Recombinant Human IL-1 alpha/IL-1F1 Protein
Recombinant Human IL-1 alpha/IL-1F1 Protein Summary
Product Specifications
Ser113-Ala271
Analysis
Customers also Viewed
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
200-LA
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 10 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
200-LA/CF
| Formulation | Supplied as a 0.2 μm filtered solution in PBS. |
| Shipping | The product is shipped with dry ice or equivalent. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
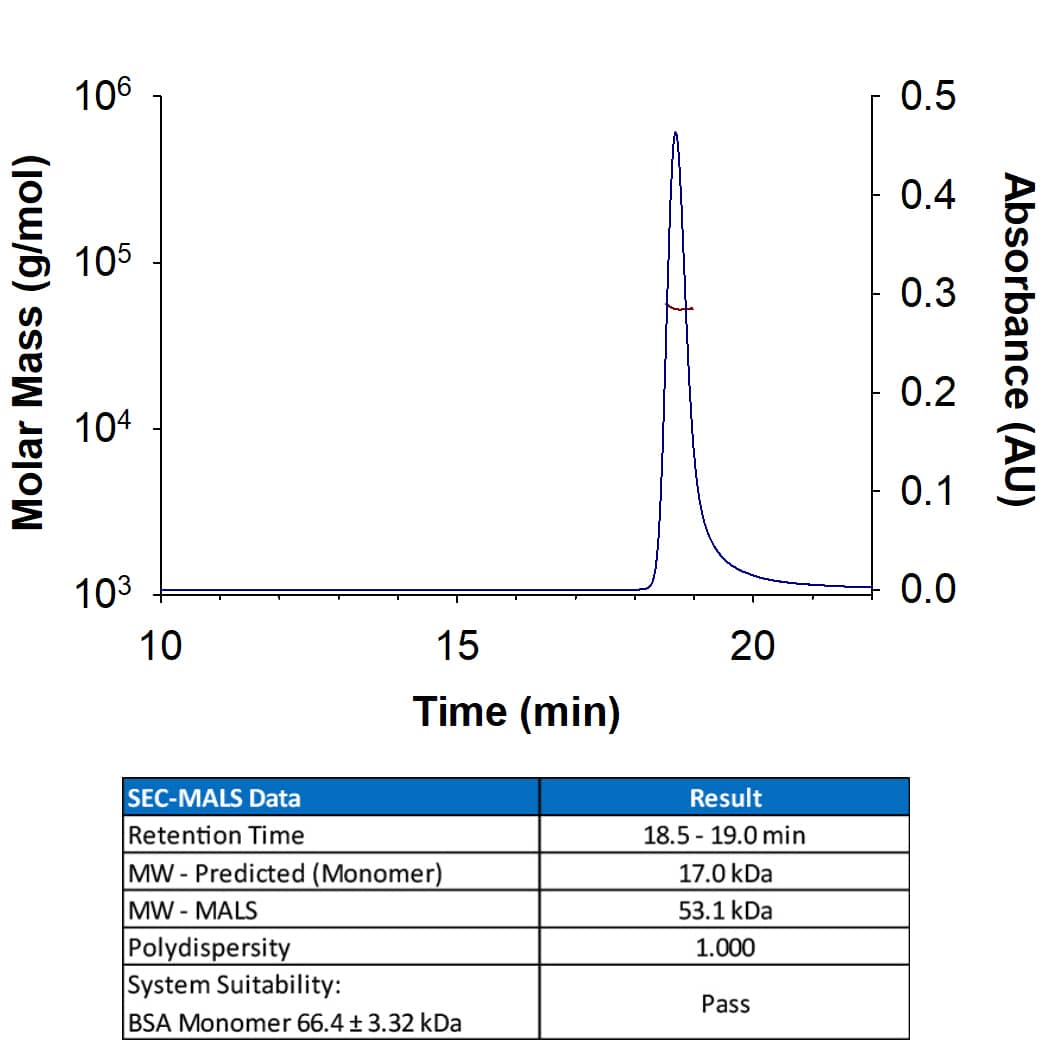
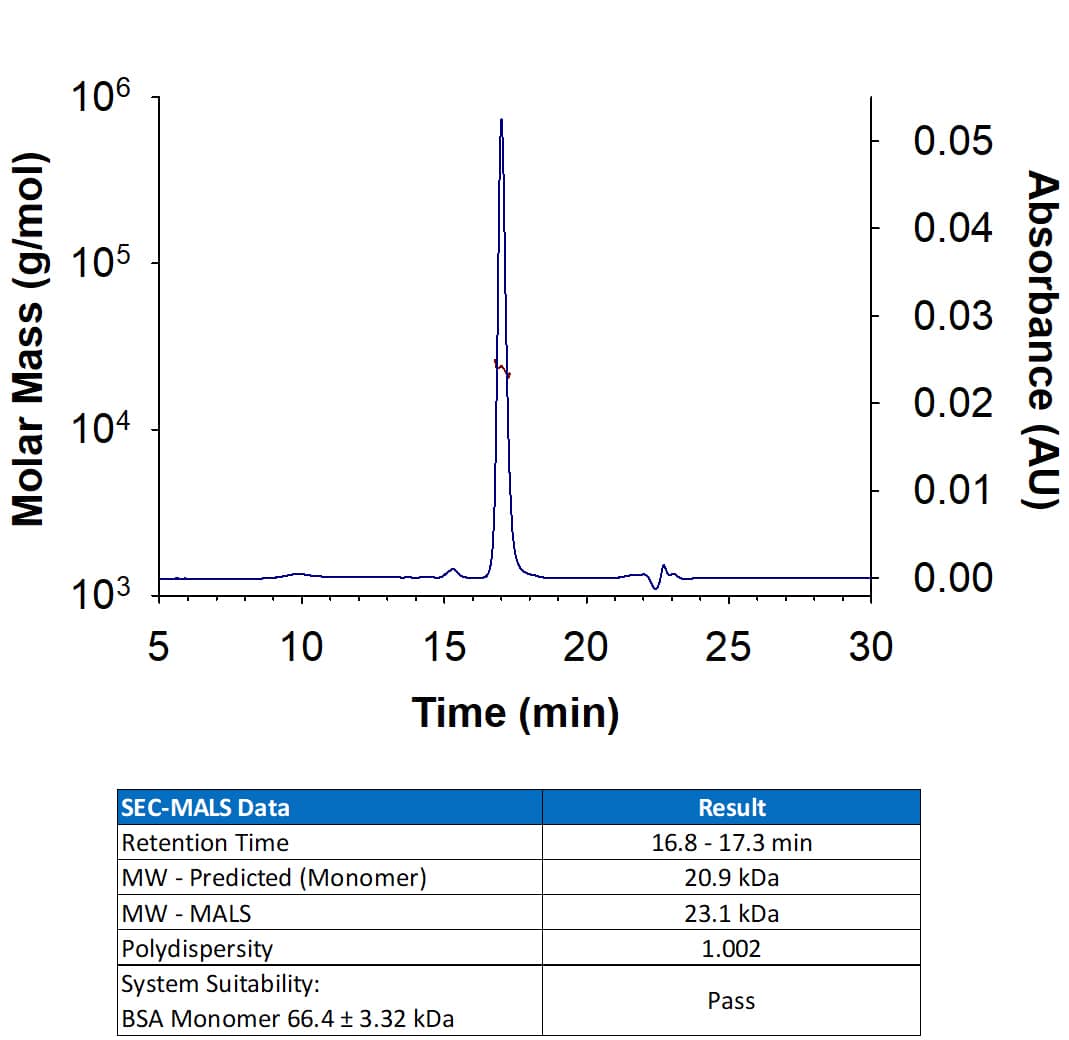
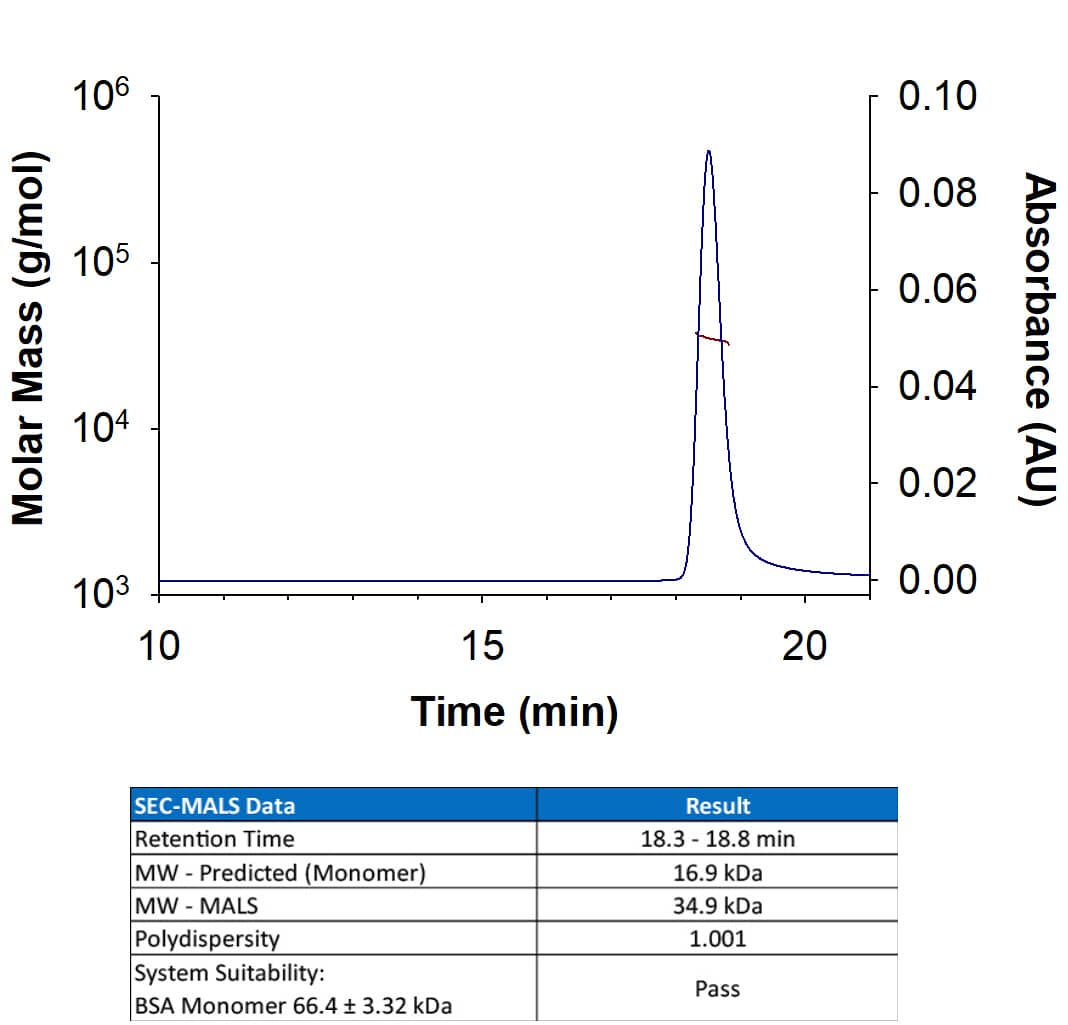
Scientific Data
 View Larger
View Larger
Recombinant Human IL-1 alpha /IL-1F1 (Catalog # 200-LA) stimulates cell proliferation of the D10.G4.1 mouse helper T cell line. The ED50 for this effect is 1-6 pg/mL.
 View Larger
View Larger
1 μg/lane of Recombinant Human IL-1 alpha /IL-1F1 was resolved with SDS-PAGE under reducing (R) conditions and visualized by silver staining, showing a single band at 18 kDa.
