Recombinant Human IL-18/IL-1F4 Protein
Recombinant Human IL-18/IL-1F4 Protein Summary
Product Specifications
Tyr37-Asp193
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
9124-IL
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS and DTT with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 200 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
9124-IL/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS and DTT. |
| Reconstitution | Reconstitute at 200 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
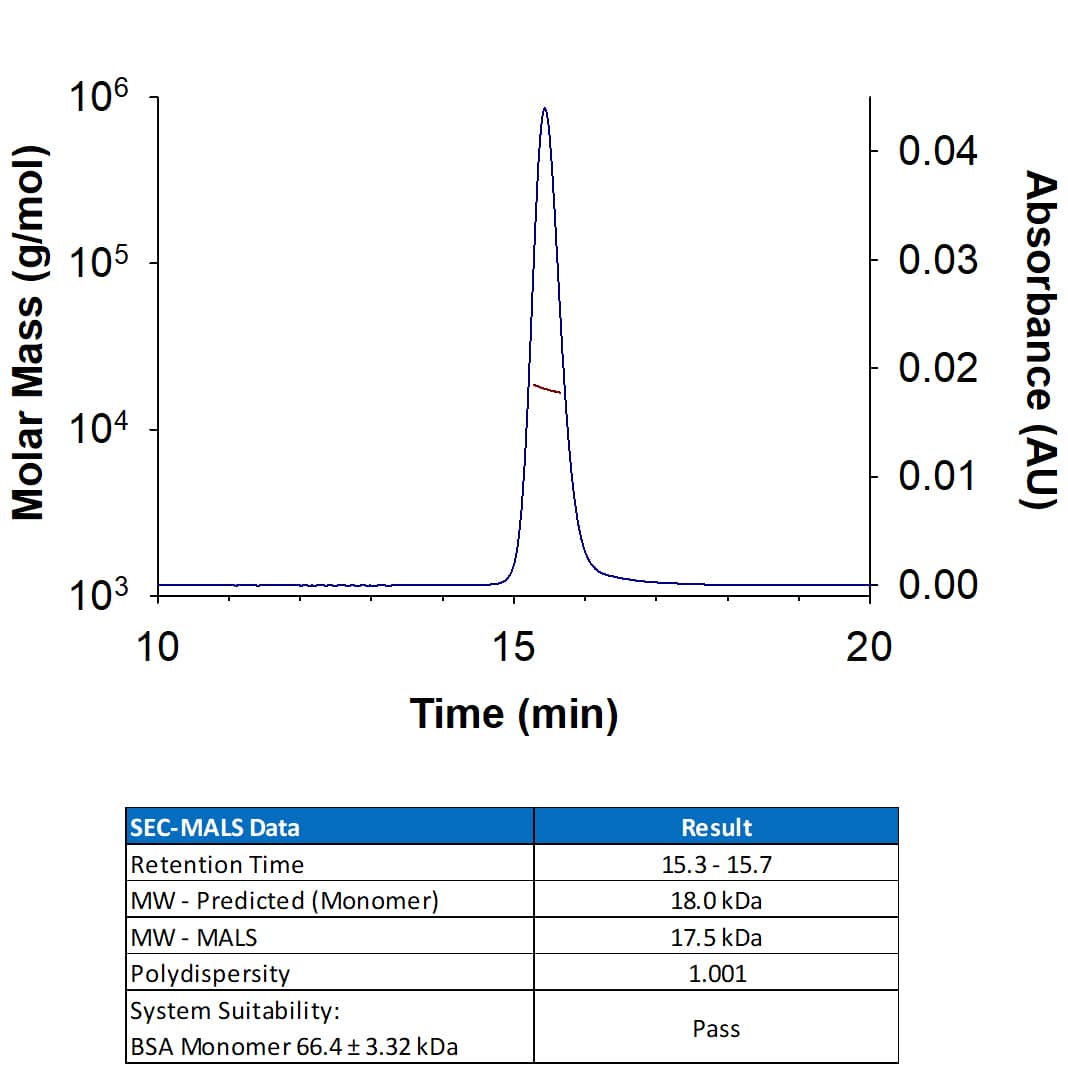
Recombinant Human IL-18 (Catalog # 9124-IL) has a molecular weight (MW) of 17.5 kDa as analyzed by SEC-MALS, suggesting that this protein is a monomer.
