Human HepaCAM Antibody Summary
Val34-Tyr242
Accession # Q14CZ8
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human HepaCAM by Western Blot. Western blot shows lysates of human brain (motor cortex) tissue and human brain (hippocampus) tissue. PVDF membrane was probed with 0.25 µg/mL of Mouse Anti-Human HepaCAM Monoclonal Antibody (Catalog # MAB4108) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF018). A specific band was detected for HepaCAM at approximately 45-70 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
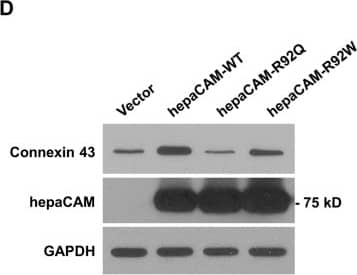
Detection of Human HepaCAM by Western Blot HepaCAM associates with connexin 43.(A) U373 MG cells were stably transfected with pcDNA3.1 vector, wild-type hepaCAM, hepaCAM-R92Q and hepaCAM-R92W. Immunofluorescent staining was performed with antibodies against the hepaCAM cytoplasmic domain (green) and connexin 43 (red). Co-localization of hepaCAM and connexin 43 is indicated by yellow fluorescence. Nuclei were stained with DAPI (blue). Insets show a higher magnification of sites of cell-cell contacts. Cells were visualized by confocal microscopy under a 60× objective. Scale bar: 10 μm. (B) Co-immunoprecipitatation of connexin 43 and hepaCAM. Cell lysates were prepared from U373 MG cells stably transfected with pcDNA3.1 vector and wild-type hepaCAM, and immunoprecipitated with antibody against the hepaCAM extracellular domain (IP hepaCAM). Immunoprecipitation with mouse IgG1 (IP IgG) was included as a negative control. Western blot analysis was performed on the immunoprecipitates and input (3%) using connexin 43 antibody. The efficiency of hepaCAM immunoprecipitation was evaluated with an HRP-conjugated FLAG antibody. The IgG heavy chain detected with an HRP-conjugated anti-mouse antibody is shown as a loading control. (C) Co-immunoprecipitation of wild-type and mutant hepaCAM with connexin 43. Cell lysates were immunoprecipitated with antibody against the hepaCAM extracellular domain (IP hepaCAM). Immunoprecipitation with mouse IgG1 (IP IgG) was included as a negative control. Western blot analysis was performed on the immunoprecipitates and input (2%) using connexin 43 antibody. (D) Expression of wild-type hepaCAM increases connexin 43 protein levels in U373 MG cells. 20 μg of cell lysates were subjected to Western blot analysis. GAPDH was used as a loading control. The result presented is a representative experiment of four independent experiments with similar results. The full view blots are shown in Supplementary Figure 1. (E) Quantification of connexin 43 protein levels in D and in three additional independent Western blot analyses. Using ImageJ the densities of the connexin 43 bands were normalized to the densities of the respective GAPDH bands for each sample, and the mean relative density over the four experiments was calculated. The data presented are the means ± SE (n = 4), **p < 0.01 as assessed by one-way ANOVA with Tukey’s multiple comparison test. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/srep36218), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
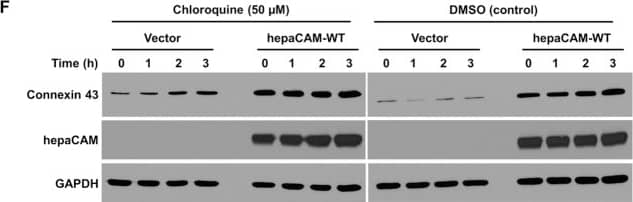
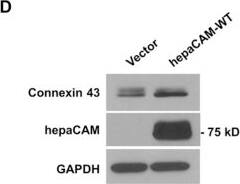
Detection of Human HepaCAM by Western Blot HepaCAM regulates connexin 43 stability.(A) Evaluation of connexin 43 mRNA expression. Total RNA was analyzed by RT-PCR. GAPDH and no template controls (NTC) were included as housekeeping gene and negative controls, respectively. (B) Evaluation of connexin 43 protein stability by a cycloheximide (CHX) chase assay. Cells treated with CHX (50 μg/ml) for the times indicated were lysed and 30 μg of cell lysates were subjected to Western blot analysis. The result presented is a representative experiment of three independent experiments with similar results. (C) Quantification of all three CHX chase experiments using ImageJ. The densities of the connexin 43 bands were normalized to the densities of the respective GAPDH bands at each time-point. The level of connexin 43 remaining at each time-point was calculated as a percentage of the initial connexin 43 level (time 0 of CHX treatment). The data presented are the means ± SE (n = 3). (D) Expression of hepaCAM in HEK293T cells increases connexin 43 protein levels. HEK293T cells were transiently transfected with pcDNA3.1 vector or wild-type hepaCAM. Two days after transfection, cells were lysed and 60 μg of cell lysates were subjected to Western blot analysis