Mouse Podoplanin Antibody Summary
Gln21-Lys133
Accession # Q546R8
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Mouse Podoplanin by Western Blot. Western blot shows lysates of mouse lung tissue. PVDF membrane was probed with 0.25 µg/mL of Goat Anti-Mouse Podoplanin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3244) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF019). A specific band was detected for Podoplanin at approximately 35-42 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Podoplanin in Mouse Kidney. Podoplanin was detected in perfusion fixed frozen sections of mouse kidney using Goat Anti-Mouse Podoplanin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3244) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
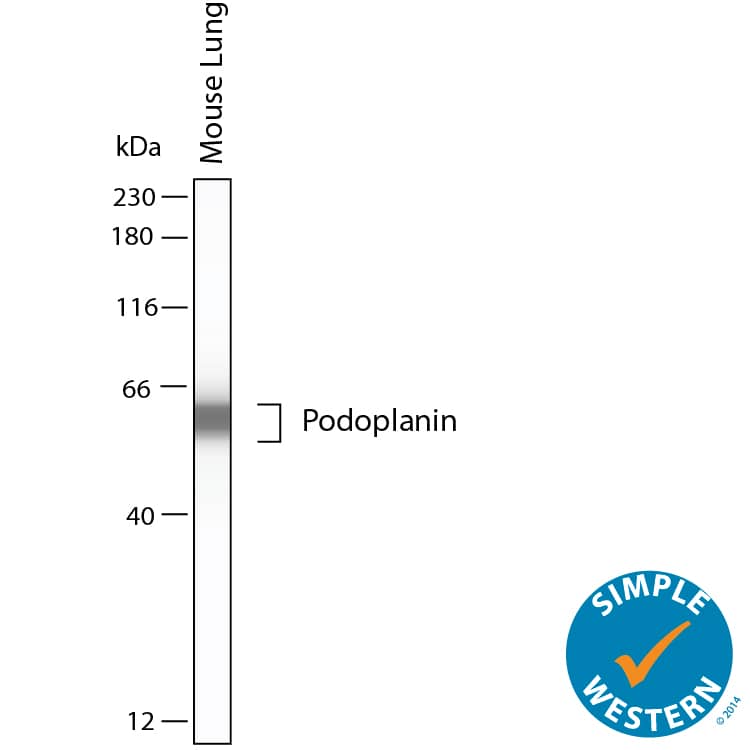
Detection of Mouse Podoplanin by Simple WesternTM. Simple Western shows lysates of mouse lung, loaded at 0.5 mg/ml. A specific band was detected for Podoplanin at approximately 60 kDa (as indicated) using 10 µg/mL of Goat Anti-Mouse Podoplanin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3244). This experiment was conducted under reducing conditions and using the 12-230kDa separation system.
 View Larger
View Larger
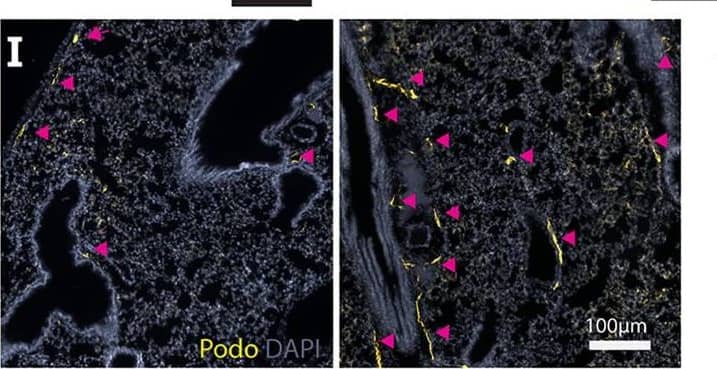
Detection of Mouse Podoplanin by Immunocytochemistry/Immunofluorescence Mfn1/2i delta AEC2 mice develop spontaneous lung fibrosis. a Schema demonstrating the generation of mice with AEC2 cell-specific tamoxifen-inducible deletion of Mfn1/2. SftpcCreERT2+/+ or SftpcCreERT2+/− mice were used as controls. b Genotyping of CD45(-)EpCAM(+) cells isolated from Mfn1/2i delta AEC2 mice (n = 3 mice; lane 1 to lane 3 serves as the positive control). c Representative immunoblots of AEC2 cell lysates obtained 6 weeks after tamoxifen-induced deletion, showing decreased protein levels of both MFN1 and MFN2 in the Mfn1/2−/− AEC2 cells (n = 3 mice per group). d Representative TEM images (upper row, ×12,000; lower row, ×50,000) show mitochondrial ultrastructural changes in SftpcCreERT2+/− and Mfn1/2−/− AEC2 cells (n = 3 mice per group) with disrupted cristae marked with white arrowheads (scale bar, upper row 2 μm, lower row 500 nm. e Kaplan–Meier survival curves of Mfn1/2i delta AEC2 (n = 22) and SftpcCreERT2+/+ (n = 23) mice (p < 0.01 by log-rank test). f Representative Masson’s trichrome-stained lung sections (upper panel, ×100 magnification; lower panel, ×200 magnification) 17 weeks post tamoxifen-induced deletion (SftpcCreERT2+/+ mice n = 6; Mfn1/2i delta AEC2 mice n = 11; scale bar, upper panel 4 mm, lower panel 200 μm). g Representative IHC staining of vimentin, alpha-smooth muscle actin ( alpha -SMA), and collagen III (Col-III) (×200 magnification; n = 3 mice per group; scale bar 200 μm). h Representative immunofluorescent staining of 5x5 tiled confocal images (using ×40 objective) of frozen murine lung sections stained for podoplanin (green), surfactant protein-C (SP-C) (yellow), ER-TR7 (magenta), and Hoechst 33342 stain (blue) (n = 3 mice per group; scale bar 50 μm). i Representative immunofluorescence staining confocal images of podoplanin (green), SP-C (yellow), ER-TR7 (magenta) and Hoechst 33342 nuclear stain (blue) using lung sections of SftpcCreERT2+/−, Mfn1i delta AEC2 and Mfn2i delta AEC2 mice (n = 2 mice per group; scale bar 20 μm). Source data (c, e) are provided as a Source Data file Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31358769), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Podoplanin by Western Blot Effect of VEGFCC156S on angiotensin II-induced cardiac dysfunction, cardiac lymphatics, and skin lymphatics.