Background: IL-1 alpha/IL-1F1
Interleukin 1 (IL-1) is a name that designates two proteins, IL-1 alpha and IL-1 beta, which are the products of distinct genes, but which show approximately 25% amino acid sequence identity and which recognize the same cell surface receptors. Although IL-1 production is generally considered to be a consequence of inflammation, recent evidence suggests that IL-1 is also temporarily upregulated during bone formation and the menstrual cycle and can be induced in response to nervous system stimulation. In response to classic stimuli produced by inflammatory agents, infections or microbial endotoxins, a dramatic increase in the production of IL-1 by macrophages and various other cells is seen. Cells in particular known to produce IL-1 include osteoblasts, monocytes, macrophages, keratinocytes, Kupffer cells, hepatocytes, thymic and salivary gland epithelium, Schwann cells, fibroblasts and glia (oligodendroglia, astrocytes and microglia).
IL-1 alpha and IL-1 beta are both synthesized as 31 kDa precursors that are subsequently cleaved into proteins with molecular weights of approximately 17,000 Da. Neither precursor contains a typical hydrophobic signal peptide sequence and most of the precursor form of IL-1 alpha remains in the cytosol of cells, although there is evidence for a membrane-bound form of the precursor form of IL-1 alpha. The IL-1 alpha precursor reportedly shows full biological activity in the EL-4 assay. Among various species, the amino acid sequence of mature IL-1 alpha is conserved 60% to 70% and human IL-1 has been found to be biologically active on murine cell lines. Both forms of IL-1 bind to the same receptors, designated type I and type II. Evidence suggests that only the type I receptor is capable of signal transduction and that the type II receptor may function as a decoy, binding IL-1 and thus preventing binding of IL-1 to the type I receptor.
