Mouse Netrin-G1a Antibody Summary
His29-Gly513
Accession # Q8R4G0.2
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
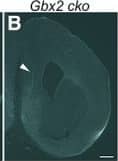
Detection of Mouse Netrin-G1a by Immunohistochemistry Transient expression of Cyp26b1 in the PFC does not occur in the absence of thalamus–cortex interactions in Gbx2 mutant mice. A–F, Thalamus–PFC disconnection in Gbx2 mutant mice. A, B, Immunostaining for NetrinG1 at E16.5. In control mice, NetrinG1-labeled thalamocortical axons are visible in coronal sections of frontal cortex. Arrowhead in A shows the medial PFC, where robust labeling is detected. In contrast, NetrinG1 labeling is barely detectable in the frontal cortex of Gbx2 mutant mice, including the medial PFC (B, arrowhead). Scale bar, 200 µm. C–F, DiI labeling at P14. C, D, DiI placement in medial PFC retrogradely labels medial thalamic nuclei in the control brains. E, F, In Gbx2 mutants, the label is severely reduced, indicating the deficiency of both thalamocortical and corticothalamic projections. G–J, Expression of ROR beta and Lmo4 is qualitatively normal in the PFC of Gbx2 mutant mice at P8. G, I, The expression of ROR beta in layer 4 is comparable between control (G) and Gbx2 mutant (cko) mice (I, arrows). H, J, Laminar expression patterns of Lmo4 also appear unchanged in Gbx2 mutants. Scale bar, 1 mm. K–Z, Transient expression of Cyp26b1 in the PFC does not occur in the absence of thalamus–cortex interactions in Gbx2 mutant mice. K–T, in situ hybridization of frontal sections through PFC at various postnatal stages with a Cyp26b1 probe. K–O, In control mice (K–O), Cyp26b1 expression starts at P2 in medial (K, arrowhead) and ventral (K, single arrow) PFC, and continues until P14 (M). N, O, At P21, expression in medial PFC is reduced (N) and is no longer detectable at P35 (O). K–O, In addition to medial and ventral PFC, Cyp26b1 is also expressed in lateral frontal cortex, including the motor and somatosensory areas (double arrows) and agranular insula (double arrowheads). P–T, In Gbx2 mutant mice, the expression of Cyp26b1 is not induced in medial or ventral PFC at P2 as well as at later stages, although ventral PFC does not appear to be affected at P14 and later. P–T, Expression in more superficial layer of lateral cortex (double arrows and double arrowheads) is not affected in Gbx2 mutant mice. U, X, Expression of the layer 6 marker Syt6 is not affected in Gbx2 mutant mice. V, Y, Expression of Aldh1a3 in layer 2 of medial PFC and anterior cingulate cortex (arrow) is not affected in Gbx2 mutant mice. Scale bar, 1 mm. W, Z, Summary schematic for this figure. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/30868103), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
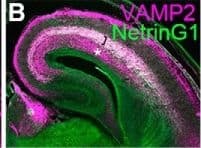
Detection of Mouse Netrin-G1a by Immunocytochemistry/ Immunofluorescence Normal induction of Cyp26b1 in PFC in mice expressing tetanus toxin light chain in thalamocortical axons. A–D, Immunostaining for VAMP2 on frontal sections of somatosensory cortex at E16.5 control (A, B) and mutant mice with ectopic expression of TeNT in thalamic neurons (C, D). TeNT expression leads to the deletion of VAMP2, specifically in thalamocortical axons at E16.5. Thalamocortical axons are shown by NetrinG1 staining (B, D, green). In control brains, both thalamocortical (bracket, A–D) and corticofugal (asterisk, A–D) axons express VAMP2, whereas in TeNT-expressing mice, VAMP2 staining is specifically diminished in thalamocortical axons (C, D, bracket). Scale bar, 500 μm. E, F, Deletion of VAMP2 in thalamocortical axons results in the lack of the characteristic pattern of ROR beta expression in the barrel field of primary somatosensory cortex at P8 (arrow), similar to the defect found in Gbx2 mutant mice (Vue et al., 2013). G–J, Immunostaining for VAMP2 on frontal sections of prefrontal cortex at P0 control (G, H) and mutant mice with ectopic expression of TeNT in thalamic neurons (I, J). Similar to the somatosensory cortex, VAMP2 staining in thalamocortical axons is diminished in TeNT-expressing mice (I, J, bracket). Scale bar, 200 μm. K, L, Expression of Cyp26b1 in medial (arrowhead) and ventral PFC is intact in TeNT-expressing mice (L), similar to control (K), at P8. Scale bars: G, H, I, J, 200 μm; E, F, K, L, 1 mm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/30868103), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
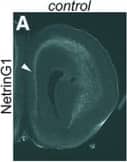