(A) Pharmacokinetic analysis of VEGFCC156S in mice (n = 2 per dose group). (B) Plasma VEGFC concentration measured by ELISA at euthanasia (n = 10–17/group). (C–S) Mice were infused with saline + BSA, angiotensin II + BSA, or angiotensin II + VEGFCC156S as described in Figure 2A. (C) Representative M-mode echocardiography images for each group. (D–G) Echocardiography parameters cardiac output (D), stroke volume (E), Left Ventricular Posterior Wall thickness at diastole (LVPWd) (F) and heart rate (G) are shown (n = 15–24/group). (H–J) Hearts were arrested in diastole, fixed, sectioned, and stained with wheat germ agglutinin (WGA). (H) Representative images of WGA stain for each group. Scale bar, 100 μm. (I) Cardiomyocyte size was assessed (n = 7–9/group). (J) Analysis of cell size variance coefficient from WGA-stained mouse heart sections (n = 21–27/group; a linear mixed model was used for statistics). (K–N) Protein lysates were prepared from mouse hearts and immunoblotted for podoplanin, p-Akt, Akt, p-Erk1/2, Erk1/2, and GAPDH. (K) Representative immunoblots. (L–N) Densitometric quantification of podoplanin (L), p-Akt to Akt ratio (M) and p-Erk to Erk ratio (N) (n = 7–9/group). (O) Representative images of the whole mount stain of lyve1-positive lymphatic vessels in ear skin for each group. Scale bar, 100 μm. (P) Quantification of lyve1-positive lymphatic vessel diameter in ear skin (n = 737–880 vessel/group; a linear mixed model was used for statistics). (Q) Ear skin samples were fixed, sectioned, and stained for lyve1(green) and DAPI (blue). Representative images of lyve1-positive lymphatic vessels in ear skin cross-section for each group. Scale bar, 200 μm. (R) Quantification of lyve1-positive lymphatic vessel density (normalized to total area) in ear skin (n = 6–8 animals/group, n = 6–8 sections/animal; a linear mixed model was used for statistics). Ctrl, control. AngII, angiotensin II. VEGFC, VEGFCc156s. Data are mean ± s.d. One-way ANOVA with Bonferroni posthoc was used for statistics for all figures except 2J, 2P and 2R (a linear mixed model was used for statistics). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, n.s. not significant. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33200983), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Mouse Podoplanin Antibody by Immunohistochemistry-Paraffin Carboplatin induces lymphangiogenesis in healthy tissues (A) Schematic of rat mesentery culture model. (B) Vehicle-treated lymphatic vessels from mesentery cultures stained with LYVE-1 (grey). (i) High magnification image of boxed area in (B). (C) Carboplatin-treated lymphatic vessels from mesentery cultures stained with LYVE-1 (grey). (ii) High magnification image of boxed area in (C) Scale bar=100µm. (D) Number of sprouts per lymphatic vessel area (n=3/group). (E) Lymphatic vessel density (podoplanin+ vessels per mm2 stroma) in whole mammary fat pads of healthy mice treated with systemic carboplatin (8 mg/kg/dose) or vehicle by IV(n=3-4/group). (F) Lymphatic vessel density measured in mammary fat pads of healthy mice 2 months after treatment with 3 doses of carboplatin or vehicle, (n=3/group). (G) Lymph nodes from healthy, tumor-naïve mice treated with vehicle and stained H&E. (H) LEC number in vehicle-treated and carboplatin-treated lymph nodes in vivo (n=6/group). (I) Representative images of lungs from mice treated with 3 doses of vehicle (left) and carboplatin (right). Podoplanin+ lymphatic vessels noted by arrowheads. (J) Lymphatic vessel density in stromal tissue of lungs of mice pre-treated with carboplatin (n=3/group). *p < 0.05, **p < 0.01, ****p < 0.001. Each data point represents one mouse. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35372032), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Podoplanin
Podoplanin, also known as T1 alpha (T1 alpha ), is a mucin type transmembrane glycoprotein with extensive O-glycosylation. It is specifically expressed by lymphatic endothelial cells but not blood vascular endothelial cells. In addition, non-endothelial cells in numerous normal tissues also express the protein. Within the region used as the immunogen, mouse Podoplanin shares 73.5% and 29% amino acid sequence homology with rat and human Podoplanin, respectively.
Product Datasheets
Citations for Mouse Podoplanin Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
74
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
FGF7-induced E11 facilitates cell-cell communication through connexin43
Authors: X Liu, M Bai, Y Sun, X Hu, C Wang, J Xie, L Ye
International journal of biological sciences, 2021-09-03;17(14):3862-3874.
-
Disrupted fibroblastic reticular cells and interleukin-7 expression in tumor draining lymph nodes
Authors: Jianbao Gao, Lintao Zhao, Lina Liu, Yang Yang, Bo Guo, Bo Zhu
Oncology Letters
-
Podoplanin neutralization improves cardiac remodeling and function after acute myocardial infarction
Authors: Maria Cimini, Venkata Naga Srikanth Garikipati, Claudio de Lucia, Zhongjian Cheng, Chunlin Wang, May M. Truongcao et al.
JCI Insight
-
Pazopanib Is a Potential Treatment for Coronavirus-Induced Lung Injuries
Authors: Yi Luan, Qianying Yuan, Qijun Wang, Susan Compton, Dianqing Wu, Wenwen Tang
The Journal of Immunology
-
Vascular endothelial growth factor-D over-expressing tumor cells induce differential effects on uterine vasculature in a mouse model of endometrial cancer
Authors: Jane E Girling, Jacqueline F Donoghue, Fiona L Lederman, Leonie M Cann, Marc G Achen, Steven A Stacker et al.
Reproductive Biology and Endocrinology
-
Mitofusins regulate lipid metabolism to mediate the development of lung fibrosis
Authors: Kuei-Pin Chung, Chia-Lang Hsu, Li-Chao Fan, Ziling Huang, Divya Bhatia, Yi-Jung Chen et al.
Nature Communications
-
The tetraspanin CD9 controls migration and proliferation of parietal epithelial cells and glomerular disease progression
Authors: Hélène Lazareth, Carole Henique, Olivia Lenoir, Victor G. Puelles, Martin Flamant, Guillaume Bollée et al.
Nature Communications
-
CD47 Activation by Thrombospondin-1 in Lymphatic Endothelial Cells Suppresses Lymphangiogenesis and Promotes Atherosclerosis
Authors: Singla B, Aithbathula RV, Pervaiz N et al.
Arteriosclerosis, thrombosis, and vascular biology
-
Immunohistochemistry in Investigative and Toxicologic Pathology
Authors: Janardhan KS, Jensen H, Clayton NP, Herbert RA
Toxicol Pathol.