 View Larger
View Larger
Recombinant Human IL-18 (Catalog # 9124-IL) induces IFN-gamma secretion by KG‑1 human acute myelogenous leukemia cells. The ED50 for this effect is 1.5-9 ng/mL.
 View Larger
View Larger
1 μg/lane of Recombinant Human IL-18/IL-1F4 (Catalog # 9124-IL) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by silver staining, showing a single band at 18 kDa.
Reconstitution Calculator
Background: IL-18/IL-1F4
Interleukin-18 (IL-18) is a proinflammatory cytokine in the IL-1 family that exerts distinct immune effects depending on the local cytokine environment. It is expressed as a 24 kDa precursor by endothelial and epithelial cells, keratinocytes, gamma δ T cells, and phagocytes. The precursor is activated intracellularly by Caspase-1 mediated proteolysis to release the 17 kDa mature cytokine. The precursor can also be released by necrotic cells for extracellular cleavage by multiple proteases.
IL-18 activation is induced by infection or tissue damage and contributes to disease pathology in chronic inflammation (1-3). IL-18 binds to the widely expressed
IL-18 R alpha which recruits IL-18 R beta to form the signaling receptor complex (4, 5). Its bioactivity is negatively regulated by interactions with IL-18 binding proteins and virally encoded IL-18BP homologs (6). In the presence of IL-12 or IL-15, IL-18 enhances anti-viral Th1 immune responses by inducing IFN-gamma production and the cytolytic activity of CD8+ T cells and NK cells (7, 8). In the absence of IL-12 or IL-15, however, IL-18 promotes production of the Th2 cytokines IL-4 and IL-13 by CD4+ T cells and basophils (9, 10). In the presence of IL-1 beta or IL-23, IL-18 induces the antigen-independent production of IL-17 by gamma δ T cells and CD4+ T cells (11).
IL-18 also promotes myeloid dendritic cell maturation and triggers neutrophil respiratory burst (12, 13). In cancer, IL-18 exhibits diverse activities including enhancing anti-tumor immunity, inhibiting or promoting angiogenesis, and promoting tumor cell metastasis (14). Mature human IL-18 shares approximately 63% amino acid sequence identity with mouse and rat IL-18 (15). Alternative splicing in human ovarian cancer generates an isoform that is resistant to Caspase-1 activation (16). A cell surface form can be expressed on M-CSF induced macrophages and released in response to bacterial endotoxin (17).
- Dinarello, C.A. et al. (2013) Front. Immunol. 4:289.
- Smith, D.E. (2011) J. Leukoc. Biol. 89:383.
- Gu, Y. et al. (1997) Science 275:206.
- Torigoe, K. et al. (1997) J. Biol. Chem. 272:25737.
- Cheung, H. et al. (2005) J. Immunol. 174:5351.
- Novick, D. et al. (1999) Immunity 10:127.
- Fehniger, T.A. et al. (1999) J. Immunol. 162:4511.
- Yoshimoto, T. et al. (1998) J. Immunol. 161:3400.
- Yoshimoto, T. et al. (2000) Nat. Immunol. 1:132.
- Kroeger, K.M. et al. (2009) J. Leukoc. Biol. 86:769.
- Lalor, S.J. et al. (2011) J. Immunol. 186:5738.
- Li, J. et al. (2004) Cell. Immunol. 227:103.
- Elbim, C. et al. (2005) Clin. Diagn. Lab. Immunol. 12:436.
- Fabbi, M. et al. (2015) J. Leukoc. Biol. 97:665.
- Ushio, S. et al. (1996) J. Immunol. 156:4274.
- Gaggero, A. et al. (2004) Oncogene 23:7552.
- Bellora, F. et al. (2012) Eur. J. Immunol. 42:1618.
