Mouse LYVE-1 Antibody Summary
Ala24-Thr234
Accession # Q8BHC0
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
LYVE‑1 in Mouse Liver. LYVE-1 was detected in perfusion fixed frozen sections of mouse liver using 15 µg/mL Goat Anti-Mouse LYVE-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2125) overnight at 4 °C. Tissue was stained with the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; CTS008) and counterstained with hematoxylin (blue). Specific labeling was localized to the cytoplasm of endothelial cells in sinusoids. View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
Detection of Mouse LYVE‑1 by Western Blot. Western blot shows lysates of mouse liver tissue and bEnd.3 mouse brain endothelial cell line. PVDF membrane was probed with 0.25 µg/mL of Goat Anti-Mouse LYVE-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2125) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF019). A specific band was detected for LYVE-1 at approximately 60-65 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of Mouse LYVE-1 by Immunocytochemistry/Immunofluorescence 17D-204 causes viscerotropic abnormalities in the absence of IFN-gamma. H&E sections (a–c) antibody-stained frozen sections (b, d) of spleen (a, b) and liver (c, d) were presented. a Spleen sections from 17D-204-infected animals were indistinguishable at 4 dpi, whereas Angola71-infected animals display loss of white pulp and red pulp architecture and increase in infiltrating macrophages and neutrophils (inset) at 4 dpi. At 11 dpi, 17D-204 infected AGB6 has increased immune infiltration and extramedullary hematopoiesis (inset) but not AB6 mice. b YFV antigen can be detected in the spleen at 4 dpi in both AB6 and AGB6 mice in the red pulp and outer marginal zone area. c 17D-204-infected AB6 mice do not display major histological changes but infected AGB6 mice and Angola71-infected animals had microsteatosis (inset) at 4 dpi. In AGB6 mice, microsteatosis only occurs transiently at 4 dpi and resolved by 11 dpi. Antibody-stained frozen liver sections (d) revealed that YFV antigen could only be detected in infected AGB6 mice at 4 dpi but not AB6 mice, parallel to titer data in Fig. 1g. Original magnification = 40x, n = 4 Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/s41541-017-0039-z), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse LYVE-1 by Immunocytochemistry/Immunofluorescence Molecular characterization of LECs in the SCS ceiling with RNA FISH.(A-B) Expression of new cLEC/cluster 2 marker genes Ackr3 (A) and Btnl9 (B) by RNA sequencing (left panels) and RNA FISH (right panels). As GFP fluorescence is lost during tissue processing for RNA FISH, immunofluorescence staining for ANXA2 (red) and LYVE1 (green) served as markers for cLECs and fLECs, respectively. Arrows point to cLECs expressing Ackr3 and Btnl9 transcripts (white). ACKR3, atypical chemokine receptor 3; ANXA2, annexin A2; Btnl9, butyrophilin like 9; cLEC, ceiling LEC; FISH, fluorescence in situ hybridization; fLEC, floor-lining LEC; LEC, lymphatic endothelial cell; LYVE1, lymphatic vessel endothelial hyaluronan receptor 1; SCS, subcapsular sinus; UMAP, Uniform Manifold Approximation and Projection. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32251437), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse LYVE-1 by Immunocytochemistry/Immunofluorescence Molecular characterization of LECs in the SCS ceiling with immunofluorescence staining.(A–C) Expression of new cLEC/cluster 2 marker genes ANXA2 (A), FABP4 (B), and CD36 (C) by RNA sequencing (left panels) and immunofluorescence staining (right panels) in Ackr4-GFP reporter mice. GFP (white) and immunofluorescence costaining for LYVE1 (green) served as markers for cLECs and fLECs, respectively. ACKR4, atypical chemokine receptor 4; ANXA2, annexin A2; CD, cluster of differentiation; cLEC, ceiling LEC; FABP4, fatty acid binding protein 4; fLEC, floor-lining LEC; GFP, green fluorescent protein; LEC, lymphatic endothelial cell; LYVE1, lymphatic vessel endothelial hyaluronan receptor 1; SCS, subcapsular sinus; UMAP, Uniform Manifold Approximation and Projection. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32251437), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse LYVE-1 by Immunocytochemistry/Immunofluorescence Molecular characterization of LECs in the SCS ceiling with RNA FISH.(A-B) Expression of new cLEC/cluster 2 marker genes Ackr3 (A) and Btnl9 (B) by RNA sequencing (left panels) and RNA FISH (right panels). As GFP fluorescence is lost during tissue processing for RNA FISH, immunofluorescence staining for ANXA2 (red) and LYVE1 (green) served as markers for cLECs and fLECs, respectively. Arrows point to cLECs expressing Ackr3 and Btnl9 transcripts (white). ACKR3, atypical chemokine receptor 3; ANXA2, annexin A2; Btnl9, butyrophilin like 9; cLEC, ceiling LEC; FISH, fluorescence in situ hybridization; fLEC, floor-lining LEC; LEC, lymphatic endothelial cell; LYVE1, lymphatic vessel endothelial hyaluronan receptor 1; SCS, subcapsular sinus; UMAP, Uniform Manifold Approximation and Projection. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32251437), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse LYVE-1 by Immunocytochemistry/Immunofluorescence POSTN is upregulated in (pre)-metastatic LN. Morphometric analysis of POSTN and LYVE1+ lymphatic vessels in experimental (pre)-metastatic LN (in the ear sponge assay using B16F10 cells). CTRLs correspond to mice implanted with a sponge without tumor cells. a–d–g Immunostaining of POSTN (red) and LYVE1 (green) in pre-metastatic (PM) (at 1 week in A, at 2 weeks in d) and in metastatic (M+) LNs (G). Bars = 250 µm. b–e–h Scatter graphs use scatter plots to represent POSTN and LYVE1 densities (in percentage) assessed by a computer assisted method (n ≥ 9). Results are expressed as mean ± SD, and statistical analyses were performed using a Wilcoxon–Mann–Whitney test (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001). c–f–i Spatial distribution analysis from tissue edge to tissue center. The blue rectangle indicates the area between 0–0.30 mm from the LN border where the cumulate normalized areas of LYVE1 and POSTN were measured and represented in the top right. Maximum distance of migration from the tissue border (Lmax) is indicated. Results are expressed as mean ± SD (Wilcoxon–Mann–Whitney test: *p < 0.05; **p < 0.01). d–i All the results represent the set of two independent experiments Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35567669), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of LYVE‑1 in Mouse Liver. Formalin-fixed paraffin-embedded tissue sections of mouse liver were probed for LYVE1 mRNA (ACD RNAScope Probe, catalog # 428451; Fast Red chromogen, ACD catalog # 322360). Adjacent tissue section was processed for immunohistochemistry using goat anti-mouse LYVE1 polyclonal antibody (R&D Systems catalog # AF2125) at 5ug/mL with overnight incubation at 4 degrees Celsius followed by incubation with anti-goat IgG VisUCyte HRP Polymer Antibody (Catalog # VC004) and DAB chromogen (yellow-brown). Tissue was counterstained with hematoxylin (blue). Specific staining was localized to endothelial cells of sinusoids.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: LYVE-1
Lymphatic vessel endothelial hyaluronan (HA) receptor-1 (LYVE-1) is a recently identified receptor of HA, a linear high molecular weight polymer composed of alternating units of D-glucuronic acid and N-acetyl-D-glucosamine. HA is found in the extracellular matrix of most animal tissues and in body fluids. It modulates cell behavior and functions during tissue remodeling, development, homeostasis, and disease. The turnover of HA (several grams/day in humans) occurs primarily in the lymphatics and liver, the two major clearance systems that catabolize approximately 85% and 15% of HA, respectively. LYVE-1 shares 41% homology with the other known HA receptor, CD44. The homology between the two proteins increases to 61% within the HA binding domain. The HA binding domain, known as the link module, is a common structural motif found in other HA binding proteins such as link protein, aggrecan and versican. Human and mouse LYVE-1 share 69% amino acid sequence identity.