Detection of Mouse Netrin-G1a by Immunohistochemistry Transient expression of Cyp26b1 in the PFC does not occur in the absence of thalamus–cortex interactions in Gbx2 mutant mice. A–F, Thalamus–PFC disconnection in Gbx2 mutant mice. A, B, Immunostaining for NetrinG1 at E16.5. In control mice, NetrinG1-labeled thalamocortical axons are visible in coronal sections of frontal cortex. Arrowhead in A shows the medial PFC, where robust labeling is detected. In contrast, NetrinG1 labeling is barely detectable in the frontal cortex of Gbx2 mutant mice, including the medial PFC (B, arrowhead). Scale bar, 200 µm. C–F, DiI labeling at P14. C, D, DiI placement in medial PFC retrogradely labels medial thalamic nuclei in the control brains. E, F, In Gbx2 mutants, the label is severely reduced, indicating the deficiency of both thalamocortical and corticothalamic projections. G–J, Expression of ROR beta and Lmo4 is qualitatively normal in the PFC of Gbx2 mutant mice at P8. G, I, The expression of ROR beta in layer 4 is comparable between control (G) and Gbx2 mutant (cko) mice (I, arrows). H, J, Laminar expression patterns of Lmo4 also appear unchanged in Gbx2 mutants. Scale bar, 1 mm. K–Z, Transient expression of Cyp26b1 in the PFC does not occur in the absence of thalamus–cortex interactions in Gbx2 mutant mice. K–T, in situ hybridization of frontal sections through PFC at various postnatal stages with a Cyp26b1 probe. K–O, In control mice (K–O), Cyp26b1 expression starts at P2 in medial (K, arrowhead) and ventral (K, single arrow) PFC, and continues until P14 (M). N, O, At P21, expression in medial PFC is reduced (N) and is no longer detectable at P35 (O). K–O, In addition to medial and ventral PFC, Cyp26b1 is also expressed in lateral frontal cortex, including the motor and somatosensory areas (double arrows) and agranular insula (double arrowheads). P–T, In Gbx2 mutant mice, the expression of Cyp26b1 is not induced in medial or ventral PFC at P2 as well as at later stages, although ventral PFC does not appear to be affected at P14 and later. P–T, Expression in more superficial layer of lateral cortex (double arrows and double arrowheads) is not affected in Gbx2 mutant mice. U, X, Expression of the layer 6 marker Syt6 is not affected in Gbx2 mutant mice. V, Y, Expression of Aldh1a3 in layer 2 of medial PFC and anterior cingulate cortex (arrow) is not affected in Gbx2 mutant mice. Scale bar, 1 mm. W, Z, Summary schematic for this figure. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/30868103), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
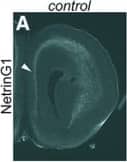
Detection of Mouse Netrin-G1a by Immunohistochemistry Transient expression of Cyp26b1 in the PFC does not occur in the absence of thalamus–cortex interactions in Gbx2 mutant mice. A–F, Thalamus–PFC disconnection in Gbx2 mutant mice. A, B, Immunostaining for NetrinG1 at E16.5. In control mice, NetrinG1-labeled thalamocortical axons are visible in coronal sections of frontal cortex. Arrowhead in A shows the medial PFC, where robust labeling is detected. In contrast, NetrinG1 labeling is barely detectable in the frontal cortex of Gbx2 mutant mice, including the medial PFC (B, arrowhead). Scale bar, 200 µm. C–F, DiI labeling at P14. C, D, DiI placement in medial PFC retrogradely labels medial thalamic nuclei in the control brains. E, F, In Gbx2 mutants, the label is severely reduced, indicating the deficiency of both thalamocortical and corticothalamic projections. G–J, Expression of ROR beta and Lmo4 is qualitatively normal in the PFC of Gbx2 mutant mice at P8. G, I, The expression of ROR beta in layer 4 is comparable between control (G) and Gbx2 mutant (cko) mice (I, arrows). H, J, Laminar expression patterns of Lmo4 also appear unchanged in Gbx2 mutants. Scale bar, 1 mm. K–Z, Transient expression of Cyp26b1 in the PFC does not occur in the absence of thalamus–cortex interactions in Gbx2 mutant mice. K–T, in situ hybridization of frontal sections through PFC at various postnatal stages with a Cyp26b1 probe. K–O, In control mice (K–O), Cyp26b1 expression starts at P2 in medial (K, arrowhead) and ventral (K, single arrow) PFC, and continues until P14 (M). N, O, At P21, expression in medial PFC is reduced (N) and is no longer detectable at P35 (O). K–O, In addition to medial and ventral PFC, Cyp26b1 is also expressed in lateral frontal cortex, including the motor and somatosensory areas (double arrows) and agranular insula (double arrowheads). P–T, In Gbx2 mutant mice, the expression of Cyp26b1 is not induced in medial or ventral PFC at P2 as well as at later stages, although ventral PFC does not appear to be affected at P14 and later. P–T, Expression in more superficial layer of lateral cortex (double arrows and double arrowheads) is not affected in Gbx2 mutant mice. U, X, Expression of the layer 6 marker Syt6 is not affected in Gbx2 mutant mice. V, Y, Expression of Aldh1a3 in layer 2 of medial PFC and anterior cingulate cortex (arrow) is not affected in Gbx2 mutant mice. Scale bar, 1 mm. W, Z, Summary schematic for this figure. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/30868103), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Netrin-G1a
