Recombinant Human IL-18/IL-1F4 Biotinylated Protein, CF
Recombinant Human IL-18/IL-1F4 Biotinylated Protein, CF Summary
Product Specifications
Tyr37-Asp193
Analysis
Customers also Viewed
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
BT9124
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS and DTT with Trehalose. |
| Reconstitution | Reconstitute at 250 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
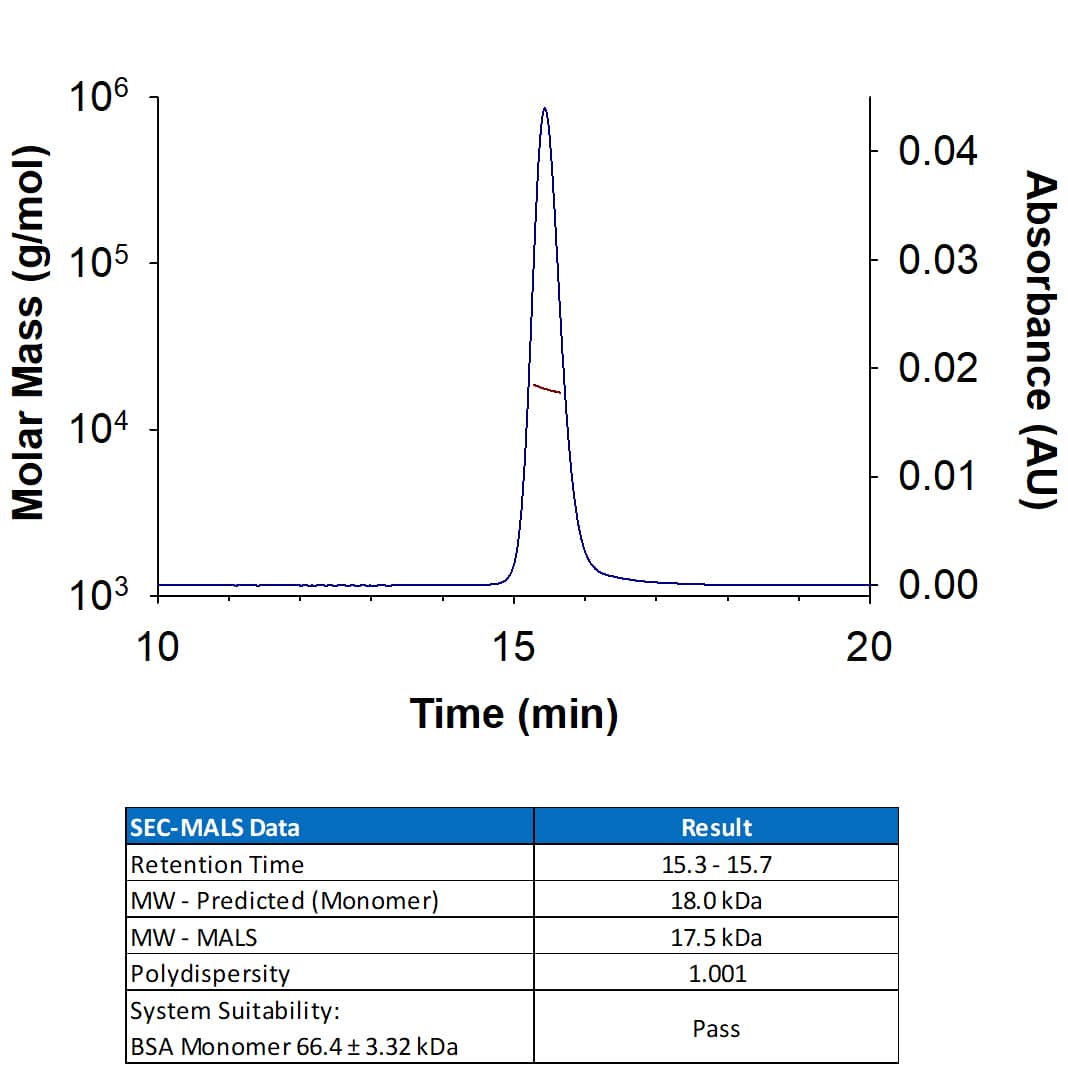
Measured by its binding ability in a functional ELISA. When Recombinant Human IL-18 BPa His-tag Protein (11236-BP) is immobilized at 0.25 μg/mL (100 μL/well), the concentration of Biotinylated Recombinant Human IL-18/IL-1F4 (Catalog # BT9124) that produces 50% of the binding response is 1.50-12.0 ng/mL.