-
Osteocyte specific responses to soluble and mechanical stimuli in a stem cell derived culture model
Authors: William R. Thompson, Gunes Uzer, Kaitlyn E. Brobst, Zhihui Xie, Buer Sen, Sherwin S. Yen et al.
Scientific Reports
-
A Novel Osteogenic Cell Line That Differentiates Into GFP‐Tagged Osteocytes and Forms Mineral With a Bone‐Like Lacunocanalicular Structure
Authors: Kun Wang, Lisa Le, Brad M. Chun, LeAnn M. Tiede‐Lewis, Lora A. Shiflett, Matthew Prideaux et al.
Journal of Bone and Mineral Research
-
Evolution of fibroblasts in the lung metastatic microenvironment is driven by stage-specific transcriptional plasticity
Authors: Ophir Shani, Yael Raz, Lea Monteran, Ye'ela Scharff, Oshrat Levi-Galibov, Or Megides et al.
eLife
-
Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen
Authors: Laurent Gautron, Joseph M. Rutkowski, Michael D. Burton, Wei Wei, Yihong Wan, Joel K. Elmquist
Journal of Comparative Neurology
-
Tumor-associated factors are enriched in lymphatic exudate compared to plasma in metastatic melanoma patients
Authors: Maria A.S. Broggi, Lea Maillat, Cristina C. Clement, Natacha Bordry, Patricia Corthésy, Aymeric Auger et al.
Journal of Experimental Medicine
-
CD4+ T cells are activated in regional lymph nodes and migrate to skin to initiate lymphedema
Authors: GD García Nor, CL Ly, DA Cuzzone, RP Kataru, GE Hespe, JS Torrisi, JJ Huang, JC Gardenier, IL Savetsky, MD Nitti, JZ Yu, S Rehal, BJ Mehrara
Nat Commun, 2018-05-17;9(1):1970.
-
Urine podoplanin heralds the onset of ischemia-reperfusion injury of the kidney
Authors: Vivek Kasinath, Osman Arif Yilmam, Mayuko Uehara, Merve Yonar, Liwei Jiang, Xiaofei Li et al.
American Journal of Physiology-Renal Physiology
-
Mitochondrial respiration controls the Prox1-Vegfr3 feedback loop during lymphatic endothelial cell fate specification and maintenance
Authors: Wanshu Ma, Hyea Jin Gil, Xiaolei Liu, Lauren P. Diebold, Marc A. Morgan, Michael J. Oxendine-Burns et al.
Science Advances
-
T helper 2 differentiation is necessary for development of lymphedema
Authors: Catherine L. Ly, Gabriela D. García García Nores, Raghu P. Kataru, Babak J. Mehrara
Translational Research
-
Spatial and Single-Cell Transcriptional Profiling Identifies Functionally Distinct Human Dermal Fibroblast Subpopulations
Authors: Christina Philippeos, Stephanie B. Telerman, Bénédicte Oulès, Angela O. Pisco, Tanya J. Shaw, Raul Elgueta et al.
Journal of Investigative Dermatology
-
Reduced Prenatal Pulmonary Lymphatic Function Is Observed in Clp1 K/K Embryos With Impaired Motor Functions Including Fetal Breathing Movements in Preparation of the Developing Lung for Inflation at Birth
Authors: Kitti Szoták-Ajtay, Dániel Szõke, Gábor Kovács, Judit Andréka, Gábor B. Brenner, Zoltán Giricz et al.
Frontiers in Bioengineering and Biotechnology
-
Platinum Chemotherapy Induces Lymphangiogenesis in Cancerous and Healthy Tissues That Can be Prevented With Adjuvant Anti-VEGFR3 Therapy
Authors: Alexandra R. Harris, Savieay Esparza, Mohammad S. Azimi, Robert Cornelison, Francesca N. Azar, Danielle C. Llaneza et al.
Frontiers in Oncology
-
The arginine methyltransferase PRMT7 promotes extravasation of monocytes resulting in tissue injury in COPD
Authors: G Günes Güns, TM Conlon, A Jeridi, R Kim, Z Ertüz, NJ Lang, M Ansari, M Novikova, D Jiang, M Strunz, M Gaianova, C Hollauer, C Gabriel, I Angelidis, S Doll, JC Pestoni, SL Edelmann, MS Kohlhepp, A Guillot, K Bassler, HP Van Eeckho, Ö Kayalar, N Konyalilar, T Kanashova, S Rodius, C Ballester-, CM Genes Robl, N Smirnova, M Rehberg, C Agarwal, I Krikki, B Piavaux, SE Verleden, B Vanaudenae, M Königshoff, G Dittmar, KR Bracke, JL Schultze, H Watz, O Eickelberg, T Stoeger, G Burgstalle, F Tacke, V Heissmeyer, Y Rinkevich, H Bayram, HB Schiller, M Conrad, R Schneider, AÖ Yildirim
Nature Communications, 2022-03-14;13(1):1303.