using antibodies against connexin 43 and the hepaCAM extracellular domain. The result presented is a representative experiment of three independent experiments with similar results. (E) Quantification of all three experiments using ImageJ. The densities of the connexin 43 bands were normalized to the densities of the respective GAPDH bands for each sample, and the mean relative density over the three experiments was calculated. The data presented are the means ± SE (n = 3), ***p < 0.0001 as assessed by t-test. (F) HepaCAM slows down connexin 43 turnover by the lysosomal pathway. U373 MG cells stably transfected with pcDNA3.1 vector or wild-type hepaCAM were treated with chloroquine (50 μM) and 30 μg of cell lysates were subjected to Western blot analysis for connexin 43. The result presented is representative of two independent experiments with similar results. The full view blots for (B,D,F) are shown in Supplementary Figures 3,4 and 5, respectively. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/srep36218), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human HepaCAM by Western Blot HepaCAM regulates connexin 43 stability.(A) Evaluation of connexin 43 mRNA expression. Total RNA was analyzed by RT-PCR. GAPDH and no template controls (NTC) were included as housekeeping gene and negative controls, respectively. (B) Evaluation of connexin 43 protein stability by a cycloheximide (CHX) chase assay. Cells treated with CHX (50 μg/ml) for the times indicated were lysed and 30 μg of cell lysates were subjected to Western blot analysis. The result presented is a representative experiment of three independent experiments with similar results. (C) Quantification of all three CHX chase experiments using ImageJ. The densities of the connexin 43 bands were normalized to the densities of the respective GAPDH bands at each time-point. The level of connexin 43 remaining at each time-point was calculated as a percentage of the initial connexin 43 level (time 0 of CHX treatment). The data presented are the means ± SE (n = 3). (D) Expression of hepaCAM in HEK293T cells increases connexin 43 protein levels. HEK293T cells were transiently transfected with pcDNA3.1 vector or wild-type hepaCAM. Two days after transfection, cells were lysed and 60 μg of cell lysates were subjected to Western blot analysis using antibodies against connexin 43 and the hepaCAM extracellular domain. The result presented is a representative experiment of three independent experiments with similar results. (E) Quantification of all three experiments using ImageJ. The densities of the connexin 43 bands were normalized to the densities of the respective GAPDH bands for each sample, and the mean relative density over the three experiments was calculated. The data presented are the means ± SE (n = 3), ***p < 0.0001 as assessed by t-test. (F) HepaCAM slows down connexin 43 turnover by the lysosomal pathway. U373 MG cells stably transfected with pcDNA3.1 vector or wild-type hepaCAM were treated with chloroquine (50 μM) and 30 μg of cell lysates were subjected to Western blot analysis for connexin 43. The result presented is representative of two independent experiments with similar results. The full view blots for (B,D,F) are shown in Supplementary Figures 3,4 and 5, respectively. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/srep36218), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human HepaCAM by Immunocytochemistry/Immunofluorescence HepaCAM enhances connexin 43 expression.(A) Treatment of hepaCAM-expressing U373 MG cells with antibodies against the hepaCAM extracellular domain prevents the association of hepaCAM with connexin 43 at cell-cell contacts. Wild-type hepaCAM-expressing U373 MG cells were treated overnight with antibody against the hepaCAM extracellular domain in soluble form (10 μg/ml). Cells were also treated with the isotype mouse IgG1 as a control. The next day, cells were fixed and immunofluorescent staining was performed with antibodies against the hepaCAM extracellular domain (green) and connexin 43 (red). Co-localization of hepaCAM and connexin 43 is indicated by yellow fluorescence. Nuclei were stained with DAPI (blue). Cells were visualized by confocal microscopy under a 60× objective. Scale bar: 10 μm. (B) Treatment of hepaCAM-expressing U373 MG cells with antibodies against the hepaCAM extracellular domain causes a downregulation of connexin 43 expression. Wild-type hepaCAM-expressing U373 MG cells were treated overnight with antibody against the hepaCAM extracellular domain in soluble form (10 μg/ml). Cells were also treated with the isotype mouse IgG1 as a control. The next day, cells were lysed and 20 μg of cell lysates were subjected to Western blot analysis using connexin 43 antibody. GAPDH was used as a loading control. The full view blots are shown in Supplementary Figure 2. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/srep36218), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human HepaCAM by Immunocytochemistry/Immunofluorescence HepaCAM associates with connexin 43.(A) U373 MG cells were stably transfected with pcDNA3.1 vector, wild-type hepaCAM, hepaCAM-R92Q and hepaCAM-R92W. Immunofluorescent staining was performed with antibodies against the hepaCAM cytoplasmic domain (green) and connexin 43 (red). Co-localization of hepaCAM and connexin 43 is indicated by yellow fluorescence. Nuclei were stained with DAPI (blue). Insets show a higher magnification of sites of cell-cell contacts. Cells were visualized by confocal microscopy under a 60× objective. Scale bar: 10 μm. (B) Co-immunoprecipitatation of connexin 43 and hepaCAM. Cell lysates were prepared from U373 MG cells stably transfected with pcDNA3.1 vector and wild-type hepaCAM, and immunoprecipitated with antibody against the hepaCAM extracellular domain (IP hepaCAM). Immunoprecipitation with mouse IgG1 (IP IgG) was included as a negative control. Western blot analysis was performed on the immunoprecipitates and input (3%) using connexin 43 antibody. The efficiency of hepaCAM immunoprecipitation was evaluated with an HRP-conjugated FLAG antibody. The IgG heavy chain detected with an HRP-conjugated anti-mouse antibody is shown as a loading control. (C) Co-immunoprecipitation of wild-type and mutant hepaCAM with connexin 43. Cell lysates were immunoprecipitated with antibody against the hepaCAM extracellular domain (IP hepaCAM). Immunoprecipitation with mouse IgG1 (IP IgG) was included as a negative control. Western blot analysis was performed on the immunoprecipitates and input (2%) using connexin 43 antibody. (D) Expression of wild-type hepaCAM increases connexin 43 protein levels in U373 MG cells. 20 μg of cell lysates were subjected to Western blot analysis. GAPDH was used as a loading control. The result presented is a representative experiment of four independent experiments with similar results. The full view blots are shown in Supplementary Figure 1. (E) Quantification of connexin 43 protein levels in D and in three additional independent Western blot analyses. Using ImageJ the densities of the connexin 43 bands were normalized to the densities of the respective GAPDH bands for each sample, and the mean relative density over the four experiments was calculated. The data presented are the means ± SE (n = 4), **p < 0.01 as assessed by one-way ANOVA with Tukey’s multiple comparison test. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/srep36218), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: HepaCAM
HepaCAM (hepatocyte cell adhesion molecule) is a 50-75 kDa, type I transmembrane glycoprotein that belongs to the Ig-superfamily. It forms homodimers on the cell surface and promotes cell spreading and motility. Human HepaCAM is 382 aa in length. It contains a 206 aa extracellular domain (ECD) (aa 35‑240) and a 152 aa cytoplasmic region. The ECD has two C2 Ig-like domains. There is one potential truncated isoform that shows an alternate start site at Met200. Over aa 34‑242, human HepaCAM shares 99% and 98% aa sequence identity with mouse and dog HepaCAM, respectively.
Product Datasheets
Citations for Human HepaCAM Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
15
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Lysine-selective molecular tweezers are cell penetrant and concentrate in lysosomes
Authors: Zizheng Li, Ibrar Siddique, Inesa Hadrović, Abbna Kirupakaran, Jiwen Li, Ye Zhang et al.
Communications Biology
-
HepaCAM controls astrocyte self-organization and coupling
Authors: Katherine T. Baldwin, Christabel X. Tan, Samuel T. Strader, Changyu Jiang, Justin T. Savage, Xabier Elorza-Vidal et al.
Neuron
-
Conservation and divergence of vulnerability and responses to stressors between human and mouse astrocytes
Authors: Jiwen Li, Lin Pan, William G. Pembroke, Jessica E. Rexach, Marlesa I. Godoy, Michael C. Condro et al.
Nature Communications
-
Astrocyte‐to‐astrocyte contact and a positive feedback loop of growth factor signaling regulate astrocyte maturation
Authors: Jiwen Li, Rana R. Khankan, Christine Caneda, Marlesa I. Godoy, Michael S. Haney, Mitchell C. Krawczyk et al.
Glia
-
Morphological diversification and functional maturation of human astrocytes in glia-enriched cortical organoid transplanted in mouse brain
Authors: Wang, M;Zhang, L;Novak, SW;Yu, J;Gallina, IS;Xu, LL;Lim, CK;Fernandes, S;Shokhirev, MN;Williams, AE;Saxena, MD;Coorapati, S;Parylak, SL;Quintero, C;Molina, E;Andrade, LR;Manor, U;Gage, FH;
Nature biotechnology
Species: Human, Xenograft
Sample Types: Organoid, Whole Tissue
Applications: Immunohistochemistry -
Astroglial exosome HepaCAM signaling and ApoE antagonization coordinates early postnatal cortical pyramidal neuronal axon growth and dendritic spine formation
Authors: Jin, S;Chen, X;Tian, Y;Jarvis, R;Promes, V;Yang, Y;
Nature communications
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Identification of ligand-receptor pairs that drive human astrocyte development
Authors: Voss, AJ;Lanjewar, SN;Sampson, MM;King, A;Hill, EJ;Sing, A;Sojka, C;Bhatia, TN;Spangle, JM;Sloan, SA;
Nature neuroscience
Species: Human
Sample Types: Organoid
Applications: Immunopanning -
Lymphocyte deficiency alters the transcriptomes of oligodendrocytes, but not astrocytes or microglia
Authors: MC Krawczyk, L Pan, AJ Zhang, Y Zhang
PLoS ONE, 2023-02-24;18(2):e0279736.
Species: Mouse
Sample Types: Whole Cells
Applications: Immunoprecipitation -
Mutations in the transcriptional regulator MeCP2 severely impact key cellular and molecular signatures of human astrocytes during maturation
Authors: J Sun, S Osenberg, A Irwin, LH Ma, N Lee, Y Xiang, F Li, YW Wan, IH Park, M Maletic-Sa, N Ballas
Cell Reports, 2023-01-05;42(1):111942.
Species: Human
Sample Types: Organoid
Applications: IHC -
Enrichment of Glial Cells From Human Post-mortem Tissue for Transcriptome and Proteome Analysis Using Immunopanning
Authors: A Nolle, I van Dijken, CM Waelti, D Calini, J Bryois, E Lezan, S Golling, A Augustin, L Foo, JJM Hoozemans
Frontiers in Cellular Neuroscience, 2021-12-13;15(0):772011.
Species: Human
Sample Types: Whole Cells
Applications: Immunopanning -
Neurotoxic reactive astrocytes induce cell death via saturated lipids
Authors: KA Guttenplan, MK Weigel, P Prakash, PR Wijewardha, P Hasel, U Rufen-Blan, AE Münch, JA Blum, J Fine, MC Neal, KD Bruce, AD Gitler, G Chopra, SA Liddelow, BA Barres
Nature, 2021-10-06;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model
Authors: KA Guttenplan, MK Weigel, DI Adler, J Couthouis, SA Liddelow, AD Gitler, BA Barres
Nat Commun, 2020-07-27;11(1):3753.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma
Authors: D Henrik Hei, VM Ravi, SP Behringer, JH Frenking, J Wurm, K Joseph, NWC Garrelfs, J Strähle, S Heynckes, J Grauvogel, P Franco, I Mader, M Schneider, AL Potthoff, D Delev, UG Hofmann, C Fung, J Beck, R Sankowski, M Prinz, O Schnell
Nat Commun, 2019-06-11;10(1):2541.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: Flow Cytometry, IHC, Immunopanning -
HepaCAM associates with connexin 43 and enhances its localization in cellular junctions
Sci Rep, 2016-11-07;6(0):36218.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Oligodendrocyte-lineage cell exocytosis and L-type prostaglandin D synthase promote oligodendrocyte development and myelination
Authors: L Pan, A Trimarco, AJ Zhang, K Fujimori, Y Urade, LO Sun, C Taveggia, Y Zhang
Elife, 2023-02-13;12(0):.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human HepaCAM Antibody
There are currently no reviews for this product. Be the first to review Human HepaCAM Antibody and earn rewards!
Have you used Human HepaCAM Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image

