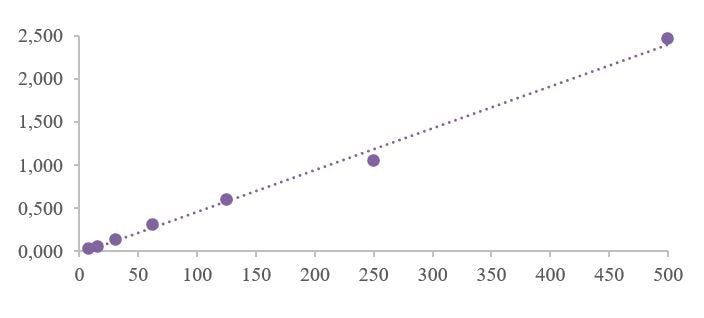
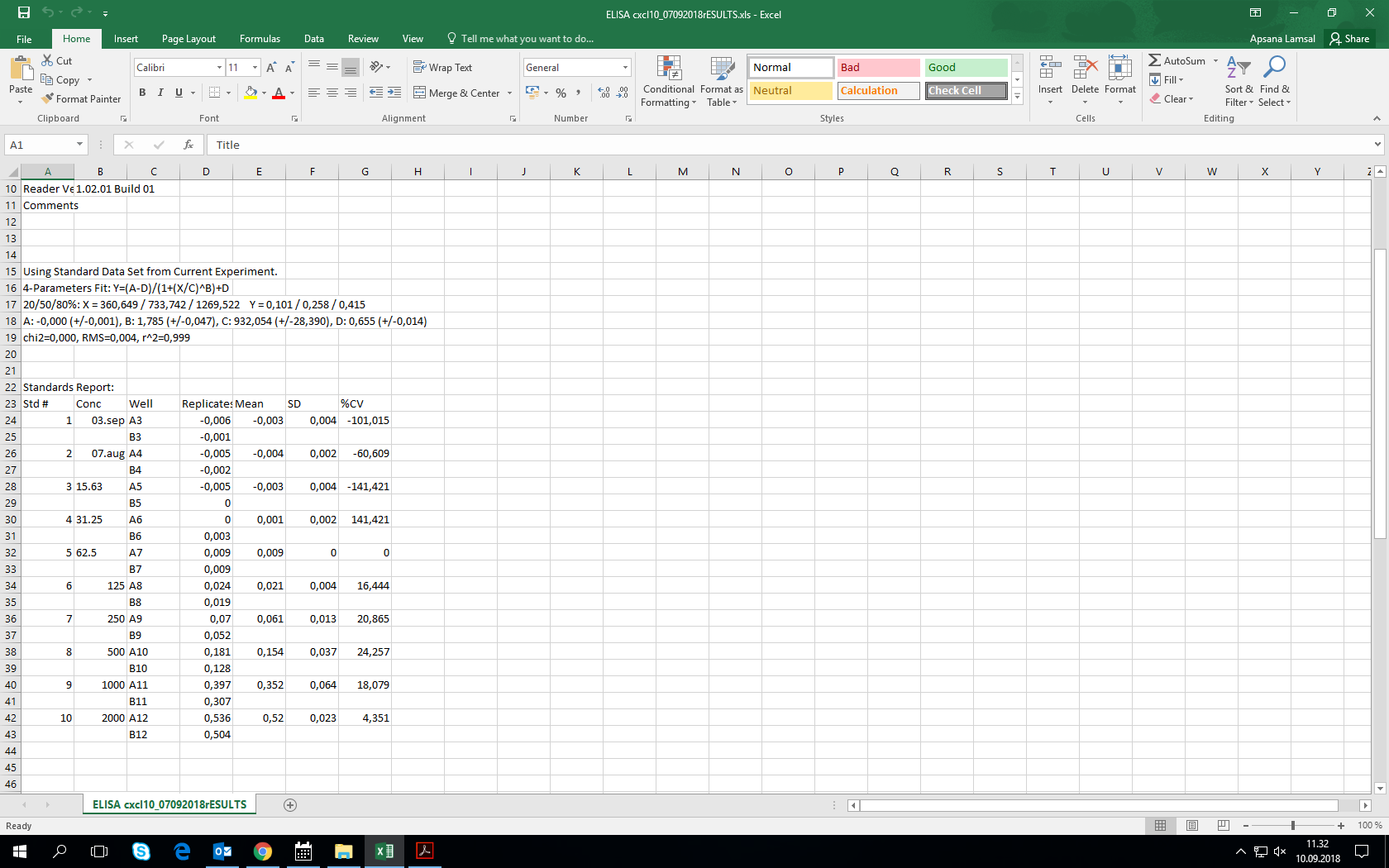
Human CXCL10/IP-10 Quantikine ELISA Kit Summary
Sample Values
Serum/Plasma/Saliva/Urine - Samples from apparently healthy volunteers were evaluated for the presence of human IP-10 in this assay. No medical histories were available for the donors used in this study.| Sample Type | Mean (pg/mL) | Range (pg/mL) |
| Serum (n=60) | 89 | 38-361 |
| EDTA plasma (n=35) | 96 | 47-382 |
| Heparin plasma (n=35) | 110 | 52-450 |
| Saliva (n=5) | 729 | 292-1340 |
| Sample Type | Mean of Detectable (pg/mL) | % Detectable | Range (pg/mL) |
| Urine (n=18) | 17.2 | 67 | ND-49.7 |
| Condition | Day 1 (pg/mL) | Day 5 (pg/mL) |
| Unstimulated | 29,774 | 21,900 |
| Stimulated | 18,456 | 11,091 |
THP-1 human acute monocyte leukemia cells were cultured in RPMI supplemented with 10% fetal bovine serum, 50 μM beta -mercaptoethanol, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin sulfate. Cells were stimulated with 1.0 μg/mL recombinant human IFN-gamma for 8 hours and then 1.0 μg/mL LPS was added. Cells were incubated for an additional 18 hours. An aliquot of the cell culture supernate was removed, diluted 600-fold and assayed, and measured 164,640 pg/mL.