Netrins/UNC-6 (netr: Sanskrit for “one who guides”) are a family of laminin-related small proteins that are involved in neurite outgrowth and axon guidance. Netrins bind to the DCC and UNC5 family of receptors to attract or repel axons. Mouse Netrin-G1a is synthesized as a 539 amino acid (aa) precursor with an 18 aa signal sequence, a 399 aa laminin-related region containing an N-terminal laminin globular domain (domain VI) followed by 3 laminin EGF-like repeats, and a 122 aa C domain with a hydrophobic signal for glycosyl phosphatidylinositol (GPI) lipid linkage. Unlike classical Netrins which have a C domain rich in basic aa residues that serves as a heparin binding site, Netrin-G1a is predominantly anchored on the cell surface via GPI linkages. By alternative splicing, at least six isoforms for Netrin-G1 have been identified. All G1a variants (G1b through G1f) are shorter than G1a and lack one or more of the EGF-like domains. Mouse Netrin-G1a shares 27%, 26%, and 31% aa sequence identity with mouse Netrins-1, 3 and 4, respectively. It also shares 60% aa identity with another GPI-anchored mouse Netrin-G2a. Although a human homolog of mouse Netrin-G1a has not been reported, a human G1f that shares 97% aa sequence identity with mouse G1f has been cloned. Netrin-G1 has widespread expression, occurring in olfactory mitral cells, cells of the inferior colliculus (hearing), dorsal thalamus (behavior), and cells of the deep cerebellar nuclei and inferior olive (motion). The G-type Netrins are found only in vertebrates and are suggested to play a role in forming circuits associated with motor activity. Conditioned media containing myc-tagged Netrin-G1a proteins did not bind to cells transiently transfected with the Netrin receptors DCC, UNC5H1, UNC5H2, or UNC5H3 (1‑4).
- McFarlane, S. (2000) Biochem. Cell. Biol. 78:563.
- Yu, T.W. and C.I. Bargmann (2001) Nature Neurosci. 4(Suppl):1169.
- Nakashiba, T. et al. (2002) Mech. Dev. 111:47.
- Nakashiba, T. et al. (2000) J. Neurosci. 20:6540.
Product Datasheets
Product Specific Notices
This product or the use of this product is covered by U.S. Patents owned by The Regents of the University of California. This product is for research use only and is not to be used for commercial purposes. Use of this product to produce products for sale or for diagnostic, therapeutic or drug discovery purposes is prohibited. In order to obtain a license to use this product for such purposes, contact The Regents of the University of California.U.S. Patent # 5,565,331, 6,096,866, 6,017,714, 6,309,638, 6,670,451, and other U.S. and international patents pending.
Citations for Mouse Netrin-G1a Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
7
Citations: Showing 1 - 7
Filter your results:
Filter by:
-
Plasticity of thalamocortical axons is regulated by serotonin levels modulated by preterm birth
Authors: Sinclair-Wilson, A;Lawrence, A;Ferezou, I;Cartonnet, H;Mailhes, C;Garel, S;Lokmane, L;
Proceedings of the National Academy of Sciences of the United States of America
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Maturation and circuit integration of transplanted human cortical organoids
Authors: O Revah, F Gore, KW Kelley, J Andersen, N Sakai, X Chen, MY Li, F Birey, X Yang, NL Saw, SW Baker, ND Amin, S Kulkarni, R Mudipalli, B Cui, S Nishino, GA Grant, JK Knowles, M Shamloo, JR Huguenard, K Deisseroth, SP Pa?ca
Nature, 2022-10-12;610(7931):319-326.
Species: Xenograft
Sample Types: Whole Tissue
Applications: ICC -
Early construction of the thalamocortical axon pathway requires c‐Jun N‐terminal kinase signaling within the ventral forebrain
Authors: Jessica G. Cunningham, James D. Scripter, Stephany A. Nti, Eric S. Tucker
Developmental Dynamics
-
The maternal microbiome modulates fetal neurodevelopment in mice
Authors: HE Vuong, GN Pronovost, DW Williams, EJL Coley, EL Siegler, A Qiu, M Kazantsev, CJ Wilson, T Rendon, EY Hsiao
Nature, 2020-09-23;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The Thalamus Regulates Retinoic Acid Signaling and Development of Parvalbumin Interneurons in Postnatal Mouse Prefrontal Cortex
Authors: Rachel Larsen, Alatheia Proue, Earl Parker Scott, Matthew Christiansen, Yasushi Nakagawa
eNeuro
-
NOVA2-mediated RNA regulation is required for axonal pathfinding during development
Elife, 2016-05-25;5(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Thalamic control of neocortical area formation in mice.
Authors: Vue T, Lee M, Tan Y, Werkhoven Z, Wang L, Nakagawa Y
J Neurosci, 2013-05-08;33(19):8442-53.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse Netrin-G1a Antibody
Average Rating: 4.5 (Based on 2 Reviews)
Have you used Mouse Netrin-G1a Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:



