Recombinant SARS-CoV-2 C.1.2 Spike RBD His-tag Protein, CF
Recombinant SARS-CoV-2 C.1.2 Spike RBD His-tag Protein, CF Summary
Product Specifications
Arg319-Phe541 (Tyr449His, Glu484Lys, Asn501Tyr), with a C-terminal 6-His tag
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
10997-CV
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
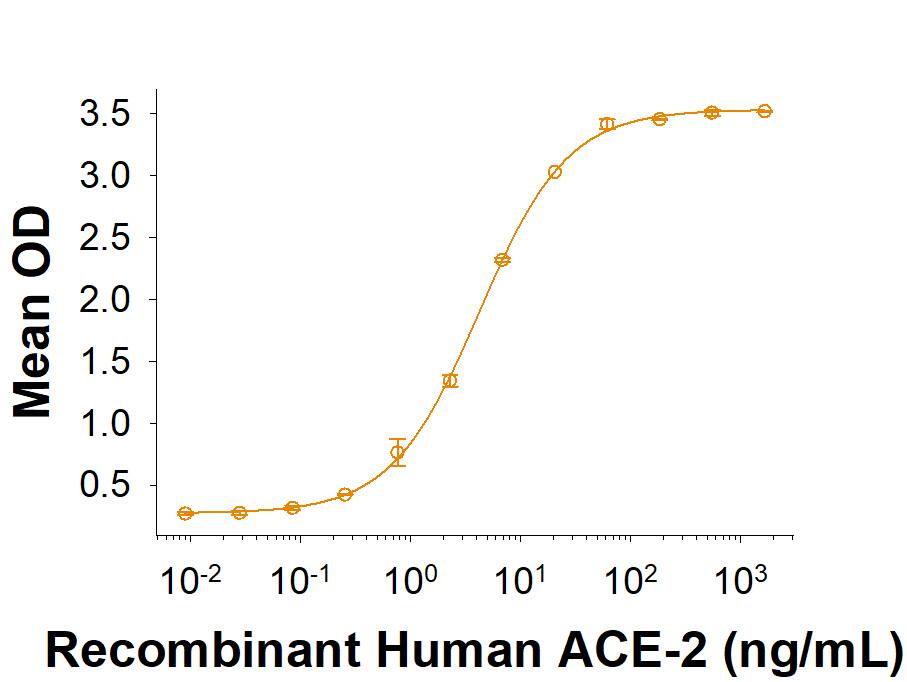 View Larger
View Larger
Recombinant SARS-CoV-2 C.1.2 Spike RBD His-tag (Catalog # 10997-CV) binds Recombinant Human ACE-2 His-tag (933-ZN) in a functional ELISA.
 View Larger
View Larger
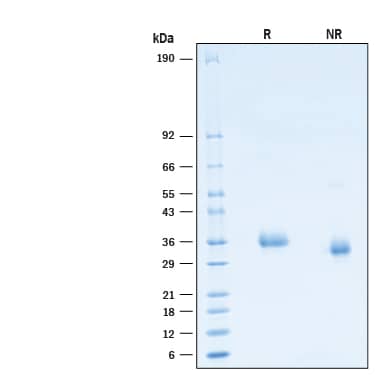
2 μg/lane of Recombinant SARS-CoV-2 C.1.2 Spike RBD His-tag Protein (Catalog # 10997-CV) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 32-38 kDa.
Reconstitution Calculator
Background: Spike RBD
SARS-CoV-2, which causes the global pandemic coronavirus disease 2019 (Covid-19), belongs to a family of viruses known as coronaviruses that also include MERS‑CoV and SARS-CoV-1. Coronaviruses are commonly comprised of four structural proteins: Spike protein (S), Envelope protein (E), Membrane protein (M) and Nucleocapsid protein (N) (1). The SARS-CoV-2 S protein is a glycoprotein that mediates membrane fusion and viral entry. The S protein is homotrimeric, with each ~180-kDa monomer consisting of two subunits, S1 and S2 (2). In SARS-CoV-2, as with most coronaviruses, proteolytic cleavage of the S protein into S1 and S2 subunits is required for activation. The S1 subunit is focused on attachment of the protein to the host receptor while the S2 subunit is involved with cell fusion (3-5). A receptor binding domain (RBD) in the C-terminus of the S1 subunit has been identified and the RBD of SARS-CoV-2 shares 73% amino acid (aa) identity with the RBD of the SARS-CoV-1, but only 22% aa identity with the RBD of MERS‑CoV (6, 7). The low aa sequence homology is consistent with the finding that SARS and MERS‑CoV bind different cellular receptors (8). The RBD of SARS-CoV-2 binds a metallopeptidase, angiotensin-converting enzyme 2 (ACE‑2), similar to SARS-CoV-1, but with much higher affinity and faster binding kinetics (9). Before binding to the ACE‑2 receptor, structural analysis of the S1 trimer shows that only one of the three RBD domains is in the "up" conformation. This is an unstable and transient state that passes between trimeric subunits but is nevertheless an exposed state to be targeted for neutralizing antibody therapy (10). Polyclonal antibodies to the RBD of the SARS-CoV-2 protein have been shown to inhibit interaction with the ACE‑2 receptor, confirming RBD as an attractive target for vaccinations or antiviral therapy (11). There is also promising work showing that the RBD may be used to detect presence of neutralizing antibodies present in a patient's bloodstream, consistent with developed immunity after exposure to the SARS-CoV-2 (12). A SARS-CoV-2 variant (C.1.2) carrying the aa substitution Tyr449His, Glu484Lys, Asn501Tyr in RBD protein was evolved from C.1. It was first identified in May 2021 in South Africa and rapidly spreaded globally. Whether these mutations in the spike protein would cause more severe symptom or decrease the efficacy of vaccine-induced immunity is still under investigation (13).
- Wu, F. et al. (2020) Nature 579:265.
- Tortorici, M.A. and D. Veesler (2019) Adv. Virus Res. 105:93.
- Bosch, B.J. et al. (2003). J. Virol 77:8801.
- Belouzard, S. et al. (2009) Proc. Natl. Acad. Sci. 106:5871.
- Millet, J.K. and G.R. Whittaker (2015) Virus Res. 202:120.
- Li, W. et al. (2003) Nature 426:450.
- Wong, S.K. et al. (2004) J. Biol. Chem. 279:3197.
- Jiang, S. et al. (2020) Trends. Immunol. https://doi.org/10.1016/j.it.2020.03.007.
- Ortega, J.T. et al. (2020) EXCLI J. 19:410.
- Wrapp, D. et al. (2020) Science 367:1260.
- Tai, W. et al. (2020) Cell. Mol. Immunol. https://doi.org/10.1016/j.it.2020.03.007.1.
- Okba, N.M.A. et al. (2020). Emerg. Infect. Dis. https://doi.org/10.3201/eid2607.200841.
- Scheepers, C. et al. (2021) medRxiv https://doi.org/10.1101/2021.08.20.21262342.
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant SARS-CoV-2 C.1.2 Spike RBD His-tag Protein, CF
There are currently no reviews for this product. Be the first to review Recombinant SARS-CoV-2 C.1.2 Spike RBD His-tag Protein, CF and earn rewards!
Have you used Recombinant SARS-CoV-2 C.1.2 Spike RBD His-tag Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