Product Summary
Precision
Cell Culture Supernates, Saliva, Urine
| Intra-Assay Precision | Inter-Assay Precision | ||||
|---|---|---|---|---|---|
| Sample | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 174 | 342 | 80.7 | 194 | 362 |
| Standard Deviation | 5.15 | 10.74 | 7.91 | 13.48 | 24.27 |
| CV% | 3 | 3.1 | 9.8 | 6.9 | 6.7 |
Recovery
The recovery of IP-10 spiked to levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=4) | 95 | 88-101 |
| EDTA Plasma (n=5) | 99 | 90-109 |
| Heparin Plasma (n=5) | 99 | 87-113 |
| Serum (n=5) | 99 | 88-112 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: CXCL10/IP-10/CRG-2
IP-10 was originally identified as an IFN-gamma-inducible gene in monocytes, fibroblasts and endothelial cells. The mouse homolog of human IP-10, CRG-2, shares approximately 67% amino acid sequence identity with human IP-10. The amino acid sequence of IP-10 identified the protein as a member of the CXC chemokine subfamily.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- For Cell Culture Supernate, Saliva, & Urine Samples: Add 150 µL of Assay Diluent to each well.
For Serum & Plasma Samples: Add 75 µL of Assay Diluent to each well. - For Cell Culture Supernate, Saliva, & Urine Samples: Add 100 µL of Standard, control, or sample to each well.
For Serum & Plasma Samples: Add 75 µL of Standard, control, or sample to each well. - Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes.
- Add 200 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours.
- Aspirate and wash 4 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Human CXCL10/IP-10 Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
155
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
A sensitive assay for measuring whole-blood responses to type I IFNs
Authors: Gervais, A;Le Floc'h, C;Le Voyer, T;Bizien, L;Bohlen, J;Celmeli, F;Al Qureshah, F;Masson, C;Rosain, J;Chbihi, M;Lévy, R;Castagnoli, R;Rothenbuhler, A;Jouanguy, E;Zhang, Q;Zhang, SY;Béziat, V;Bustamante, J;Puel, A;Bastard, P;Casanova, JL;
Proceedings of the National Academy of Sciences of the United States of America
Species: Human
Sample Types: Plasma
-
Transforming kidney transplant monitoring with urine CXCL9 and CXCL10: practical clinical implementation
Authors: Tinel, C;Sauvaget, V;Aouni, L;Lamarthée, B;Terzi, F;Legendre, C;Rabant, M;Anglicheau, D;
Scientific reports
Species: Human
Sample Types: Urine
-
Characterization of IL-6R-expressing monocytes in the lung of patients with chronic obstructive pulmonary disease
Authors: Ono, Y;Fujino, N;Saito, T;Matsumoto, S;Konno, S;Endo, T;Suzuki, M;Yamada, M;Okada, Y;Sugiura, H;
Respiratory investigation
Species: Human
Sample Types: Cell Culture Supernates
-
The inhibitory effect of DIF-3 on polyinosinic-polycytidylic acid-induced innate immunity activation in human cerebral microvascular endothelial cells
Authors: Araya, R;Men, S;Uekusa, Y;Yu, Z;Kikuchi, H;Daitoku, K;Minakawa, M;Kawaguchi, S;Furukawa, KI;Oshima, Y;Imaizumi, T;Seya, K;
Journal of pharmacological sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Blood-brain barrier damage associates with glia-related cytokines in the cerebrospinal fluid of patients with Multiple Sclerosis
Authors: Puthenparampil, M;Marin, A;Zanotelli, G;Mauceri, VA;De Napoli, F;Gaggiola, M;Miscioscia, A;Ponzano, M;Bovis, F;Perini, P;Rinaldi, F;Molon, B;Gallo, P;