Citations for Recombinant Human IL-1 alpha/IL-1F1 Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
65
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Landscape of Interactions between Stromal and Myeloid Cells in Ileal Crohn's Disease; Indications of an Important Role for Fibroblast-Derived CCL-2
Authors: Dovrolis, N;Valatas, V;Drygiannakis, I;Filidou, E;Spathakis, M;Kandilogiannakis, L;Tarapatzi, G;Arvanitidis, K;Bamias, G;Vradelis, S;Manolopoulos, VG;Paspaliaris, V;Kolios, G;
Biomedicines
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A keratinocyte-adipocyte signaling loop is reprogrammed by loss of BTG3 to augment skin carcinogenesis
Authors: Cheng, YC;Acedera, JD;Li, YJ;Shieh, SY;
Cell death and differentiation
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Monitoring phosphorylation and acetylation of CRISPR-mediated HiBiT-tagged endogenous proteins
Authors: Alves, J;Schwinn, M;Machleidt, T;Goueli, SA;Cali, JJ;Zegzouti, H;
Scientific reports
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Blockade of IL-6R prevents preterm birth and adverse neonatal outcomes
Authors: Farias-Jofre, M;Romero, R;Galaz, J;Xu, Y;Miller, D;Garcia-Flores, V;Arenas-Hernandez, M;Winters, AD;Berkowitz, BA;Podolsky, RH;Shen, Y;Kanninen, T;Panaitescu, B;Glazier, CR;Pique-Regi, R;Theis, KR;Gomez-Lopez, N;
EBioMedicine
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Dietary Polyphenols Decrease Chemokine Release by Human Primary Astrocytes Responding to Pro-Inflammatory Cytokines
Authors: Grabarczyk, M;Ksiazek-Winiarek, D;Glabinski, A;Szpakowski, P;
Pharmaceutics
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Crosstalk of protein clearance, inflammasome, and Ca2+ channels in retinal pigment epithelium derived from age-related macular degeneration patients
Authors: Karema-Jokinen, V;Koskela, A;Hytti, M;Hongisto, H;Viheri�l�, T;Liukkonen, M;Torsti, T;Skottman, H;Kauppinen, A;Nymark, S;Kaarniranta, K;
The Journal of biological chemistry
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Human Primary Astrocytes Differently Respond to Pro- and Anti-Inflammatory Stimuli
Authors: P Szpakowski, D Ksiazek-Wi, M Turniak-Ku, I Pacan, A Glabinski
Biomedicines, 2022-07-22;10(8):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Inflammasome Activation in Retinal Pigment Epithelium from Human Donors with Age-Related Macular Degeneration
Authors: MC Ebeling, CR Fisher, RJ Kapphahn, MR Stahl, S Shen, J Qu, SR Montezuma, DA Ferrington
Cells, 2022-06-30;11(13):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Anti-Inflammatory and Anti-Fibrotic Effect of Immortalized Mesenchymal-Stem-Cell-Derived Conditioned Medium on Human Lung Myofibroblasts and Epithelial Cells
Authors: E Filidou, L Kandilogia, G Tarapatzi, M Spathakis, P Steiropoul, D Mikroulis, K Arvanitidi, V Paspaliari, G Kolios
International Journal of Molecular Sciences, 2022-04-20;23(9):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Orally-active, clinically-translatable senolytics restore alpha-Klotho in mice and humans
Authors: Y Zhu, LGPL Prata, EOW Gerdes, JME Netto, T Pirtskhala, N Giorgadze, U Tripathi, CL Inman, KO Johnson, A Xue, AK Palmer, T Chen, K Schaefer, JN Justice, AM Nambiar, N Musi, SB Kritchevsk, J Chen, S Khosla, D Jurk, MJ Schafer, T Tchkonia, JL Kirkland
EBioMedicine, 2022-03-13;0(0):103912.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
The Activin/FLRG pathway associates with poor COVID-19 outcomes in hospitalized patients