Citations for Recombinant Human IL-18/IL-1F4 Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
23
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Alveolar hypoxia induces organ-specific inflammasome-related inflammation in male mouse lungs
Authors: Udjus, C;Halvorsen, B;Kong, XY;Sagen, EL;Martinsen, M;Yang, K;Løberg, EM;Christensen, G;Skjønsberg, OH;Larsen, KO;
Physiological reports
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Regulation of HHLA2 expression in kidney cancer and myeloid cells
Authors: Shigemura, T;Perrot, N;Huang, Z;Bhatt, RS;Sheshdeh, AB;Ahmar, NE;Ghandour, F;Signoretti, S;McDermott, DF;Freeman, GJ;Mahoney, KM;
BMC cancer
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
TNF? induced by DNA-sensing in macrophage compromises retinal pigment epithelial (RPE) barrier function
Authors: Twarog, M;Schustak, J;Xu, Y;Coble, M;Dolan, K;Esterberg, R;Huang, Q;Saint-Geniez, M;Bao, Y;
Scientific reports
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Chemokine positioning determines mutually exclusive roles for their receptors in extravasation of pathogenic human T cells
Authors: F Parween, SP Singh, HH Zhang, N Kathuria, FA Otaizo-Car, A Shamsaddin, PJ Gardina, S Ganesan, J Kabat, HA Lorenzi, TG Myers, JM Farber
bioRxiv : the preprint server for biology, 2023-02-13;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Differential IL18 signaling via IL18 receptor and Na-Cl co-transporter discriminating thermogenesis and glucose metabolism regulation
Authors: X Zhang, S Luo, M Wang, Q Cao, Z Zhang, Q Huang, J Li, Z Deng, T Liu, CL Liu, M Meppen, A Vromman, RA Flavell, GS Hotam??l?g, J Liu, P Libby, Z Liu, GP Shi
Nature Communications, 2022-12-08;13(1):7582.
Species: Human
Sample Types: Whole Tissue
Applications: Bioassay -
In�vivo G-CSF treatment activates the GR-SOCS1 axis to suppress IFN-gamma secretion by natural killer cells
Authors: X Zhao, T Peng, X Cao, Y Hou, R Li, T Han, Z Fan, M Zhao, Y Chang, H Chen, C Li, X Huang
Cell Reports, 2022-09-13;40(11):111342.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
DLL4 and VCAM1 enhance the emergence of T cell-competent hematopoietic progenitors from human pluripotent stem cells
Authors: YS Michaels, JM Edgar, MC Major, EL Castle, C Zimmerman, T Yin, A Hagner, C Lau, HH Hsu, MI Ibañez-Rio, LJ Durland, DJHF Knapp, PW Zandstra
Science Advances, 2022-08-24;8(34):eabn5522.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Imprint of unconventional T cell response in acute hepatitis C persists despite successful early antiviral treatment
Authors: Y Du, T Khera, B Strunz, K Deterding, D Todt, N Woller, SA Engelskirc, S Hardtke, K Port, A Ponzetta, E Steinmann, M Cornberg, J Hengst, NK Björkström, H Wedemeyer, HepNet Acu
European Journal of Immunology, 2021-12-13;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Mucosal-Associated Invariant T (MAIT) Cells Are Highly Activated and Functionally Impaired in COVID-19 Patients
Authors: S Deschler, J Kager, J Erber, L Fricke, P Koyumdzhie, A Georgieva, T Lahmer, JR Wiessner, F Voit, J Schneider, J Horstmann, R Iakoubov, M Treiber, C Winter, J Ruland, DH Busch, PA Knolle, U Protzer, CD Spinner, RM Schmid, M Quante, K Böttcher
Viruses, 2021-02-03;13(2):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Human Thymic CD10+ PD-1+ Intraepithelial Lymphocyte Precursors Acquire Interleukin-15 Responsiveness at the CD1a- CD95+ CD28- CCR7- Developmental Stage
Authors: L Billiet, G Goetgeluk, S Bonte, S De Munter, L De Cock, M Pille, J Ingels, H Jansen, K Weening, F Van Nieuwe, T Kerre, T Taghon, G Leclercq, B Vandekerck
Int J Mol Sci, 2020-11-20;21(22):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Boosting NAD Level Suppresses Inflammatory Activation of PBMC in Heart Failure
Authors: B Zhou, DD Wang, Y Qiu, S Airhart, Y Liu, A Stempien-O, KD O'Brien, R Tian
J. Clin. Invest., 2020-11-02;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Inflammatory Signals Induce AT2 Cell-Derived Damage-Associated Transient Progenitors that Mediate Alveolar Regeneration
Authors: J Choi, JE Park, G Tsagkogeor, M Yanagita, BK Koo, N Han, JH Lee
Cell Stem Cell, 2020-08-03;27(3):366-382.e7.