-
Radioproteomics stratifies molecular response to antifibrotic treatment in pulmonary fibrosis
Authors: Lauer, D;Magnin, CY;Kolly, LR;Wang, H;Brunner, M;Chabria, M;Cereghetti, GM;Gabry?, HS;Tanadini-Lang, S;Uldry, AC;Heller, M;Verleden, SE;Klein, K;Sarbu, AC;Funke-Chambour, M;Ebner, L;Distler, O;Maurer, B;Gote-Schniering, J;
JCI insight
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Glycogen Synthase Kinase-3 Inhibition by CHIR99021 Promotes Alveolar Epithelial Cell Proliferation and Lung Regeneration in the Lipopolysaccharide-Induced Acute Lung Injury Mouse Model
Authors: Fernandes, R;Barbosa-Matos, C;Borges-Pereira, C;Carvalho, ALRT;Costa, S;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Desmoplastic stroma restricts T cell extravasation and mediates immune exclusion and immunosuppression in solid tumors
Authors: Xiao, Z;Todd, L;Huang, L;Noguera-Ortega, E;Lu, Z;Huang, L;Kopp, M;Li, Y;Pattada, N;Zhong, W;Guo, W;Scholler, J;Liousia, M;Assenmacher, CA;June, CH;Albelda, SM;Puré, E;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC/IF -
Hydrogen Sulfide Attenuates Lymphedema Via the Induction of Lymphangiogenesis Through a PI3K/Akt-Dependent Mechanism
Authors: J Suzuki, Y Shimizu, T Hayashi, Y Che, Z Pu, K Tsuzuki, S Narita, R Shibata, I Ishii, JW Calvert, T Murohara
Journal of the American Heart Association, 2022-10-26;11(21):e026889.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Sirt3 mediates the benefits of exercise on bone in aged mice
Authors: Q Li, R Wang, Z Zhang, H Wang, X Lu, J Zhang, AP Kong, XY Tian, HF Chan, AC Chung, JC Cheng, Q Jiang, WY Lee
Cell Death and Differentiation, 2022-09-24;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Omega-3 fatty acid epoxides produced by PAF-AH2 in mast cells regulate pulmonary vascular remodeling
Authors: H Moriyama, J Endo, M Kataoka, Y Shimanaka, N Kono, Y Sugiura, S Goto, H Kitakata, T Hiraide, N Yoshida, S Isobe, T Yamamoto, K Shirakawa, A Anzai, Y Katsumata, M Suematsu, K Kosaki, K Fukuda, H Arai, M Sano
Nature Communications, 2022-05-31;13(1):3013.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A High-Fat Diet Activates the BAs-FXR Axis and Triggers Cancer-Associated Fibroblast Properties in the Colon
Authors: TY Kim, S Kim, Y Kim, YS Lee, S Lee, SH Lee, MN Kweon
Cellular and Molecular Gastroenterology and Hepatology, 2021-12-29;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Expanded renal lymphatics improve recovery following kidney injury
Authors: G Baranwal, HA Creed, LM Black, A Auger, AM Quach, R Vegiraju, HE Eckenrode, A Agarwal, JM Rutkowski
Physiological Reports, 2021-11-01;9(22):e15094.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Transcriptome-Wide Analysis Reveals a Role for Extracellular Matrix and Integrin Receptor Genes in Otic Neurosensory Differentiation from Human iPSCs
Authors: L Johnson Ch, H Lahlou, C Steinacher, S Assou, Y Messat, J Dudás, A Edge, B Crespo, M Crosier, C Sergi, A Schrott-Fi, A Zine
International Journal of Molecular Sciences, 2021-10-07;22(19):.