Authors: M McAleavy, Q Zhang, PJ Ehmann, J Xu, MF Wipperman, D Ajithdoss, L Pan, M Wakai, R Simonson, A Gadi, A Oyejide, SC Hamon, A Boyapati, LG Morton, T Shavlakadz, CA Kyratsous, DJ Glass
Molecular and Cellular Biology, 2021-11-01;0(0):MCB0046721.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
ELTD1 Activation Induces an Endothelial-EMT Transition to a Myofibroblast Phenotype
Authors: H Sheldon, J Alexander, E Bridges, L Moreira, S Reilly, KH Ang, D Wang, S Lin, S Haider, AH Banham, AL Harris
International Journal of Molecular Sciences, 2021-10-19;22(20):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Oncogene-induced senescence in hematopoietic progenitors features myeloid restricted hematopoiesis, chronic inflammation and histiocytosis
Authors: R Biavasco, E Lettera, K Giannetti, D Gilioli, S Beretta, A Conti, S Scala, D Cesana, P Gallina, M Norelli, L Basso-Ricc, A Bondanza, G Cavalli, M Ponzoni, L Dagna, C Doglioni, A Aiuti, I Merelli, R Di Micco, E Montini
Nature Communications, 2021-07-27;12(1):4559.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Senolytics reduce coronavirus-related mortality in old mice
Authors: Christina D. Camell, Matthew J. Yousefzadeh, Yi Zhu, Larissa G. P. Langhi Prata, Matthew A. Huggins, Mark Pierson et al.
Science
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Hydroquinone Induces NLRP3-Independent IL-18 Release from ARPE-19 Cells
Authors: N Bhattarai, E Korhonen, Y Mysore, K Kaarnirant, A Kauppinen
Cells, 2021-06-06;10(6):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
TAS-116, a Well-Tolerated Hsp90 Inhibitor, Prevents the Activation of the NLRP3 Inflammasome in Human Retinal Pigment Epithelial Cells
Authors: S Ranta-Aho, N Piippo, E Korhonen, K Kaarnirant, M Hytti, A Kauppinen
International Journal of Molecular Sciences, 2021-05-05;22(9):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Systematic functional analysis of SARS-CoV-2 proteins uncovers viral innate immune antagonists and remaining vulnerabilities
Authors: M Hayn, M Hirschenbe, L Koepke, R Nchioua, JH Straub, S Klute, V Hunszinger, F Zech, C Prelli Boz, W Aftab, MH Christense, C Conzelmann, JA Müller, S Srinivasac, CM Stürzel, I Forne, S Stenger, KK Conzelmann, J Münch, FI Schmidt, D Sauter, A Imhof, F Kirchhoff, KMJ Sparrer
Cell Reports, 2021-04-27;35(7):109126.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-1&alpha Is a Critical Mediator of the Response of Human Bronchial Fibroblasts to Eosinophilic Inflammation
Authors: K Bernau, JP Leet, H Floerke, EM Bruhn, AL Noll, IS McDermott, S Esnault, NN Jarjour, N Sandbo
Cells, 2021-03-02;10(3):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
18F-FHBG PET-CT Reporter Gene Imaging of Adoptive CIK Cell Transfer Immunotherapy for Breast Cancer in a Mouse Model
Authors: X Li, G Yin, W Ji, J Liu, Y Zhang, J Wang, X Zhu, L Zhu, D Dai, W Ma, W Xu
Onco Targets Ther, 2020-11-13;13(0):11659-11668.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Soluble Compounds Released by Hypoxic Stroma Confer Invasive Properties to Pancreatic Ductal Adenocarcinoma
Authors: D Liu, A Steins, R Klaassen, AP van der Za, RJ Bennink, G van Tienho, MG Besselink, MF Bijlsma, HWM van Laarho
Biomedicines, 2020-10-22;8(11):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The Pseudomonas aeruginosa protease LasB directly activates IL-1&beta
Authors: J Sun, DL LaRock, EA Skowronski, JM Kimmey, J Olson, Z Jiang, AJ O'Donoghue, V Nizet, CN LaRock
EBioMedicine, 2020-09-23;60(0):102984.
