Types: Plasma
-
Sulfisoxazole inhibits the secretion of small extracellular vesicles by targeting the endothelin receptor A
Authors: EJ Im, CH Lee, PG Moon, GG Rangaswamy, B Lee, JM Lee, JC Lee, JG Jee, JS Bae, TK Kwon, KW Kang, MS Jeong, JE Lee, HS Jung, HJ Ro, S Jun, W Kang, SY Seo, YE Cho, BJ Song, MC Baek
Nat Commun, 2019-03-27;10(1):1387.
Species: Human
Sample Types: Cell Culture Supernates
-
HIV-specific Fc effector function early in infection predicts the development of broadly neutralizing antibodies
Authors: SI Richardson, AW Chung, H Natarajan, B Mabvakure, NN Mkhize, N Garrett, S Abdool Kar, PL Moore, ME Ackerman, G Alter, L Morris
PLoS Pathog., 2018-04-09;14(4):e1006987.
Species: Human
Sample Types: Plasma
-
Point-of-care testing for CXCL13 in Lyme neuroborreliosis
Authors: A Pietikäine, J Oksi, J Hytönen
Diagn. Microbiol. Infect. Dis., 2018-02-23;0(0):.
Species: Human
Sample Types: CSF
-
Lower Baseline Germinal Center Activity and Preserved Th1 Immunity Are Associated With Hepatitis B Vaccine Response in Treated HIV Infection
Authors: RM Paris, LG Milagres, E Moysi, JF Okulicz, BK Agan, A Ganesan, C Petrovas, RA Koup
Pathog Immun, 2017-03-14;2(1):66-88.
Species: Human
Sample Types: Plasma
-
Elevated C-X-C motif ligand 13 and B-cell-activating factor levels in neuromyelitis optica during remission
Authors: S Wang, T Yang, J Wan, Y Zhang, Y Fan
Brain Behav, 2017-03-10;7(4):e00648.
Species: Human
Sample Types: Serum
-
Intrathecal Th17- and B cell-associated cytokine and chemokine responses in relation to clinical outcome in Lyme neuroborreliosis: a large retrospective study
Authors: P Gyllemark, P Forsberg, J Ernerudh, AJ Henningsso
J Neuroinflammation, 2017-02-01;14(1):27.
Species: Human
Sample Types: Serum
-
BAFF Index and CXCL13 levels in the cerebrospinal fluid associate respectively with intrathecal IgG synthesis and cortical atrophy in multiple sclerosis at clinical onset
Authors: M Puthenpara, L Federle, S Miante, A Zito, E Toffanin, S Ruggero, M Ermani, S Pravato, D Poggiali, P Perini, F Rinaldi, P Gallo
J Neuroinflammation, 2017-01-17;14(1):11.
Species: Human
Sample Types: Serum
-
CXCL13 is a plasma biomarker of germinal center activity
Proc. Natl. Acad. Sci. U.S.A., 2016-02-23;113(10):2702-7.
Species: Human, Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Plasma
-
Predictive Value of Cytokines and Immune Activation Biomarkers in AIDS-Related Non-Hodgkin Lymphoma Treated with Rituximab plus Infusional EPOCH (AMC-034 trial).
Authors: Epeldegui M, Lee J, Martinez A, Widney D, Magpantay L, Regidor D, Mitsuyasu R, Sparano J, Ambinder R, Martinez-Maza O
Clin Cancer Res, 2015-09-17;22(2):328-36.
Species: Human
Sample Types: Plasma
-
Biomarkers of inflammation and axonal degeneration/damage in patients with newly diagnosed multiple sclerosis: contributions of the soluble CD163 CSF/serum ratio to a biomarker panel.
Authors: Stilund M, Gjelstrup M, Petersen T, Moller H, Rasmussen P, Christensen T
PLoS ONE, 2015-04-10;10(4):e0119681.
Species: Human
Sample Types: Serum
-
HIV-1 single-stranded RNA induces CXCL13 secretion in human monocytes via TLR7 activation and plasmacytoid dendritic cell-derived type I IFN.
Authors: Cohen K, Dugast A, Alter G, McElrath M, Stamatatos L
J Immunol, 2015-02-09;194(6):2769-75.
Species: Human
Sample Types: Plasma
-
CXCL13 and neopterin concentrations in cerebrospinal fluid of patients with Lyme neuroborreliosis and other diseases that cause neuroinflammation.
Authors: Hytonen J, Kortela E, Waris M, Puustinen J, Salo J, Oksi J
J Neuroinflammation, 2014-06-11;11(0):103.
Species: Human
Sample Types: CSF
-
Cerebrospinal fluid immunoglobulin kappa light chain in clinically isolated syndrome and multiple sclerosis.
Authors: Senel M, Tumani H, Lauda F, Presslauer S, Mojib-Yezdani R, Otto M, Brettschneider J
PLoS ONE, 2014-04-02;9(4):e88680.
Species: Human
Sample Types: CSF
-
BAFF/APRIL system in pediatric OMS: relation to severity, neuroinflammation, and immunotherapy.
Authors: Pranzatelli, Michael, Tate, Elizabet, McGee, Nathan R, Travelstead, Anna L, Colliver, Jerry A, Ness, Jayne M, Ransohoff, Richard
J Neuroinflammation, 2013-01-16;10(0):10.
Species: Human
Sample Types: Serum
-
Cerebrospinal Fluid Neopterin as Marker of the Meningo-Encephalitic Stage of Trypanosoma brucei gambiense Sleeping Sickness.
Authors: Tiberti N, Hainard A, Lejon V, Courtioux B, Matovu E, Enyaru JC, Robin X, Turck N, Kristensson K, Ngoyi DM, Vatunga GM, Krishna S, Buscher P, Bisser S, Ndung'u JM, Sanchez JC
PLoS ONE, 2012-07-18;7(7):e40909.
Species: Human
Sample Types: CSF
-
The chemokine CXCL13 is a prognostic marker in clinically isolated syndrome (CIS).
Authors: Brettschneider J, Czerwoniak A, Senel M
PLoS ONE, 2010-08-05;5(8):e11986.
Species: Human
Sample Types: Serum
-
Serum CXCL13 positively correlates with prostatic disease, prostate-specific antigen and mediates prostate cancer cell invasion, integrin clustering and cell adhesion.
Authors: Singh S, Singh R, Sharma PK, Singh UP, Rai SN, Chung LW, Cooper CR, Novakovic KR, Grizzle WE, Lillard JW
Cancer Lett., 2009-04-17;283(1):29-35.
Species: Human
Sample Types: Serum
-
Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease.
Authors: Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, Kim JS
J. Immunol., 2009-03-15;182(6):3919-27.
Species: Human
Sample Types: Plasma
-
Chemokine CXCL13 is overexpressed in the tumour tissue and in the peripheral blood of breast cancer patients.
Authors: Panse J, Friedrichs K, Marx A, Hildebrandt Y, Luetkens T, Bartels K, Horn C, Stahl T, Cao Y, Milde-Langosch K, Niendorf A, Kroger N, Wenzel S, Leuwer R, Bokemeyer C, Hegewisch-Becker S, Atanackovic D
Br. J. Cancer, 2008-09-16;99(6):930-8.
Species: Human
Sample Types: Serum
-
Altered expression of the receptor-ligand pair CXCR5/CXCL13 in B cells during chronic HIV-1 infection.
Authors: Cagigi A, Mowafi F, Phuong Dang LV, Tenner-Racz K, Atlas A, Grutzmeier S, Racz P, Chiodi F, Nilsson A
Blood, 2008-09-09;112(12):4401-10.
Species: Human
Sample Types: Cell Culture Supernates
-
Potential novel biomarkers of disease activity in rheumatoid arthritis patients: CXCL13, CCL23, transforming growth factor alpha, tumor necrosis factor receptor superfamily member 9, and macrophage colony-stimulating factor.
Authors: Rioja I, Hughes FJ, Sharp CH, Warnock LC, Montgomery DS, Akil M, Wilson AG, Binks MH, Dickson MC
Arthritis Rheum., 2008-08-01;58(8):2257-67.
Species: Human
Sample Types: Plasma
-
Increased serum levels of the chemokine CXCL13 and up-regulation of its gene expression are distinctive features of HCV-related cryoglobulinemia and correlate with active cutaneous vasculitis.
Authors: Sansonno D, Tucci FA, Troiani L, Lauletta G, Montrone M, Conteduca V, Sansonno L, Dammacco F
Blood, 2008-06-12;112(5):1620-7.
Species: Human
Sample Types: Serum
-
Lymphoid chemokines in chronic neuroinflammation.
Authors: Aloisi F, Columba-Cabezas S, Franciotta D, Rosicarelli B, Magliozzi R, Reynolds R, Ambrosini E, Coccia E, Salvetti M, Serafini B
J. Neuroimmunol., 2008-06-09;198(1):106-12.
Species: Human
Sample Types: CSF
-
Loss of IL-7 receptor alpha on CD4+ T cells defines terminally differentiated B cell-helping effector T cells in a B cell-rich lymphoid tissue.
Authors: Lim HW, Kim CH
J. Immunol., 2007-12-01;179(11):7448-56.
Species: Human
Sample Types: Cell Culture Supernates
-
Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia.
Authors: Burkle A, Niedermeier M, Schmitt-Graff A, Wierda WG, Keating MJ, Burger JA
Blood, 2007-07-25;110(9):3316-25.
Species: Human
Sample Types: Serum
-
High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory lyme arthritis.
Authors: Shin JJ, Glickstein LJ, Steere AC
Arthritis Rheum., 2007-04-01;56(4):1325-35.
Species: Human
Sample Types: Synovial Fluid
-
Follicular dendritic cells are related to bone marrow stromal cell progenitors and to myofibroblasts.
Authors: Munoz-Fernandez R, Blanco FJ, Frecha C, Martin F, Kimatrai M, Abadia-Molina AC, Garcia-Pacheco JM, Olivares EG
J. Immunol., 2006-07-01;177(1):280-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses.
Authors: Lim HW, Hillsamer P, Kim CH
J. Clin. Invest., 2004-12-01;114(11):1640-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis.
Authors: Casazza S, Zappia E, Pistorio A, Gambini C
Proc. Natl. Acad. Sci. U.S.A., 2004-07-19;101(30):11064-9.
Species: Human
Sample Types: CSF
-
IL1beta and TNFalpha differently modulate CXCL13 chemokine in stromal cells and osteoblasts isolated from osteoarthritis patients: evidence of changes associated to cell maturation.
Authors: Lisignoli G, Cristino S, Toneguzzi S, Grassi F, Piacentini A, Cavallo C, Facchini A, Mariani E
Exp. Gerontol., 2004-04-01;39(4):659-65.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human CXCL13/BLC/BCA-1 Quantikine ELISA Kit
Average Rating: 4.8 (Based on 5 Reviews)
Have you used Human CXCL13/BLC/BCA-1 Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
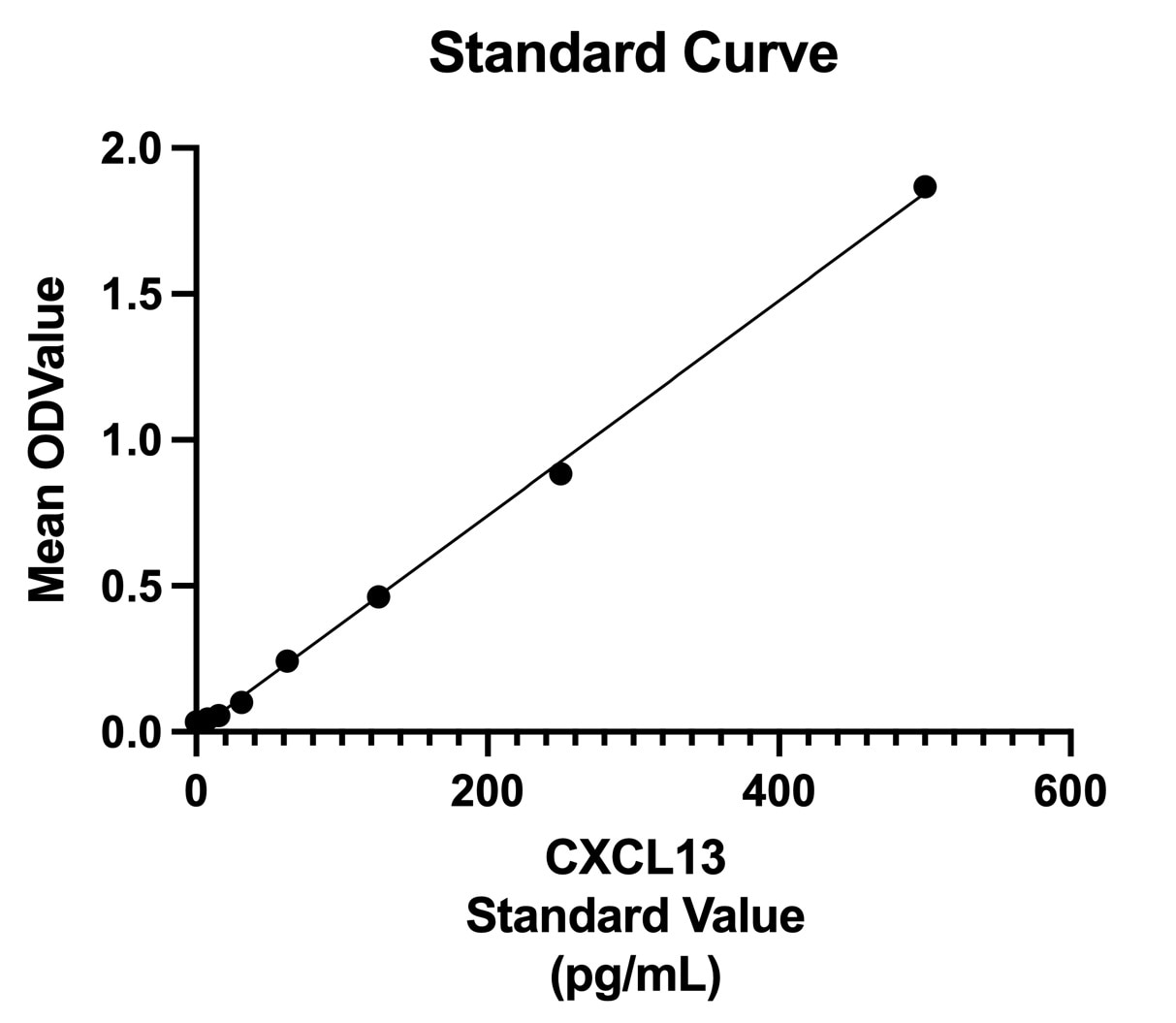
The standard curve shows a good fit.
Easy to use, and take ~5hours from start to get a result.