Species: Mouse
Sample Types: Cell Lysates
Applications: IF -
Mesenchymal stromal cell aging impairs the self-organizing capacity of lung alveolar epithelial stem cells
Authors: D Chanda, M Rehan, SR Smith, KG Dsouza, Y Wang, K Bernard, D Kurundkar, V Memula, K Kojima, JA Mobley, GA Benavides, V Darley-Usm, YI Kim, JW Zmijewski, JS Deshane, S De Langhe, VJ Thannickal
Elife, 2021-09-16;10(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Nucleoside-modified VEGFC mRNA induces organ-specific lymphatic growth and reverses experimental lymphedema
Authors: D Sz?ke, G Kovács, É Kemecsei, L Bálint, K Szoták-Ajt, P Aradi, A Styevkóné, BL Mui, YK Tam, TD Madden, K Karikó, RP Kataru, MJ Hope, D Weissman, BJ Mehrara, N Pardi, Z Jakus
Nature Communications, 2021-06-08;12(1):3460.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Tertiary lymphoid organs are associated with the progression of kidney damage and regulated by interleukin-17A
Authors: R Luo, Y Cheng, D Chang, T Liu, L Liu, G Pei, N Zhang, Z Wang, K Guo, W Chen, M Li, L Fan, C Zhang, Y Li, W Dai, M Zuo, Y Xu, Y Yao, S Ge, G Xu
Theranostics, 2021-01-01;11(1):117-131.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
TRPV4 channels are essential for alveolar epithelial barrier function as protection from lung edema
Authors: J Weber, S Rajan, C Schremmer, YK Chao, G Krasteva-C, M Kannler, AÖ Yildirim, M Brosien, J Schredelse, N Weissmann, C Grimm, T Gudermann, A Dietrich
JCI Insight, 2020-10-15;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Western Blot -
Lymph node fibroblastic reticular cells deposit fibrosis-associated collagen following organ transplantation
Authors: X Li, J Zhao, V Kasinath, M Uehara, L Jiang, N Banouni, MM McGrath, T Ichimura, P Fiorina, DR Lemos, SR Shin, CF Ware, JS Bromberg, R Abdi
J. Clin. Invest., 2020-08-03;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IF -
Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis
Authors: M Strunz, LM Simon, M Ansari, JJ Kathiriya, I Angelidis, CH Mayr, G Tsidiridis, M Lange, LF Mattner, M Yee, P Ogar, A Sengupta, I Kukhtevich, R Schneider, Z Zhao, C Voss, T Stoeger, JHL Neumann, A Hilgendorf, J Behr, M O'Reilly, M Lehmann, G Burgstalle, M Königshoff, HA Chapman, FJ Theis, HB Schiller
Nat Commun, 2020-07-16;11(1):3559.
Species: Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: IHC, Western Blot -
Keratinocyte-Expressed Podoplanin is Dispensable for Multi-Step Skin Carcinogenesis
Authors: M Sesarti?, K Ikenberg, SY Yoon, M Detmar
Cells, 2020-06-24;9(6):.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Characterizing Lymphangiogenesis and Concurrent Inflammation in Adipose Tissue in Response to VEGF-D
Authors: A Chakrabort, CK Scogin, K Rizwan, TS Morley, JM Rutkowski
Front Physiol, 2020-04-22;11(0):363.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Overproduction of thrombopoietin by BRAFV600E-mutated mouse hepatocytes and contribution of thrombopoietin to hepatocarcinogenesis
Authors: H Tanaka, K Horioka, M Yamamoto, M Asari, K Okuda, K Yamazaki, K Shimizu, K Ogawa
Cancer Sci., 2019-07-30;110(9):2748-2759.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Conditional deletion of E11/podoplanin in bone protects against load-induced osteoarthritis
Authors: KA Staines, E Ikpegbu, AE Törnqvist, S Dillon, B Javaheri, AK Amin, DN Clements, DJ Buttle, AA Pitsillide, C Farquharso
BMC Musculoskelet Disord, 2019-07-27;20(1):344.