Species: Mouse
Sample Types: Reference Standard
-
Systemic conditioned medium treatment from interleukin-1 primed mesenchymal stem cells promotes recovery after stroke
Authors: CJ Cunningham, R Wong, J Barrington, S Tamburrano, E Pinteaux, SM Allan
Stem Cell Res Ther, 2020-01-21;11(1):32.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
PASylation of IL-1 Receptor antagonist (IL-1Ra) retains IL-1 blockade and extends its duration in mouse urate crystal-induced peritonitis
Authors: NE Powers, B Swartzwelt, C Marchetti, DM de Graaf, A Lerchner, M Schlapschy, R Datar, U Binder, CK Edwards, A Skerra, CA Dinarello
J. Biol. Chem., 2019-12-09;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Oxidative Stress is the Principal Contributor to Inflammasome Activation in Retinal Pigment Epithelium Cells with Defunct Proteasomes and Autophagy
Authors: N Piippo, E Korhonen, M Hytti, K Kinnunen, K Kaarnirant, A Kauppinen
Cell. Physiol. Biochem., 2018-08-23;49(1):359-367.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Chemoproteomics of matrix metalloproteases in a model of cartilage degeneration suggests functional biomarkers associated with posttraumatic osteoarthritis
Authors: KC Ravindra, CC Ahrens, Y Wang, JY Ramseier, JS Wishnok, LG Griffith, AJ Grodzinsky, SR Tannenbaum
J. Biol. Chem., 2018-05-23;0(0):.
Species: Bovine
Sample Types: Whole Tissue
Applications: Bioassay -
Interleukin-17A-induced production of acute serum amyloid A by keratinocytes contributes to psoriasis pathogenesis
Authors: E Couderc, F Morel, P Levillain, A Buffière-M, M Camus, C Paquier, C Bodet, JF Jégou, M Pohin, L Favot, M Garcia, V Huguier, J Mcheik, C Lacombe, H Yssel, G Guillet, FX Bernard, JC Lecron
PLoS ONE, 2017-07-14;12(7):e0181486.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Prevalence and correlation of cytokine-specific autoantibodies with epidemiological factors and C-reactive protein in 8,972 healthy individuals: Results from the Danish Blood Donor Study
Authors: JH von Steman, AS Rigas, LW Thørner, DGK Rasmussen, OB Pedersen, K Rostgaard, C Erikstrup, H Ullum, MB Hansen
PLoS ONE, 2017-06-30;12(6):e0179981.
Species: Human
Sample Types: Plasma
Applications: Control -
Atorvastatin Promotes Phagocytosis and Attenuates Pro-Inflammatory Response in Human Retinal Pigment Epithelial Cells
Authors: B Tian, A Al-Moujahe, P Bouzika, Y Hu, S Notomi, P Tsoka, JW Miller, H Lin, DG Vavvas
Sci Rep, 2017-05-24;7(1):2329.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro
Authors: E Redondo-Ca, C Cunningham, J Miller, L Martuscell, S Aoulad-Ali, NJ Rothwell, CM Kielty, SM Allan, E Pinteaux
Stem Cell Res Ther, 2017-04-17;8(1):79.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells
Authors: KM Perrott, CD Wiley, PY Desprez, J Campisi
Geroscience, 2017-04-04;39(2):161-173.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Inflammatory hypoxia induces syndecan-2 expression through IL-1?-mediated FOXO3a activation in colonic epithelia
Authors: Sojoong Choi
FASEB J, 2016-12-28;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Inflammatory Adipokines Decrease Expression of Two High Molecular Weight Isoforms of Tropomyosin Similar to the Change in Type 2 Diabetic Patients
PLoS ONE, 2016-09-20;11(9):e0162908.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Smooth Muscle Enriched Long Non-Coding RNA (SMILR) Regulates Cell Proliferation
Authors: MD Ballantyne, K Pinel, R Dakin, AT Vesey, L Diver, R Mackenzie, R Garcia, P Welsh, N Sattar, G Hamilton, N Joshi, MR Dweck, JM Miano, MW McBride, DE Newby, RA McDonald, AH Baker
Circulation, 2016-04-06;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Characterization of the MMP/TIMP Imbalance and Collagen Production Induced by IL-1? or TNF-? Release from Human Hepatic Stellate Cells
Authors: S Robert, T Gicquel, A Bodin, V Lagente, E Boichot
PLoS ONE, 2016-04-05;11(4):e0153118.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice.
Authors: Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C, Beckmann D, Paruzel P, Bertrand J, Redlich K, Koers-Wunrau C, Stratis A, Korb-Pap A, Pap T
Nat Med, 2015-08-03;21(9):1085-90.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Brief Glutamine Pretreatment Increases Alveolar Macrophage CD163/Heme Oxygenase-1/p38-MAPK Dephosphorylation Pathway and Decreases Capillary Damage but Not Neutrophil Recruitment in IL-1/LPS-Insufflated Rats.