LYVE-1 is primarily expressed on both the luminal and abluminal surfaces of lymphatic vessels. In addition, LYVE-1 is also present in normal hepatic blood sinusoidal endothelial cells. LYVE-1 mediates the endocytosis of HA and may transport HA from tissue to lymph by transcytosis, delivering HA to lymphatic capillaries for removal and degradation in the regional lymph nodes. Because of its restricted expression patterns, LYVE-1, along with other lymphatic proteins such as VEGF R3, podoplanin and the homeobox protein propero-related (Prox-1), constitute a set of markers useful for distinguishing between lymphatic and blood microvasculature.
Product Datasheets
Citations for Mouse LYVE-1 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
144
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Carbon nanotubes exhibit fibrillar pharmacology in primates
Authors: S Alidori, DLJ Thorek, BJ Beattie, D Ulmert, BA Almeida, S Monette, DA Scheinberg, MR McDevitt
PLoS ONE, 2017-08-28;12(8):e0183902.
-
Single-cell mapping reveals new markers and functions of lymphatic endothelial cells in lymph nodes
Authors: Fujimoto N, He Y, D'Addio M et al.
PLoS Biol.
-
Lymphatic activation of ACKR3 signaling regulates lymphatic response after ischemic heart injury
Authors: Balint, L;Patel, S;Serafin, DS;Zhang, H;Quinn, KE;Aghajanian, A;Kistner, BM;Caron, KM;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Inhibiting corneal transplantation rejection via lymphatic vessel ligation in a novel murine model
Authors: Igarashi, A;Hayashi, T;Shimizu, T;Yuda, K;Yamagami, S;
Scientific reports
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
GDF2 and BMP10 coordinate liver cellular crosstalk to maintain liver health
Authors: Zhao, D;Huang, Z;Li, X;Wang, H;Hou, Q;Wang, Y;Yan, F;Yang, W;Liu, D;Yi, S;Han, C;Hao, Y;Tang, L;
eLife
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Redefining the ontogeny of hyalocytes as yolk sac-derived tissue-resident macrophages of the vitreous body
Authors: Rosmus, DD;Koch, J;Hausmann, A;Chiot, A;Arnhold, F;Masuda, T;Kierdorf, K;Hansen, SM;Kuhrt, H;Fröba, J;Wolf, J;Boneva, S;Gericke, M;Ajami, B;Prinz, M;Lange, C;Wieghofer, P;
Journal of neuroinflammation
Species: Mouse
Sample Types:
Applications: Immunofluorescence -
Lipid-siRNA conjugate accesses a perivascular transport mechanism and achieves widespread and durable knockdown in the central nervous system
Authors: Sorets, AG;Schwensen, KR;Francini, N;Kjar, A;Abdulrahman, AM;Shostak, A;Katdare, KA;Schoch, KM;Cowell, RP;Park, JC;Ligocki, AP;Ford, WT;Ventura-Antunes, L;Hoogenboezem, EN;Prusky, A;Castleberry, M;Michell, DL;Miller, TM;Vickers, KC;Schrag, MS;Duvall, CL;Lippmann, ES;
bioRxiv : the preprint server for biology
Species: Rat
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
First Application of a Mixed Porcine-Human Repopulated Bioengineered Liver in a Preclinical Model of Post-Resection Liver Failure
Authors: Felgendreff, P;Hosseiniasl, SM;Minshew, A;Amiot, BP;Wilken, S;Ahmadzada, B;Huebert, RC;Sakrikar, NJ;Engles, NG;Halsten, P;Mariakis, K;Barry, J;Riesgraf, S;Fecteau, C;Ross, JJ;Nyberg, SL;
Biomedicines
Species: Human, Porcine
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Yerba Mate (Ilex Paraguariensis) Reduces Colitis Severity by Promoting Anti-Inflammatory Macrophage Polarization
Authors: Olate?Briones, A;Albornoz-Muñoz, S;Rodríguez-Arriaza, F;Rodríguez-Vergara, V;Machuca, J;Liu, C;Peña?Farfal, C;Escobedo, N;Herrada, A;
Nutrients
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Liver Sinusoidal Endothelial Cells Contribute to Portal Hypertension Through Collagen Type IV-Driven Sinusoidal Remodeling
Authors: Gan, C;Yaqoob, U;Lu, J;Xie, M;Anwar, AA;Jalan-Sakrikar, N;Jerez, S;Sehrawat, T;Navarro-Corcuera, A;Kostallari, E;Habash, NW;Cao, S;Shah, VH;
JCI insight
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Vegfc-expressing cells form heterotopic bone after musculoskeletal injury
Authors: Vishlaghi, N;Guo, L;Griswold-Wheeler, D;Sun, Y;Booker, C;Crossley, JL;Bancroft, AC;Juan, C;Korlakunta, S;Ramesh, S;Pagani, CA;Xu, L;James, AW;Tower, RJ;Dellinger, M;Levi, B;
Cell reports
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
An autophagy program that promotes T cell egress from the lymph node controls responses to immune checkpoint blockade
Authors: Houbaert, D;Nikolakopoulos, A;Jacobs, K;Meçe, O;Roels, J;Shankar, G;Agrawal, M;More, S;Ganne, M;Rillaerts, K;Boon, L;Swoboda, M;Nobis, M;Mourão, L;Bosisio, F;Vandamme, N;Bergers, G;Scheele, C;Agostinis, P;
Cell Reports
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
15-Lipoxygenase promotes resolution of inflammation in lymphedema by controlling Treg cell function through IFN-?
Authors: Zamora, A;Nougué, M;Verdu, L;Balzan, E;Draia-Nicolau, T;Benuzzi, E;Pujol, F;Baillif, V;Lacazette, E;Morfoisse, F;Galitzky, J;Bouloumié, A;Dubourdeau, M;Chaput, B;Fazilleau, N;Malloizel-Delaunay, J;Bura-Rivière, A;Prats, AC;Garmy-Susini, B;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: IF/IHC -
Apelin-VEGF-C mRNA delivery as therapeutic for the treatment of secondary lymphedema
Authors: Creff, J;Lamaa, A;Benuzzi, E;Balzan, E;Pujol, F;Draia-Nicolau, T;Nougué, M;Verdu, L;Morfoisse, F;Lacazette, E;Valet, P;Chaput, B;Gross, F;Gayon, R;Bouillé, P;Malloizel-Delaunay, J;Bura-Rivière, A;Prats, AC;Garmy-Susini, B;
EMBO molecular medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Spatial Distribution of Non-Immune Cells Expressing Glycoprotein A Repetitions Predominant in Human and Murine Metastatic Lymph Nodes
Authors: Rouaud, L;Baudin, L;Gautier-Isola, M;Van Meerbeeck, P;Feyereisen, E;Blacher, S;van Baren, N;Kridelka, F;Lucas, S;Noel, A;
Cancers
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Rediscovering the Rete Ovarii: a secreting auxiliary structure to the ovary
Authors: Anbarci, DN;McKey, J;Levic, DS;Bagnat, M;Capel, B;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Inhibition of TP signaling promotes endometriosis growth and neovascularization
Authors: Furue, A;Hattori, K;Hosono, K;Tanabe, M;Sato, E;Honda, M;Sekiguchi, K;Ito, Y;Majima, M;Narumiya, S;Kato, K;Amano, H;
Molecular medicine reports
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
LFA-1/ICAM-1 Adhesion Pathway Mediates the Homeostatic Migration of Lymphocytes from Peripheral Tissues into Lymph Nodes through Lymphatic Vessels
Authors: Guo, J;Xu, Z;Gunderson, RC;Xu, B;Michie, SA;
Biomolecules
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
hESC‐Derived Epicardial Cells Promote Repair of Infarcted Hearts in Mouse and Swine
Authors: Xiao‐Ling Luo, Yun Jiang, Qiang Li, Xiu‐Jian Yu, Teng Ma, Hao Cao et al.