Species: Canine, Human, Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
An important role of cutaneous lymphatic vessels in coordinating and promoting anagen hair follicle growth
Authors: SY Yoon, LC Dieterich, S Karaman, ST Proulx, SB Bachmann, C Sciaroni, M Detmar
PLoS ONE, 2019-07-25;14(7):e0220341.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
An important role of podoplanin in hair follicle growth
Authors: SY Yoon, LC Dieterich, C Tacconi, M Sesartic, Y He, L Brunner, O Kwon, M Detmar
PLoS ONE, 2019-07-23;14(7):e0219938.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Efficient CD4Cre-Mediated Conditional KRas Expression in Alveolar Macrophages and Alveolar Epithelial Cells Causes Fatal Hyperproliferative Pneumonitis
Authors: P Chen, S Wang, KS Janardhan, RL Zemans, W Deng, P Karmaus, S Shen, M Sunday, LG Que, MB Fessler, XP Zhong
J. Immunol., 2019-07-17;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Immune heterogeneity of head and tail pancreatic lymph nodes in non-obese diabetic mice
Authors: X Li, A Bean, M Uehara, N Banouni, M Ben Nasr, V Kasinath, L Jiang, P Fiorina, R Abdi
Sci Rep, 2019-07-05;9(1):9778.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Anti-IL-6 eluting immunomodulatory biomaterials prolong skin allograft survival
Authors: M Uehara, X Li, A Sheikhi, N Zandi, B Walker, B Saleh, N Banouni, L Jiang, F Ordikhani, L Dai, M Yonar, I Vohra, V Kasinath, DP Orgill, A Khademhoss, N Annabi, R Abdi
Sci Rep, 2019-04-25;9(1):6535.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Cytotoxic chemotherapy reduces T cell trafficking to the spleen by downregulating the expression of C-C motif chemokine ligand 21 and C-C motif chemokine ligand 19
Authors: L Liu, L Zhao, Y Yang, J Gao, C Hu, B Guo, B Zhu
Oncol Lett, 2018-08-09;16(4):5013-5019.
-
Unexpected contribution of lymphatic vessels to promotion of distant metastatic tumor spread
Authors: Q Ma, LC Dieterich, K Ikenberg, SB Bachmann, J Mangana, ST Proulx, VC Amann, MP Levesque, R Dummer, P Baluk, DM McDonald, M Detmar
Sci Adv, 2018-08-08;4(8):eaat4758.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Repetitive ischemic injuries to the kidneys result in lymph node fibrosis and impaired healing
Authors: OH Maarouf, M Uehara, V Kasinath, Z Solhjou, N Banouni, B Bahmani, L Jiang, OA Yilmam, I Guleria, SB Lovitch, JL Grogan, P Fiorina, PT Sage, JS Bromberg, MM McGrath, R Abdi
JCI Insight, 2018-07-12;3(13):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Docetaxel facilitates lymphatic-tumor crosstalk to promote lymphangiogenesis and cancer progression
Authors: AR Harris, MJ Perez, JM Munson
BMC Cancer, 2018-07-06;18(1):718.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Salient type 1 interleukin 1 receptor expression in peripheral non-immune cells
Authors: A Song, L Zhu, G Gorantla, O Berdysz, SA Amici, M Guerau-de-, KM Madalena, JK Lerch, X Liu, N Quan
Sci Rep, 2018-01-15;8(1):723.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Effects of Orally Ingested Arsenic on Respiratory Epithelial Permeability to Bacteria and Small Molecules in Mice
Authors: MW Henderson, JH Madenspach, GS Whitehead, SY Thomas, JJ Aloor, KM Gowdy, MB Fessler
Environ. Health Perspect., 2017-09-28;125(9):097024.
Species: Mouse
Sample Types: BALF
Applications: Western Blot -
Indian Hedgehog Suppresses a Stromal Cell-Driven Intestinal Immune Response
Authors: BF Westendorp, NVJA Büller, ON Karpus, WA van Dop, J Koster, R Versteeg, PJ Koelink, CY Snel, S Meisner, JJTH Roelofs, A Uhmann, E Ver Loren, J Heijmans, H Hahn, V Muncan, ME Wildenberg, GR van den Br
Cell Mol Gastroenterol Hepatol, 2017-09-05;5(1):67-82.e1.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1
Authors: LA Johnson, S Banerji, W Lawrance, U Gileadi, G Prota, KA Holder, YM Roshorm, T Hanke, V Cerundolo, NW Gale, DG Jackson
Nat. Immunol., 2017-05-15;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
CD8+ T cell evasion mandates CD4+ T cell control of chronic gamma-herpesvirus infection
Authors: CSE Tan, C Lawler, PG Stevenson
PLoS Pathog., 2017-04-10;13(4):e1006311.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Differentiation of Club Cells to Alveolar Epithelial Cells In Vitro
Authors: D Zheng, BS Soh, L Yin, G Hu, Q Chen, H Choi, J Han, VT Chow, J Chen
Sci Rep, 2017-01-27;7(0):41661.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD
Authors: Hoeke A Baarsma
J. Exp. Med, 2016-12-15;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Postnatal Deletion of Podoplanin in Lymphatic Endothelium Results in Blood Filling of the Lymphatic System and Impairs Dendritic Cell Migration to Lymph Nodes
Authors: Michael Detmar
Arterioscler. Thromb. Vasc. Biol., 2016-11-03;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Type I Interferons Direct Gammaherpesvirus Host Colonization
PLoS Pathog, 2016-05-25;12(5):e1005654.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Murine junctional adhesion molecules JAM-B and JAM-C mediate endothelial and stellate cell interactions during hepatic fibrosis
Authors: E Hintermann, M Bayer, J Ehser, M Aurrand-Li, JM Pfeilschif, BA Imhof, U Christen
Cell Adh Migr, 2016-04-25;10(4):419-33.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Aging exacerbates damage and delays repair of alveolar epithelia following influenza viral pneumonia.