Authors: Fernandez-Bustamante A, Agazio A, Wilson P, Elkins N, Domaleski L, He Q, Baer K, Moss A, Wischmeyer P, Repine J
PLoS ONE, 2015-07-06;10(7):e0130764.
Species: Rat
Sample Types: In Vivo
Applications: In Vivo -
MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation.
Authors: Laberge R, Sun Y, Orjalo A, Patil C, Freund A, Zhou L, Curran S, Davalos A, Wilson-Edell K, Liu S, Limbad C, Demaria M, Li P, Hubbard G, Ikeno Y, Javors M, Desprez P, Benz C, Kapahi P, Nelson P, Campisi J
Nat Cell Biol, 2015-07-06;17(8):1049-61.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Inhibition of keratinocyte differentiation by the synergistic effect of IL-17A, IL-22, IL-1alpha, TNFalpha and oncostatin M.
Authors: Rabeony H, Petit-Paris I, Garnier J, Barrault C, Pedretti N, Guilloteau K, Jegou J, Guillet G, Huguier V, Lecron J, Bernard F, Morel F
PLoS ONE, 2014-07-10;9(7):e101937.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Fetal human keratinocytes produce large amounts of antimicrobial peptides: involvement of histone-methylation processes.
Authors: Gschwandtner M, Zhong S, Tschachler A, Mlitz V, Karner S, Elbe-Burger A, Mildner M
J Invest Dermatol, 2014-04-02;134(8):2192-201.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Proinflammatory stimuli induce galectin-9 in human mesenchymal stromal cells to suppress T-cell proliferation.
Authors: Gieseke F, Kruchen A, Tzaribachev N, Bentzien F, Dominici M, Muller I
Eur J Immunol, 2013-07-23;43(10):2741-9.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors.
Authors: Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J
Nature, 2012-07-26;487(7408):505-9.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33.
J. Exp. Med., 2012-07-16;209(8):1505-17.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7.
Authors: Jung DW, Che ZM, Kim J, Kim K, Kim KY, Williams D, Kim J
Int. J. Cancer, 2010-07-15;127(2):332-44.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Evidence for altered Wnt signaling in psoriatic skin.
Authors: Gudjonsson JE, Johnston A, Stoll SW
J. Invest. Dermatol., 2010-04-08;130(7):1849-59.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1alpha, and TNF-alpha Recapitulates Some Features of Psoriasis.
Authors: Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, Guillet G, Bernard FX, Lecron JC, Morel F
J. Immunol., 2010-03-24;184(0):5263-70.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Development and validation of sandwich ELISA microarrays with minimal assay interference.
Authors: Gonzalez RM, Seurynck-Servoss SL, Crowley SA
J. Proteome Res., 2008-04-19;7(6):2406-14.
Applications: ELISA (Standard) -
Upregulation of human cytomegalovirus by HIV type 1 in human lymphoid tissue ex vivo.
Authors: Biancotto A, Iglehart SJ, Lisco A, Vanpouille C, Grivel JC, Lurain NS, Reichelderfer PS, Margolis LB
AIDS Res. Hum. Retroviruses, 2008-03-01;24(3):453-62.
Applications: ELISA (Standard) -
Effect of serum content and diluent selection on assay sensitivity and signal intensity in multiplex bead-based immunoassays.
Authors: Pfleger C, Schloot N, ter Veld F
J. Immunol. Methods, 2007-10-22;329(1):214-8.
Applications: ELISA (Standard) -
Ultrasensitive flow-based immunoassays using single-molecule counting.
Authors: Todd J, Freese B, Lu A, Held D, Morey J, Livingston R, Goix P
Clin. Chem., 2007-09-21;53(11):1990-5.
Applications: ELISA (Standard) -
Synergism of TNF and IL-1 in the induction of matrix metalloproteinase-3 in trabecular meshwork.
Authors: Kelley MJ, Rose AY, Song K, Chen Y, Bradley JM, Rookhuizen D, Acott TS
Invest. Ophthalmol. Vis. Sci., 2007-06-01;48(6):2634-43.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
P2X receptor characterization and IL-1/IL-1Ra release from human endothelial cells.
Authors: Wilson HL, Varcoe RW, Stokes L, Holland KL, Francis SE, Dower SK, Surprenant A, Crossman DC
Br. J. Pharmacol., 2007-03-12;151(1):115-27.