Species: Mouse
Sample Types: Organoid, Organoids
Applications: Bioassay -
Metabolic Reprograming via Deletion of CISH in Human iPSC-Derived NK Cells Promotes In�Vivo Persistence and Enhances Anti-tumor Activity
Authors: H Zhu, RH Blum, D Bernareggi, EH Ask, Z Wu, HJ Hoel, Z Meng, C Wu, KL Guan, KJ Malmberg, DS Kaufman
Cell Stem Cell, 2020-06-11;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The TL1A-DR3 Axis Selectively Drives Effector Functions in Human MAIT Cells
Authors: A Sattler, LG Thiel, AH Ruhm, N Souidi, M Seifert, G Herberth, K Kotsch
J. Immunol., 2019-10-18;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Tumor-infiltrating mucosal-associated invariant T (MAIT) cells retain expression of cytotoxic effector molecules
Authors: P Sundström, L Szeponik, F Ahlmanner, M Sundquist, JSB Wong, EB Lindskog, B Gustafsson, M Quiding-Jä
Oncotarget, 2019-04-19;10(29):2810-2823.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Induction of antiinflammatory purinergic signaling in activated human iNKT cells
Authors: JC Yu, G Lin, JJ Field, J Linden
JCI Insight, 2018-09-06;3(17):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Increased NK Cell Function after Cessation of Long-Term Nucleos(t)ide Analogue Treatment in Chronic Hepatitis B Associates with Liver Damage and HBsAg Loss
Authors: CL Zimmer, F Rinker, C Höner Zu S, MP Manns, H Wedemeyer, M Cornberg, NK Björkström
J. Infect. Dis., 2018-04-23;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
An anti-TL1A antibody for the treatment of asthma and inflammatory bowel disease
Authors: AW Clarke, L Poulton, D Shim, D Mabon, D Butt, M Pollard, V Pande, J Husten, J Lyons, C Tian, AG Doyle
MAbs, 2018-03-05;0(0):1-43.
Species: Human
Sample Types: Whole Blood
Applications: Bioassay -
Early and late changes in natural killer cells in response to ledipasvir/sofosbuvir treatment
Authors: L Golden-Mas, RH McMahan, MS Kriss, AL Kilgore, L Cheng, RJ Dran, A Wieland, HR Rosen
Hepatol Commun, 2018-03-01;2(4):364-375.
Species: Human
Sample Types:
-
Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect
Authors: M Felices, AJ Lenvik, R McElmurry, S Chu, P Hinderlie, L Bendzick, MA Geller, J Tolar, BR Blazar, JS Miller
JCI Insight, 2018-02-08;3(3):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
EBI3 regulates the NK cell response to mouse cytomegalovirus infection
Authors: H Jensen, SY Chen, L Folkersen, GP Nolan, LL Lanier
Proc. Natl. Acad. Sci. U.S.A, 2017-01-31;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Mass cytometry analytical approaches reveal cytokine-induced changes in natural killer cells
Authors: Elena Vendrame
Cytometry B Clin Cytom, 2017-01-01;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Selective IRAK4 Inhibition Attenuates Disease in Murine Lupus Models and Demonstrates Steroid Sparing Activity
Authors: Shailesh Dudhgaonka
J. Immunol, 2016-12-21;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay
FAQs
-
What is the difference between the two versions of Recombinant Human IL-18, Catalog # 9124-IL and Catalog # B001-5?
Catalog # 9124-IL is manufactured by R&D Systems, while Catalog # B001-5 is manufactured by MBL. Catalog # 9124-IL is lyophilized powder whereas Catalog # B001-5 is supplied in solution (PBS with BSA and sucrose). Activity of the two proteins is measured in similar cell-based assays; please consult the respective product-specific pages for assay details. Catalog # 9124-IL is competitively priced and has higher purity and lower endotoxin than Catalog # B001-5.
Reviews for Recombinant Human IL-18/IL-1F4 Protein
Average Rating: 4.5 (Based on 8 Reviews)
Have you used Recombinant Human IL-18/IL-1F4 Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Reason for Rating: Worked nicely in human serum samples in elisa method
Reason for Rating: it works great in my assay
I am looking at the inhibition of IFN-r production by the compound with the presence of IL23 and IL-18.
Performed similarly to Competitor1 and Competitor2 proteins in immunoassay