Authors: Yin L, Zheng D, Limmon G, Leung N, Xu S, Rajapakse J, Yu H, Chow V, Chen J
Respir Res, 2014-09-30;15(0):116.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Effects of adenosine on lymphangiogenesis.
Authors: Lenoir B, Wagner D, Blacher S, Sala-Newby G, Newby A, Noel A, Devaux Y
PLoS ONE, 2014-03-20;9(3):e92715.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Key role of microRNA in the regulation of granulocyte macrophage colony-stimulating factor expression in murine alveolar epithelial cells during oxidative stress.
Authors: Sturrock, Anne, Mir-Kasimov, Mustafa, Baker, Jessica, Rowley, Jesse, Paine, Robert 3
J Biol Chem, 2013-12-26;289(7):4095-105.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Distinct fibroblast lineages determine dermal architecture in skin development and repair.
Authors: Driskell R, Lichtenberger B, Hoste E, Kretzschmar K, Simons B, Charalambous M, Ferron S, Herault Y, Pavlovic G, Ferguson-Smith A, Watt F
Nature, 2013-12-12;504(7479):277-81.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Regeneration of alveolar type I and II cells from Scgb1a1-expressing cells following severe pulmonary damage induced by bleomycin and influenza.
Authors: Zheng, Dahai, Limmon, Gino V, Yin, Lu, Leung, Nicola H, Yu, Hanry, Chow, Vincent, Chen, Jianzhu
PLoS ONE, 2012-10-31;7(10):e48451.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage.
J. Immunol., 2012-07-27;189(5):2181-90.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation.
Authors: Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, Wucherpfennig K, Turley S, Carroll MC, Sobel RA, Bettelli E, Kuchroo VK
Immunity, 2011-12-15;35(6):986-96.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
The Appearance and Modulation of Osteocyte Marker Expression during Calcification of Vascular Smooth Muscle Cells.
Authors: Zhu D, Mackenzie NC, Millan JL, Farquharson C, MacRae VE
PLoS ONE, 2011-05-17;6(5):e19595.
Species: Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: IHC-P, Western Blot -
Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3.
Authors: Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, Larrivee B, Del Toro R, Suchting S, Medvinsky A, Silva J, Yang J, Thomas JL, Koch AW, Alitalo K, Eichmann A, Bagri A
J. Cell Biol., 2010-01-11;188(1):115-30.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Platelet-derived SDF-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration
Authors: Shahin Rafii, Zhongwei Cao, Raphael Lis, Ilias I. Siempos, Deebly Chavez, Koji Shido et al.
Nature Cell Biology
-
IFN gamma -activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin
Authors: Ryan S. Lane, Julia Femel, Alec P. Breazeale, Christopher P. Loo, Guillaume Thibault, Andy Kaempf et al.
Journal of Experimental Medicine
-
Lymphangiogenic therapy prevents cardiac dysfunction by ameliorating inflammation and hypertension
Authors: LouJin Song, Xian Chen, Terri A Swanson, Brianna LaViolette, Jincheng Pang, Teresa Cunio et al.
eLife
-
Glycoprotein B Cleavage Is Important for Murid Herpesvirus 4 To Infect Myeloid Cells
Authors: Daniel L. Glauser, Ricardo Milho, Bruno Frederico, Janet S. May, Anne-Sophie Kratz, Laurent Gillet et al.
Journal of Virology
-
Characterization of Leptin Receptor+ Stromal Cells in Lymph Node
Authors: Jiang L, Yilmaz M, Uehara M et al.
Frontiers in Immunology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse Podoplanin Antibody
There are currently no reviews for this product. Be the first to review Mouse Podoplanin Antibody and earn rewards!
Have you used Mouse Podoplanin Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image