Applications: Western Blot -
Bradykinin potentiates cytokine-induced prostaglandin biosynthesis in osteoblasts by enhanced expression of cyclooxygenase 2, resulting in increased RANKL expression.
Authors: Brechter AB, Lerner UH
Arthritis Rheum., 2007-03-01;56(3):910-23.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1.
Authors: Biancotto A, Grivel JC, Iglehart SJ, Vanpouille C, Lisco A, Sieg SF, Debernardo R, Garate K, Rodriguez B, Margolis LB, Lederman MM
Blood, 2007-02-08;109(10):4272-9.
Applications: ELISA (Standard) -
IL-10 inhibits endothelium-dependent T cell costimulation by up-regulation of ILT3/4 in human vascular endothelial cells.
Authors: Gleissner CA, Zastrow A, Klingenberg R, Kluger MS, Konstandin M, Celik S, Haemmerling S, Shankar V, Giese T, Katus HA, Dengler TJ
Eur. J. Immunol., 2007-01-01;37(1):177-92.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium.
Authors: Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG
J. Exp. Med., 2006-11-20;203(12):2763-77.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
L5, the most electronegative subfraction of plasma LDL, induces endothelial vascular cell adhesion molecule 1 and CXC chemokines, which mediate mononuclear leukocyte adhesion.
Authors: Abe Y, Fornage M, Yang CY, Bui-Thanh NA, Wise V, Chen HH, Rangaraj G, Ballantyne CM
Atherosclerosis, 2006-10-04;192(1):56-66.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Transforming growth factor-alpha: a major human serum factor that promotes human keratinocyte migration.
Authors: Li Y, Fan J, Chen M, Li W, Woodley DT
J. Invest. Dermatol., 2006-05-11;126(9):2096-105.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Pre-interleukin-1alpha expression reduces cell growth and increases interleukin-6 production in SaOS-2 osteosarcoma cells: Differential inhibitory effect of interleukin-1 receptor antagonist (icIL-1Ra1).
Authors: Palmer G, Trolliet S, Talabot-Ayer D, Mezin F, Magne D, Gabay C
Cytokine, 2005-07-21;31(2):153-60.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
CYP7B expression and activity in fibroblast-like synoviocytes from patients with rheumatoid arthritis: regulation by proinflammatory cytokines.
Authors: Dulos J, van der Vleuten MA, Kavelaars A, Heijnen CJ, Boots AM
Arthritis Rheum., 2005-03-01;52(3):770-8.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways.
Authors: Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R
J. Immunol., 2004-09-01;173(5):3482-91.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-1 inhibits voltage-dependent P/Q-type Ca2+ channel associated with the inhibition of the rise of intracellular free Ca2+ concentration and catecholamine release in adrenal chromaffin cells.
Authors: Morita K, Miyasako T, Kitayama S, Dohi T
Biochim. Biophys. Acta, 2004-08-04;1673(3):160-9.
Species: Bovine
Sample Types: Whole Cells
Applications: Bioassay -
The effect of inflammatory cytokines on secretion of macrophage colony-stimulating factor and monocyte chemoattractant protein-1 in human granulosa cells.
Authors: Kawano Y, Fukuda J, Itoh H, Takai N, Nasu K, Miyakawa I
Am. J. Reprod. Immunol., 2004-08-01;52(2):124-8.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action.
Authors: Smith DE, Hanna R, Della Friend, Moore H, Chen H, Farese AM, MacVittie TJ, Virca GD, Sims JE
Immunity, 2003-01-01;18(1):87-96.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
c-Jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin.
Authors: Szabowski A, Maas-Szabowski N, Andrecht S, Kolbus A, Schorpp-Kistner M, Fusenig NE, Angel P
Cell, 2000-11-22;103(5):745-55.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Gingival crevicular fluid from patients with periodontitis contains bone resorbing activity.
Authors: Lerner UH, Modeer T, Krekmanova L, Claesson R, Rasmussen L
Eur. J. Oral Sci., 1998-06-01;106(3):778-87.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human IL-1 alpha/IL-1F1 Protein
Average Rating: 4.9 (Based on 8 Reviews)
Have you used Recombinant Human IL-1 alpha/IL-1F1 Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Reason for Rating: We used this in chondral explants as a positive control for osteoarthritis. It increases activity of comp, MMP13, and many other marks for OA. This is our labs staple IL and it works prefect every time.