Advanced Science
Species: Mouse
Sample Types: Whole Cells
Applications: Immunocytochemistry -
KRAS activation in gastric cancer stem-like cells promotes tumor angiogenesis and metastasis
Authors: Yoon C, Lu J, Jun Y et al.
BMC cancer
-
An organoid-based CRISPR-Cas9 screen for regulators of intestinal epithelial maturation and cell fate
Authors: Hansen, SL;Larsen, HL;Pikkupeura, LM;Maciag, G;Guiu, J;Müller, I;Clement, DL;Mueller, C;Johansen, JV;Helin, K;Lerdrup, M;Jensen, KB;
Science advances
Species: Mouse
Sample Types: Organoids
Applications: IHC -
Hepatic passaging of NRAS-mutant melanoma influences adhesive properties and metastatic pattern
Authors: Dietsch, B;Weller, C;Sticht, C;de la Torre, C;Kramer, M;Goerdt, S;G�raud, C;Wohlfeil, SA;
BMC cancer
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Characteristic Features of Deep Brain Lymphatic Vessels and Their Regulation by Chronic Stress
Authors: Junzhuang Chang, Bingqing Guo, Yan Gao, Wei Li, Xiaoyu Tong, Yi Feng et al.
Research (Wash D C)
-
Visualization of Organ-Specific Lymphatic Growth: An Efficient Approach to Labeling Molecular Markers in Cleared Tissues
Authors: C Christ, Z Jakus
International Journal of Molecular Sciences, 2023-03-07;24(6):.
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Increased adipose tissue lymphatic vessel density inhibits thermogenesis through elevated neurotensin levels
Authors: Thien T. Phan, Adri Chakraborty, Madison A. Tatum, Ana Lima-Orellana, Andrea J. Reyna, Joseph M. Rutkowski
Frontiers in Cell and Developmental Biology
-
VLA-4 suppression by senescence signals regulates meningeal immunity and leptomeningeal metastasis
Authors: J Li, D Huang, B Lei, J Huang, L Yang, M Nie, S Su, Q Zhao, Y Wang
Elife, 2022-12-09;11(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Three-dimensional characterization of developing and adult ocular vasculature in mice using in toto clearing
Authors: Marie Darche, Anna Verschueren, Morgane Belle, Leyna Boucherit, Stéphane Fouquet, José Alain Sahel et al.
Communications Biology
-
Single and combined impacts of irradiation and surgery on lymphatic vasculature and fibrosis associated to secondary lymphedema
Authors: F. Buntinx, A. Lebeau, L. Gillot, L. Baudin, R. Ndong Penda, F. Morfoisse et al.
Frontiers in Pharmacology
-
The cardiopharyngeal mesoderm contributes to lymphatic vessel development in mouse
Authors: Kazuaki Maruyama, Sachiko Miyagawa-Tomita, Yuka Haneda, Mayuko Kida, Fumio Matsuzaki, Kyoko Imanaka-Yoshida et al.
eLife
-
Imaging Blood Vessels and Lymphatics in Mouse Trachea Wholemounts
Authors: Peter Baluk, Donald M. McDonald
Methods in Molecular Biology
-
Liver sinusoidal endothelial cell expressed vascular cell adhesion molecule 1 promotes liver fibrosis
Authors: Qianqian Guo, Kunimaro Furuta, Shahidul Islam, Nunzia Caporarello, Enis Kostallari, Kobe Dielis et al.
Frontiers in Immunology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Ablation of lysophosphatidic acid receptor 1 attenuates hypertrophic cardiomyopathy in a mouse model
Authors: A Axelsson R, H Wakimoto, DM DeLaughter, D Reichart, J Gorham, DA Conner, M Lun, CK Probst, N Sakai, RS Knipe, SB Montesi, B Shea, LP Adam, LA Leinwand, W Wan, ES Choi, EL Lindberg, G Patone, M Noseda, N Hübner, CE Seidman, AM Tager, JG Seidman, CY Ho
Proceedings of the National Academy of Sciences of the United States of America, 2022-07-05;119(28):e2204174119.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Periostin in lymph node pre-metastatic niches governs lymphatic endothelial cell functions and metastatic colonization
Authors: Lionel Gillot, Alizée Lebeau, Louis Baudin, Charles Pottier, Thomas Louis, Tania Durré et al.
Cellular and Molecular Life Sciences
-
Orphan G-Protein Coupled Receptor GPRC5B Is Critical for Lymphatic Development
Authors: W Xu, NP Nelson-Man, L Bálint, HB Kwon, RB Davis, DCM Dy, JM Dunleavey, B St Croix, KM Caron
International Journal of Molecular Sciences, 2022-05-20;23(10):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lipid droplet degradation by autophagy connects mitochondria metabolism to Prox1-driven expression of lymphatic genes and lymphangiogenesis
Authors: O Meçe, D Houbaert, ML Sassano, T Durré, H Maes, M Schaaf, S More, M Ganne, M García-Cab, M Borri, J Verhoeven, M Agrawal, K Jacobs, G Bergers, S Blacher, B Ghesquière, M Dewerchin, JV Swinnen, S Vinckier, MS Soengas, P Carmeliet, A Noël, P Agostinis
Nature Communications, 2022-05-19;13(1):2760.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Circulating MicroRNA-505 May Serve as a Prognostic Biomarker for Hypertension-Associated Endothelial Dysfunction and Inflammation
Authors: Qinbo Yang, Peiwei Wang, Yiqing Cai, Yimeng Cui, Jingang Cui, Xiaoye Du et al.
Frontiers in Cardiovascular Medicine
-
ADAMTS2 and ADAMTS14 substitute ADAMTS3 in adults for proVEGFC activation and lymphatic homeostasis
Authors: L Dupont, L Joannes, F Morfoisse, S Blacher, C Monseur, CF Deroanne, A Noël, AC Colige
JCI Insight, 2022-04-22;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Remodeling lymphatic vessels in intrinsically aged skin on SKH-1 mouse using low dose 5-aminolevulinic acid photodynamic therapy via VEGF-C/VEGFR3 pathway
Authors: Y Yang, S Shen, Y Cao, D Wang, Z Kang, P Wang, X Wang
Photodiagnosis and photodynamic therapy, 2022-04-06;0(0):102851.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Specification of fetal liver endothelial progenitors to functional zonated adult sinusoids requires c-Maf induction
Authors: Jesus Maria Gómez-Salinero, Franco Izzo, Yang Lin, Sean Houghton, Tomer Itkin, Fuqiang Geng et al.
Cell Stem Cell
-
Temporal analyses of postnatal liver development and maturation by single-cell transcriptomics
Authors: Yan Liang, Kota Kaneko, Bing Xin, Jin Lee, Xin Sun, Kun Zhang et al.
Developmental Cell
-
Angiogenic and molecular diversity determine hepatic melanoma metastasis and response to anti-angiogenic treatment
Authors: SA Wohlfeil, V Häfele, B Dietsch, C Weller, C Sticht, AS Jauch, M Winkler, CD Schmid, AL Irkens, A Olsavszky, K Schledzews, PS Reiners-Ko, S Goerdt, C Géraud
Journal of Translational Medicine, 2022-02-02;20(1):62.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC/IF -
GPR182 limits antitumor immunity via chemokine scavenging in mouse melanoma models
Authors: RJ Torphy, Y Sun, R Lin, A Caffrey-Ca, Y Fujiwara, F Ho, EN Miller, MD McCarter, TR Lyons, RD Schulick, RM Kedl, Y Zhu
Nature Communications, 2022-01-10;13(1):97.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Bone marrow sinusoidal endothelium controls terminal erythroid differentiation and reticulocyte maturation
Authors: J Heil, V Olsavszky, K Busch, K Klapproth, C de la Torr, C Sticht, K Sandorski, J Hoffmann, H Schönhaber, J Zierow, M Winkler, CD Schmid, T Staniczek, DE Daniels, J Frayne, G Metzgeroth, D Nowak, S Schneider, M Neumaier, V Weyer, C Groden, HJ Gröne, K Richter, C Mogler, MM Taketo, K Schledzews, C Géraud, S Goerdt, PS Koch
Nature Communications, 2021-11-29;12(1):6963.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Npr2 mutant mice show vasodilation and undeveloped adipocytes in mesentery
Authors: C Sogawa-Fuj, Y Fujiwara, A Hanagata, Q Yang, T Mihara, N Kaji, T Kunieda, M Hori
Bmc Research Notes, 2021-11-27;14(1):438.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Expanded renal lymphatics improve recovery following kidney injury
Authors: G Baranwal, HA Creed, LM Black, A Auger, AM Quach, R Vegiraju, HE Eckenrode, A Agarwal, JM Rutkowski
Physiological Reports, 2021-11-01;9(22):e15094.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Gene Signatures Detect Damaged Liver Sinusoidal Endothelial Cells in Chronic Liver Diseases
Authors: Stefaan Verhulst, Elise Anne van Os, Vincent De Smet, Nathalie Eysackers, Inge Mannaerts, Leo A. van Grunsven
Frontiers in Medicine
-
Imbalanced Activation of Wnt-/ beta -Catenin-Signaling in Liver Endothelium Alters Normal Sinusoidal Differentiation
Authors: Philipp-Sebastian Koch, Kajetan Sandorski, Joschka Heil, Christian D. Schmid, Sina W. Kürschner, Johannes Hoffmann et al.
Frontiers in Physiology
-
Bone development and fracture healing is normal in mice that have a defect in the development of the lymphatic system
Authors: Anna L. McCarter, Aysha Khalid, Yating Yi, Marco Monroy, Hu Zhao, Jonathan J. Rios et al.
Lymphology
-
Vegfr3-tdTomato, a reporter mouse for microscopic visualization of lymphatic vessel by multiple modalities
Authors: E Redder, N Kirschnick, S Bobe, R Hägerling, NR Hansmeier, F Kiefer
PLoS ONE, 2021-09-20;16(9):e0249256.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Dual targeting of lymphocyte homing and retention through alpha 4 beta 7 and alpha E beta 7 inhibition in inflammatory bowel disease
Authors: Bingbing Dai, Jason A. Hackney, Ryan Ichikawa, Allen Nguyen, Justin Elstrott, Luz D. Orozco et al.
Cell Reports Medicine
-
KRAS-driven model of Gorham-Stout disease effectively treated with trametinib
Authors: N Homayun Se, AL McCarter, R Helaers, C Galant, LM Boon, P Brouillard, M Vikkula, MT Dellinger
JCI Insight, 2021-08-09;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
3-hydroxy-L-kynurenamine is an immunomodulatory biogenic amine
Authors: CC Clement, A D'Alessand, S Thangaswam, S Chalmers, R Furtado, S Spada, G Mondanelli, F Ianni, S Gehrke, M Gargaro, G Manni, LCL Cara, P Runge, WL Tsai, S Karaman, J Arasa, R Fernandez-, A Beck, A Macchiarul, M Gadina, C Halin, F Fallarino, M Skobe, M Veldhoen, S Moretti, S Formenti, S Demaria, RK Soni, R Galarini, R Sardella, G Lauvau, C Putterman, K Alitalo, U Grohmann, L Santambrog
Nature Communications, 2021-07-21;12(1):4447.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC -
Nucleoside-modified VEGFC mRNA induces organ-specific lymphatic growth and reverses experimental lymphedema
Authors: D Sz?ke, G Kovács, É Kemecsei, L Bálint, K Szoták-Ajt, P Aradi, A Styevkóné, BL Mui, YK Tam, TD Madden, K Karikó, RP Kataru, MJ Hope, D Weissman, BJ Mehrara, N Pardi, Z Jakus
Nature Communications, 2021-06-08;12(1):3460.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Single-Cell Transcriptional Heterogeneity of Lymphatic Endothelial Cells in Normal and Inflamed Murine Lymph Nodes
Authors: E Sibler, Y He, L Ducoli, N Keller, N Fujimoto, LC Dieterich, M Detmar
Cells, 2021-06-02;10(6):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Ex Vivo-Induced Bone Marrow-Derived Myeloid Suppressor Cells Prevent Corneal Allograft Rejection in Mice
Authors: J Zhu, T Inomata, K Fujimoto, K Uchida, K Fujio, K Nagino, M Miura, N Negishi, Y Okumura, Y Akasaki, K Hirosawa, M Kuwahara, A Eguchi, H Shokirova, A Yanagawa, A Midorikawa, A Murakami
Investigative Ophthalmology & Visual Science, 2021-06-01;62(7):3.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
New lymphatic cell formation is associated with damaged brain tissue clearance after penetrating traumatic brain injury
Authors: FW Meng, JT Yu, JY Chen, PF Yang
Scientific Reports, 2021-05-13;11(1):10193.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A cellular and spatial map of the choroid plexus across brain ventricles and ages
Authors: Neil Dani, Rebecca H. Herbst, Cristin McCabe, Gilad S. Green, Karol Kaiser, Joshua P. Head et al.
Cell
-
Mitochondrial respiration controls the Prox1-Vegfr3 feedback loop during lymphatic endothelial cell fate specification and maintenance
Authors: Wanshu Ma, Hyea Jin Gil, Xiaolei Liu, Lauren P. Diebold, Marc A. Morgan, Michael J. Oxendine-Burns et al.