 View Larger
View Larger
2 μg/lane of Biotinylated Recombinant Human IL-18/IL-1F4 Protein (Catalog # BT9124) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 15-21 kDa.
Background: IL-18/IL-1F4
Interleukin-18 (IL-18) is a proinflammatory cytokine in the IL-1 family that exerts distinct immune effects depending on the local cytokine environment. It is expressed as a 24 kDa precursor by endothelial and epithelial cells, keratinocytes, gamma δ T cells, and phagocytes. The precursor is activated intracellularly by Caspase-1 mediated proteolysis to release the 17 kDa mature cytokine. The precursor can also be released by necrotic cells for extracellular cleavage by multiple proteases. IL‑18 activation is induced by infection or tissue damage and contributes to disease pathology in chronic inflammation (1-3). IL-18 binds to the widely expressed IL-18 R alpha which recruits IL-18 R beta to form the signaling receptor complex (4, 5). Its bioactivity is negatively regulated by interactions with IL-18 binding proteins and virally encoded IL-18BP homologs (6). In the presence of IL-12 or IL-15, IL-18 enhances anti-viral Th1 immune responses by inducing IFN-gamma production and the cytolytic activity of CD8+ T cells and NK cells (7, 8). In the absence of IL-12 or IL-15, however, IL-18 promotes production of the Th2 cytokines IL-4 and IL-13 by CD4+ T cells and basophils (9, 10). In the presence of IL-1 beta or IL-23, IL-18 induces the antigen-independent production of IL-17 by gamma δ T cells and CD4+ T cells (11). IL-18 also promotes myeloid dendritic cell maturation and triggers neutrophil respiratory burst (12, 13). In cancer, IL-18 exhibits diverse activities including enhancing anti-tumor immunity, inhibiting or promoting angiogenesis, and promoting tumor cell metastasis (14). Mature human IL-18 shares approximately 63% amino acid sequence identity with mouse and rat IL-18 (15). Alternative splicing in human ovarian cancer generates an isoform that is resistant to Caspase-1 activation (16). A cell surface form can be expressed on M-CSF induced macrophages and released in response to bacterial endotoxin (17).
- Dinarello, C.A. et al. (2013) Front. Immunol. 4:289.
- Smith, D.E. (2011) J. Leukoc. Biol. 89:383.
- Gu, Y. et al. (1997) Science 275:206.
- Torigoe, K. et al. (1997) J. Biol. Chem. 272:25737.
- Cheung, H. et al. (2005) J. Immunol. 174:5351.
- Novick, D. et al. (1999) Immunity 10:127.
- Fehniger, T.A. et al. (1999) J. Immunol. 162:4511.
- Yoshimoto, T. et al. (1998) J. Immunol. 161:3400.
- Yoshimoto, T. et al. (2000) Nat. Immunol. 1:132.
- Kroeger, K.M. et al. (2009) J. Leukoc. Biol. 86:769.
- Lalor, S.J. et al. (2011) J. Immunol. 186:5738.
- Li, J. et al. (2004) Cell. Immunol. 227:103.
- Elbim, C. et al. (2005) Clin. Diagn. Lab. Immunol. 12:436.
- Fabbi, M. et al. (2015) J. Leukoc. Biol. 97:665.
- Ushio, S. et al. (1996) J. Immunol. 156:4274.
- Gaggero, A. et al. (2004) Oncogene 23:7552.
- Bellora, F. et al. (2012) Eur. J. Immunol. 42:1618.
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human IL-18/IL-1F4 Biotinylated Protein, CF
There are currently no reviews for this product. Be the first to review Recombinant Human IL-18/IL-1F4 Biotinylated Protein, CF and earn rewards!
Have you used Recombinant Human IL-18/IL-1F4 Biotinylated Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image