Science Advances
-
Mesenchymal stem cells induce tumor stroma formation and epithelial?mesenchymal transition through SPARC expression in colorectal cancer
Authors: T Naito, R Yuge, Y Kitadai, H Takigawa, Y Higashi, T Kuwai, K Kuraoka, S Tanaka, K Chayama
Oncology reports, 2021-04-28;45(6):.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Topical administration of the kappa opioid receptor agonist nalfurafine suppresses corneal neovascularization and inflammation
Authors: H Shokirova, T Inomata, T Saitoh, J Zhu, K Fujio, Y Okumura, A Yanagawa, K Fujimoto, J Sung, A Eguchi, M Miura, K Nagino, K Hirosawa, M Kuwahara, Y Akasaki, H Nagase, A Murakami
Scientific Reports, 2021-04-21;11(1):8647.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Tertiary lymphoid organs are associated with the progression of kidney damage and regulated by interleukin-17A
Authors: R Luo, Y Cheng, D Chang, T Liu, L Liu, G Pei, N Zhang, Z Wang, K Guo, W Chen, M Li, L Fan, C Zhang, Y Li, W Dai, M Zuo, Y Xu, Y Yao, S Ge, G Xu
Theranostics, 2021-01-01;11(1):117-131.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Blood and lymphatic systems are segregated by the FLCN tumor suppressor
Authors: I Tai-Nagara, Y Hasumi, D Kusumoto, H Hasumi, K Okabe, T Ando, F Matsuzaki, F Itoh, H Saya, C Liu, W Li, YS Mukouyama, W Marston Li, X Liu, M Hirashima, Y Suzuki, S Funasaki, Y Satou, M Furuya, M Baba, Y Kubota
Nature Communications, 2020-12-09;11(1):6314.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Novel immunotherapeutic effects of topically administered ripasudil (K-115) on corneal allograft survival
Authors: T Inomata, K Fujimoto, Y Okumura, J Zhu, K Fujio, H Shokirova, M Miura, M Okano, T Funaki, J Sung, N Negishi, A Murakami
Sci Rep, 2020-11-13;10(1):19817.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Eye lymphatic defects induced by bone morphogenetic protein 9 deficiency have no functional consequences on intraocular pressure
Authors: M Subileau, N Acar, A Carret, L Bretillon, I Vilgrain, S Bailly, D Vittet
Sci Rep, 2020-09-29;10(1):16040.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Diverse Macrophage Populations Contribute to the Inflammatory Microenvironment in Premalignant Lesions During Localized Invasion
Authors: Ayman M. Ibrahim, Matthew A. Moss, Zane Gray, Michelle D. Rojo, Caitlin M. Burke, Kathryn L. Schwertfeger et al.
Frontiers in Oncology
-
Dental cell type atlas reveals stem and differentiated cell types in mouse and human teeth
Authors: Krivanek J, Soldatov RA, Kastriti ME et al.
Nature Communications
-
Defective development and microcirculation of intestine in Npr2 mutant mice
Authors: C Sogawa-Fuj, A Hanagata, Y Fujiwara, Y Ishida, H Tomiyasu, T Kunieda, H Nakatomi, M Hori
Sci Rep, 2020-09-08;10(1):14761.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Inhibition of receptor activity-modifying protein 1 suppresses the development of endometriosis and the formation of blood and lymphatic vessels
Authors: M Honda, Y Ito, K Hattori, K Hosono, K Sekiguchi, K Tsujikawa, N Unno, M Majima
J. Cell. Mol. Med., 2020-09-01;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
S1PR1 regulates the quiescence of lymphatic vessels by inhibiting laminar shear stress-dependent VEGF-C signaling
Authors: X Geng, K Yanagida, RG Akwii, D Choi, L Chen, Y Ho, B Cha, MR Mahamud, K Berman de, H Ichise, H Chen, J Wythe, CM Mikelis, T Hla, RS Srinivasan
JCI Insight, 2020-07-23;0(0):.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Tissue-resident macrophages promote extracellular matrix homeostasis in the mammary gland stroma of nulliparous mice
Authors: Ying Wang, Thomas S Chaffee, Rebecca S LaRue, Danielle N Huggins, Patrice M Witschen, Ayman M Ibrahim et al.
eLife
-
Characterizing Lymphangiogenesis and Concurrent Inflammation in Adipose Tissue in Response to VEGF-D
Authors: A Chakrabort, CK Scogin, K Rizwan, TS Morley, JM Rutkowski
Front Physiol, 2020-04-22;11(0):363.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Progesterone Receptor Serves the Ovary as a Trigger of Ovulation and a Terminator of Inflammation
Authors: CJ Park, PC Lin, S Zhou, R Barakat, ST Bashir, JM Choi, JA Cacioppo, OR Oakley, DM Duffy, JP Lydon, CJ Ko
Cell Rep, 2020-04-14;31(2):107496.
Species: Mouse
Sample Types: Granulosa Cells
Applications: IHC -
Reduced Prenatal Pulmonary Lymphatic Function Is Observed in Clp1 K/K Embryos With Impaired Motor Functions Including Fetal Breathing Movements in Preparation of the Developing Lung for Inflation at Birth
Authors: Kitti Szoták-Ajtay, Dániel Szõke, Gábor Kovács, Judit Andréka, Gábor B. Brenner, Zoltán Giricz et al.
Frontiers in Bioengineering and Biotechnology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Donor-host Lymphatic Anastomosis After Murine Lung Transplantation
Authors: Hasina Outtz Outtz Reed, Liqing Wang, Mark L. Kahn, Wayne W. Hancock
Transplantation
-
Sphingosine 1-phosphate-regulated transcriptomes in heterogenous arterial and lymphatic endothelium of the aorta
Authors: Eric Engelbrecht, Michel V Levesque, Liqun He, Michael Vanlandewijck, Anja Nitzsche, Hira Niazi et al.
eLife
-
A Second Heart Field-Derived Vasculogenic Niche Contributes to Cardiac Lymphatics
Authors: Lioux G, Liu X, Temi�o S et al.
Developmental Cell
-
VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours
Authors: E Song, T Mao, H Dong, LSB Boisserand, S Antila, M Bosenberg, K Alitalo, JL Thomas, A Iwasaki
Nature, 2020-01-15;577(7792):689-694.
Species: Mouse
Sample Types: Tissue
Applications: IF -
Lymph Flow Induces the Postnatal Formation of Mature and Functional Meningeal Lymphatic Vessels
Authors: László Bálint, Zsombor Ocskay, Bálint András Deák, Petra Aradi, Zoltán Jakus
Frontiers in Immunology
-
Spatiotemporal dynamics and heterogeneity of renal lymphatics in mammalian development and cystic kidney disease
Authors: Daniyal J Jafree, Dale Moulding, Maria Kolatsi-Joannou, Nuria Perretta Tejedor, Karen L Price, Natalie J Milmoe et al.
eLife
-
Receptor?interacting serine/threonine?protein kinase 1 promotes the progress and lymph metastasis of gallbladder cancer
Authors: G Zhu, Q Du, X Chen, X Wang, N Tang, F She, Y Chen
Oncol. Rep., 2019-09-23;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Macrophage S1PR1 Signaling Alters Angiogenesis and Lymphangiogenesis During Skin Inflammation
Authors: Shahzad Nawaz Syed, Rebecca Raue, Andreas Weigert, Andreas von Knethen, Bernhard Brüne
Cells
-
An important role of cutaneous lymphatic vessels in coordinating and promoting anagen hair follicle growth
Authors: SY Yoon, LC Dieterich, S Karaman, ST Proulx, SB Bachmann, C Sciaroni, M Detmar
PLoS ONE, 2019-07-25;14(7):e0220341.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Placental chemokine compartmentalisation: A novel mammalian molecular control mechanism
Authors: KM Lee, GJ Wilson, M Pingen, A Fukuoka, CAH Hansell, R Bartolini, L Medina-Rui, GJ Graham
PLoS Biol., 2019-05-29;17(5):e3000287.
Species: Mouse, Transgenic Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Flow Cytometry, IHC -
Imaging Lymphatics in Mouse Lungs
Authors: Peter Baluk, Donald M. McDonald
Methods in Molecular Biology
-
Compartmentalized gut lymph node drainage dictates adaptive immune responses
Authors: D Esterházy, MCC Canesso, L Mesin, PA Muller, TBR de Castro, A Lockhart, M ElJalby, AMC Faria, D Mucida
Nature, 2019-04-15;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lymphatic impairment leads to pulmonary tertiary lymphoid organ formation and alveolar damage
Authors: HO Reed, L Wang, J Sonett, M Chen, J Yang, L Li, P Aradi, Z Jakus, J D'Armiento, WW Hancock, ML Kahn
J. Clin. Invest., 2019-04-04;129(6):2514-2526.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Brg1 promotes liver regeneration after partial hepatectomy via regulation of cell cycle
Authors: Baocai Wang, Benedikt Kaufmann, Thomas Engleitner, Miao Lu, Carolin Mogler, Victor Olsavszky et al.
Scientific Reports
-
Somatic activating mutations in PIK3CA cause generalized lymphatic anomaly
Authors: Lara Rodriguez-Laguna, Noelia Agra, Kristina Ibañez, Gloria Oliva-Molina, Gema Gordo, Noor Khurana et al.
Journal of Experimental Medicine
-
Lymph/angiogenesis contributes to sex differences in lung cancer through oestrogen receptor alpha signalling
Authors: Charline Dubois, Natacha Rocks, Silvia Blacher, Irina Primac, Anne Gallez, Melissa García-Caballero et al.
Endocrine-Related Cancer
-
Associating liver partition and portal vein ligation for staged hepatectomy: establishment of an animal model with insufficient liver remnant
Authors: A Dili, V Lebrun, C Bertrand, IA Leclercq
Lab. Invest., 2019-01-21;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Identification of ILK as a critical regulator of VEGFR 3 signalling and lymphatic vascular growth
Authors: Sofia Urner, Lara Planas‐Paz, Laura Sophie Hilger, Carina Henning, Anna Branopolski, Molly Kelly‐Goss et al.
The EMBO Journal
-
Bone Morphogenetic Protein 9 Regulates Early Lymphatic-Specified Endothelial Cell Expansion during Mouse Embryonic Stem Cell Differentiation
Authors: M Subileau, G Merdzhanov, D Ciais, V Collin-Fau, JJ Feige, S Bailly, D Vittet
Stem Cell Reports, 2018-12-27;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Lymphatic Vascular Structures: A New Aspect in Proliferative Diabetic Retinopathy
Authors: E Gucciardo, S Loukovaara, P Salven, K Lehti
Int J Mol Sci, 2018-12-13;19(12):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
uPARAP/Endo180 receptor is a gatekeeper of VEGFR-2/VEGFR-3 heterodimerisation during pathological lymphangiogenesis
Authors: T Durré, F Morfoisse, C Erpicum, M Ebroin, S Blacher, M García-Cab, C Deroanne, T Louis, C Balsat, M Van de Vel, S Kaijalaine, F Kridelka, L Engelholm, I Struman, K Alitalo, N Behrendt, J Paupert, A Noel
Nat Commun, 2018-12-05;9(1):5178.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Distinct roles of VE ‐cadherin for development and maintenance of specific lymph vessel beds
Authors: René Hägerling, Esther Hoppe, Cathrin Dierkes, Martin Stehling, Taija Makinen, Stefan Butz et al.
The EMBO Journal
-
Angiocrine Wnt signaling controls liver growth and metabolic maturation in mice
Authors: Thomas Leibing, Cyrill Géraud, Iris Augustin, Michael Boutros, Hellmut G. Augustin, Jürgen G. Okun et al.
Hepatology
-
Targeted therapy in patients with PIK3CA-related overgrowth syndrome
Authors: Quitterie Venot, Thomas Blanc, Smail Hadj Rabia, Laureline Berteloot, Sophia Ladraa, Jean-Paul Duong et al.
Nature
-
CD4+ T cells are activated in regional lymph nodes and migrate to skin to initiate lymphedema
Authors: GD García Nor, CL Ly, DA Cuzzone, RP Kataru, GE Hespe, JS Torrisi, JJ Huang, JC Gardenier, IL Savetsky, MD Nitti, JZ Yu, S Rehal, BJ Mehrara
Nat Commun, 2018-05-17;9(1):1970.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
RIP1 regulates TNF-?-mediated lymphangiogenesis and lymphatic metastasis in gallbladder cancer by modulating the NF-?B-VEGF-C pathway
Authors: CZ Li, XJ Jiang, B Lin, HJ Hong, SY Zhu, L Jiang, XQ Wang, NH Tang, FF She, YL Chen
Onco Targets Ther, 2018-05-16;11(0):2875-2890.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VEGF-C promotes the development of lymphatics in bone and bone loss
Authors: D Hominick, A Silva, N Khurana, Y Liu, PC Dechow, JQ Feng, B Pytowski, JM Rutkowski, K Alitalo, MT Dellinger
Elife, 2018-04-05;7(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Mir-126 is a conserved modulator of lymphatic development
Authors: Z Kontarakis, A Rossi, S Ramas, MT Dellinger, DYR Stainier
Dev. Biol., 2018-03-15;0(0):.
Species: Mouse
Sample Types: Embryo
Applications: Western Blot -
Gamma-interferon exerts a critical early restriction on replication and dissemination of yellow fever virus vaccine strain 17D-204
Authors: LKM Lam, AM Watson, KD Ryman, WB Klimstra
Npj Vaccines, 2018-01-23;3(0):5.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Indian Hedgehog Suppresses a Stromal Cell-Driven Intestinal Immune Response
Authors: BF Westendorp, NVJA Büller, ON Karpus, WA van Dop, J Koster, R Versteeg, PJ Koelink, CY Snel, S Meisner, JJTH Roelofs, A Uhmann, E Ver Loren, J Heijmans, H Hahn, V Muncan, ME Wildenberg, GR van den Br
Cell Mol Gastroenterol Hepatol, 2017-09-05;5(1):67-82.e1.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Targeting VEGFR-3/-2 signaling pathways with AD0157: a potential strategy against tumor-associated lymphangiogenesis and lymphatic metastases
Authors: M García-Cab, J Paupert, S Blacher, M Van de Vel, AR Quesada, MA Medina, A Noël
J Hematol Oncol, 2017-06-19;10(1):122.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
cIAP2 promotes gallbladder cancer invasion and lymphangiogenesis by activating the NF-?B pathway
Authors: X Jiang, C Li, B Lin, H Hong, L Jiang, S Zhu, X Wang, N Tang, X Li, F She, Y Chen
Cancer Sci., 2017-05-31;108(6):1144-1156.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
TNF-alpha promotes lymphangiogenesis and lymphatic metastasis of gallbladder cancer through the ERK1/2/AP-1/VEGF-D pathway
Authors: HaiJie Hong, Lei Jiang, YanFei Lin, CaiLong He, GuangWei Zhu, Qiang Du et al.
BMC Cancer
-
Diphtheria toxin–mediated ablation of lymphatic endothelial cells results in progressive lymphedema
Authors: Jason C. Gardenier, Geoffrey E. Hespe, Raghu P. Kataru, Ira L. Savetsky, Jeremy S. Torrisi, Gabriela D. García Nores et al.
JCI Insight
-
Mechanotransduction activates canonical Wnt/?-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves
Authors: Boksik Cha
Genes Dev, 2016-06-16;30(12):1454-69.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD
J Clin Invest, 2016-05-09;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
CHD4-regulated plasmin activation impacts lymphovenous hemostasis and hepatic vascular integrity
J Clin Invest, 2016-05-03;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Immunoprecipitation -
Thy1+IL-7+ lymphatic endothelial cells in iBALT provide a survival niche for memory T-helper cells in allergic airway inflammation
Proc Natl Acad Sci USA, 2016-05-02;113(20):E2842-51.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Somatic activating mutations in Pik3ca cause sporadic venous malformations in mice and humans
Authors: Sandra D. Castillo, Elena Tzouanacou, May Zaw-Thin, Inma M. Berenjeno, Victoria E.R. Parker, Iñigo Chivite et al.
Science Translational Medicine
-
Identification of Gene Expression Differences between Lymphangiogenic and Non-Lymphangiogenic Non-Small Cell Lung Cancer Cell Lines
Authors: Erin Regan, Robert C. Sibley, Bercin Kutluk Cenik, Asitha Silva, Luc Girard, John D. Minna et al.
PLOS ONE
-
Vascular Endothelial Growth Factor C for Polycystic Kidney Diseases
Authors: Jennifer L. Huang, Adrian S. Woolf, Maria Kolatsi-Joannou, Peter Baluk, Richard N. Sandford, Dorien J.M. Peters et al.
Journal of the American Society of Nephrology
-
Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina.
Authors: Zhou Y, Williams J, Smallwood P, Nathans J
PLoS ONE, 2015-12-02;10(12):e0143650.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development.
Authors: Geng X, Cha B, Mahamud M, Lim K, Silasi-Mansat R, Uddin M, Miura N, Xia L, Simon A, Engel J, Chen H, Lupu F, Srinivasan R
Dev Biol, 2015-11-02;409(1):218-33.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lymph flow regulates collecting lymphatic vessel maturation in vivo.
Authors: Sweet D, Jimenez J, Chang J, Hess P, Mericko-Ishizuka P, Fu J, Xia L, Davies P, Kahn M
J Clin Invest, 2015-07-27;125(8):2995-3007.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
A transgenic Prox1-Cre-tdTomato reporter mouse for lymphatic vessel research.
Authors: Bianchi R, Teijeira A, Proulx S, Christiansen A, Seidel C, Rulicke T, Makinen T, Hagerling R, Halin C, Detmar M
PLoS ONE, 2015-04-07;10(4):e0122976.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
SH2B3 Is a Genetic Determinant of Cardiac Inflammation and Fibrosis
Authors: Michael J. Flister, Matthew J. Hoffman, Angela Lemke, Sasha Z. Prisco, Nathan Rudemiller, Caitlin C. O’Meara et al.
Circulation: Cardiovascular Genetics
-
Machine-based method for multiplex in situ molecular characterization of tissues by immunofluorescence detection
Authors: Dmitry Yarilin, Ke Xu, Mesruh Turkekul, Ning Fan, Yevgeniy Romin, Sho Fijisawa et al.
Scientific Reports
-
Bone marrow-derived mesenchymal stem cells drive lymphangiogenesis.
Authors: Maertens L, Erpicum C, Detry B, Blacher S, Lenoir B, Carnet O, Pequeux C, Cataldo D, Lecomte J, Paupert J, Noel A
PLoS ONE, 2014-09-15;9(9):e106976.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease.
Authors: D'Alessio S, Correale C, Tacconi C, Gandelli A, Pietrogrande G, Vetrano S, Genua M, Arena V, Spinelli A, Peyrin-Biroulet L, Fiocchi C, Danese S
J Clin Invest, 2014-08-08;124(9):3863-78.
Species: Human, Mouse
Sample Types: Whole Tissue
Applications: IHC -
Schlemm's canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process.
Authors: Kizhatil, Krishnak, Ryan, Margaret, Marchant, Jeffrey, Henrich, Stephen, John, Simon W
PLoS Biol, 2014-07-22;12(7):e1001912.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lymphatic function is required prenatally for lung inflation at birth.
Authors: Jakus Z, Gleghorn J, Enis D, Sen A, Chia S, Liu X, Rawnsley D, Yang Y, Hess P, Zou Z, Yang J, Guttentag S, Nelson C, Kahn M
J Exp Med, 2014-04-14;211(5):815-26.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Vascular Endothelial Growth Factor Receptor-2 Promotes the Development of the Lymphatic Vasculature
Authors: Michael T. Dellinger, Stryder M. Meadows, Katherine Wynne, Ondine Cleaver, Rolf A. Brekken
PLoS ONE
-
Obesity Impairs Lymphatic Fluid Transport and Dendritic Cell Migration to Lymph Nodes
Authors: Evan S. Weitman, Seth Z. Aschen, Gina Farias-Eisner, Nicholas Albano, Daniel A. Cuzzone, Swapna Ghanta et al.
PLoS ONE
-
Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation
Authors: Sandrine Levet, Delphine Ciais, Galina Merdzhanova, Christine Mallet, Teresa A. Zimmers, Se-Jin Lee et al.
Blood
-
Deletion of tetraspanin CD9 diminishes lymphangiogenesis in vivo and in vitro.
Authors: Iwasaki T, Takeda Y, Maruyama K, Yokosaki Y, Tsujino K, Tetsumoto S, Kuhara H, Nakanishi K, Otani Y, Jin Y, Kohmo S, Hirata H, Takahashi R, Suzuki M, Inoue K, Nagatomo I, Goya S, Kijima T, Kumagai T, Tachibana I, Kawase I, Kumanogoh A
J Biol Chem, 2012-12-05;288(4):2118-31.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Selection -
Rho-family GTPase Cdc42 controls migration of Langerhans cells in vivo.
Authors: Luckashenak N, Wahe A, Breit K, Brakebusch C, Brocker T
J Immunol, 2012-12-03;190(1):27-35.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Inducible Tertiary Lymphoid Structures, Autoimmunity, and Exocrine Dysfunction in a Novel Model of Salivary Gland Inflammation in C57BL/6 Mice
Authors: Michele Bombardieri, Francesca Barone, Davide Lucchesi, Saba Nayar, Wim B. van den Berg, Gordon Proctor et al.
The Journal of Immunology
-
Mechanoinduction of lymph vessel expansion
Authors: Lara Planas-Paz, Boris Strilić, Axel Goedecke, Georg Breier, Reinhard Fässler, Eckhard Lammert
The EMBO Journal
Species: Mouse
Sample Types: Embryo, Whole Tissue
Applications: Immunohistochemistry -
Angiopoietin-1 is essential in mouse vasculature during development and in response to injury.
Authors: Jeansson M, Gawlik A, Anderson G
J. Clin. Invest., 2011-05-23;121(6):2278-89.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr, IHC-P -
Sphingosine 1-Phosphate-Induced Motility and Endocytosis of Dendritic Cells Is Regulated by SWAP-70 through RhoA.
Authors: Ocana-Morgner C, Reichardt P, Chopin M, Braungart S, Wahren C, Gunzer M, Jessberger R
J. Immunol., 2011-03-18;186(9):5345-55.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3.
Authors: Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, Larrivee B, Del Toro R, Suchting S, Medvinsky A, Silva J, Yang J, Thomas JL, Koch AW, Alitalo K, Eichmann A, Bagri A
J. Cell Biol., 2010-01-11;188(1):115-30.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr -
ALK1 signaling regulates early postnatal lymphatic vessel development.
Authors: Niessen K, Zhang G, Ridgway JB, Chen H, Yan M
Blood, 2009-11-10;115(8):1654-61.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth.
Authors: Cho WG, Albuquerque RJ, Kleinman ME, Tarallo V, Greco A, Nozaki M, Green MG, Baffi JZ, Ambati BK, De Falco M, Alexander JS, Brunetti A, De Falco S, Ambati J
Proc. Natl. Acad. Sci. U.S.A., 2009-04-09;106(17):7137-42.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Mst1 controls lymphocyte trafficking and interstitial motility within lymph nodes.
Authors: Katagiri K, Katakai T, Ebisuno Y, Ueda Y, Okada T, Kinashi T
EMBO J., 2009-04-02;28(9):1319-31.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs.
Authors: Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A
J. Immunol., 2008-11-01;181(9):6189-200.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Dynamic changes occur in patterns of endometrial EFNB2/EPHB4 expression during the period of spiral arterial modification in mice.
Authors: Zhang J, Dong H, Wang B, Zhu S, Croy BA
Biol. Reprod., 2008-05-07;79(3):450-8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Thymic stromal lymphopoietin transgenic mice develop cryoglobulinemia and hepatitis with similarities to human hepatitis C liver disease.
Authors: Kowalewska J, Muhlfeld AS, Hudkins KL, Yeh MM, Farr AG, Ravetch JV, Alpers CE
Am. J. Pathol., 2007-03-01;170(3):981-9.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Pericytes and endothelial precursor cells: cellular interactions and contributions to malignancy.
Authors: Bagley RG, Weber W, Rouleau C, Teicher BA
Cancer Res., 2005-11-01;65(21):9741-50.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Angiopoietin-1 is required for Schlemm's canal development in mice and humans
Authors: BR Thomson, T Souma, SW Tompson, T Onay, K Kizhatil, OM Siggs, L Feng, KN Whisenhunt, TL Yanovitch, L Kalaydjiev, DN Azmanov, S Finzi, CE Tanna, AW Hewitt, DA Mackey, YS Bradfield, E Souzeau, S Javadiyan, JL Wiggs, F Pasutto, X Liu, SW John, JE Craig, J Jin, TL Young, SE Quaggin
J. Clin. Invest., 2017-11-06;0(0):.
-
Stellate Cells, Hepatocytes, and Endothelial Cells Imprint the Kupffer Cell Identity on Monocytes Colonizing the Liver Macrophage Niche
Authors: Bonnardel J, T'Jonck W, Gaublomme D et al..
Immunity.
-
Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches
Authors: Guilliams M, Bonnardel J, Haest B Et al.
Cell
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse LYVE-1 Antibody
Average Rating: 5 (Based on 3 Reviews)
Have you used Mouse LYVE-1 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
In paraffin embedded sample.
Antigen retrieval with Tris-EDTA Buffer pH 9.0 and fixation with Methanol.
Blocking with TBS 2% BSA -0.5% Triton X-100.
Incubation of Lyve1 (AF2125) at a dilution of 1:400 (O/N at 4C).
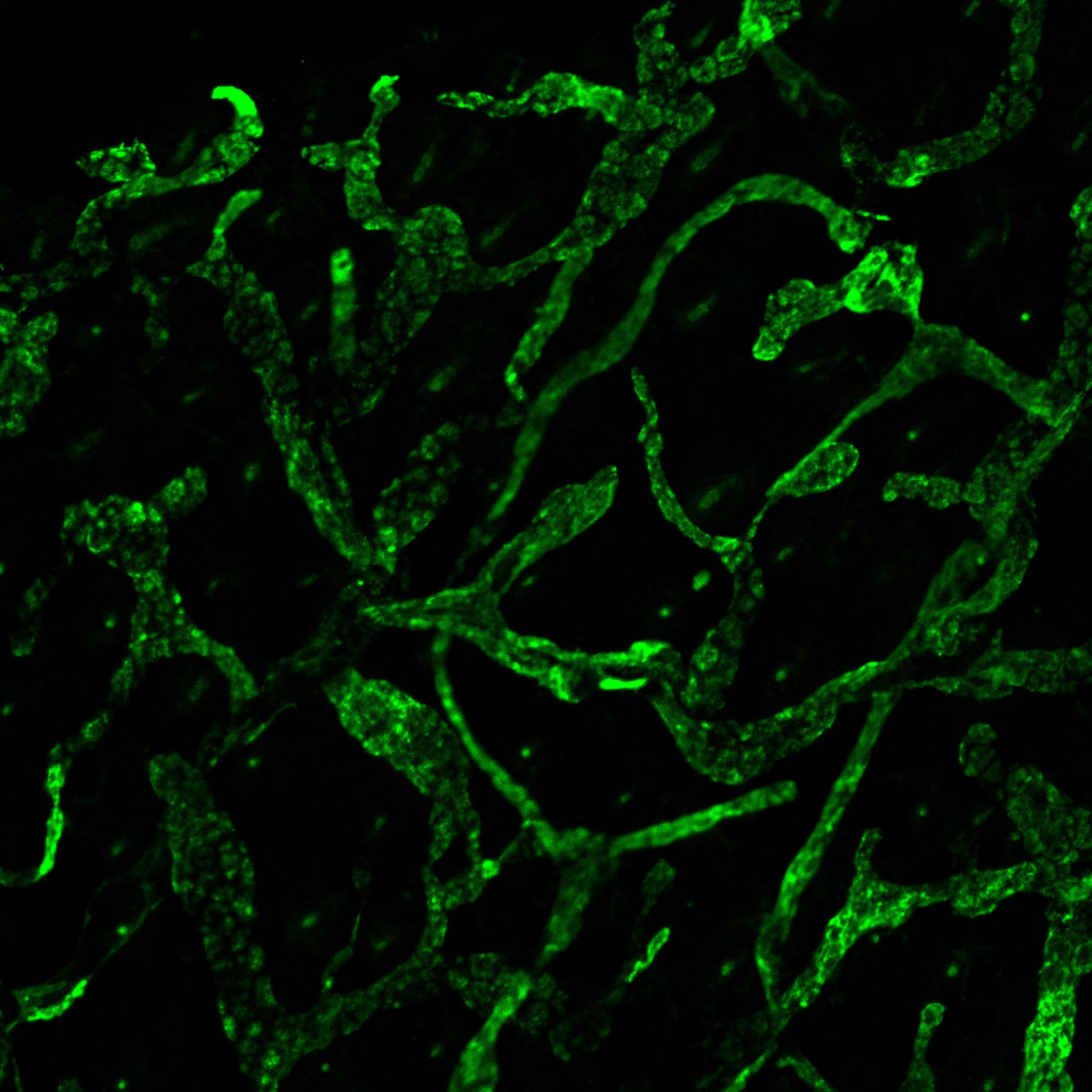
Mouse ear dermis was fixed in 2%PFA and blocked/permeabilized overnight in TBS/5% donkey serum/0.5% Tx100 before incubation in AF2125 at a dilution of 1:250 (O/N at 4C). Antibody was then detected using an alexafluor 488 labeled donkey anti goat secondary.