Multiple sclerosis and related disorders
Species: Human
Sample Types: Serum, Cerebrospinal Fluid
-
Field performance and cost-effectiveness of a point-of-care triage test for HIV virological failure in Southern Africa
Authors: Saura-Lázaro, A;Bock, P;Bogaart, EVD;van Vliet, J;Granés, L;Nel, K;Naidoo, V;Scheepers, M;Saunders, Y;Leal, N;Ramponi, F;Paulussen, R;de Wit, TR;Naniche, D;López-Varela, E;
Journal of the International AIDS Society
Species: Human
Sample Types: Plasma
-
Retrospective Study Shows That Serum Levels of Chemokine CXCL10 and Cytokine GDF15 Support a Diagnosis of Sporadic Inclusion Body Myositis and Immune-Mediated Necrotizing Myopathy
Authors: De Paepe, B;Bracke, KR;De Bleecker, JL;
Brain sciences
Species: Human
Sample Types: Serum
-
C9orf72-ALS human iPSC microglia are pro-inflammatory and toxic to co-cultured motor neurons via MMP9
Authors: Vahsen, BF;Nalluru, S;Morgan, GR;Farrimond, L;Carroll, E;Xu, Y;Cramb, KML;Amein, B;Scaber, J;Katsikoudi, A;Candalija, A;Carcolé, M;Dafinca, R;Isaacs, AM;Wade-Martins, R;Gray, E;Turner, MR;Cowley, SA;Talbot, K;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Diagnostic Performance of Serum MicroRNAs for ST-Segment Elevation Myocardial Infarction in the Emergency Department
Authors: Amezcua-Guerra, B;Amezcua-Castillo, LM;Guerra-López, JA;Díaz-Domínguez, KA;Sánchez-Gloria, JL;Cruz-Melendez, A;Hernández-Díazcouder, A;Juárez-Vicuña, Y;Sánchez-Muñoz, F;Huang, F;Tavera-Alonso, C;Brianza-Padilla, M;Varela-López, E;Sierra-Lara, D;Arias-Mendoza, A;Fonseca-Camarillo, G;Márquez-Velasco, R;González-Pacheco, H;Springall, R;Amezcua-Guerra, LM;
Biomedicines
Species: Human
Sample Types: Serum
-
An integrated organoid omics map extends modeling potential of kidney disease
Authors: Lassé, M;El Saghir, J;Berthier, CC;Eddy, S;Fischer, M;Laufer, SD;Kylies, D;Hutzfeldt, A;Bonin, LL;Dumoulin, B;Menon, R;Vega-Warner, V;Eichinger, F;Alakwaa, F;Fermin, D;Billing, AM;Minakawa, A;McCown, PJ;Rose, MP;Godfrey, B;Meister, E;Wiech, T;Noriega, M;Chrysopoulou, M;Brandts, P;Ju, W;Reinhard, L;Hoxha, E;Grahammer, F;Lindenmeyer, MT;Huber, TB;Schlüter, H;Thiel, S;Mariani, LH;Puelles, VG;Braun, F;Kretzler, M;Demir, F;Harder, JL;Rinschen, MM;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates, Cell Lysates
-
Endotoxemia Associated with Liver Disease Correlates with Systemic Inflammation and T Cell Exhaustion in Hepatitis C Virus Infection
Authors: Shive, CL;Kowal, CM;Desotelle, AF;Nguyen, Y;Carbone, S;Kostadinova, L;Davitkov, P;O'Mara, M;Reihs, A;Siddiqui, H;Wilson, BM;Anthony, DD;
Cells
Species: Human
Sample Types: Plasma
-
Inhibition of METTL3 results in a cell-intrinsic interferon response that enhances anti-tumour immunity
Authors: Guirguis, AA;Ofir-Rosenfeld, Y;Knezevic, K;Blackaby, W;Hardick, D;Chan, YC;Motazedian, A;Gillespie, A;Vassiliadis, D;Lam, EY;Tran, K;Andrews, B;Harbour, ME;Vasiliauskaite, L;Saunders, CJ;Tsagkogeorga, G;Azevedo, A;Obacz, J;Pilka, ES;Carkill, M;MacPherson, L;Wainwright, EN;Liddicoat, B;Blyth, BJ;Albertella, MR;Rausch, O;Dawson, MA;
Cancer discovery
Species: Human
Sample Types: Cell Culture Supernates
-
NR1D1 stimulates antitumor immune responses in breast cancer by activating cGAS-STING signaling
Authors: Ka, NL;Park, MK;Kim, SS;Jeon, Y;Hwang, S;Kim, SM;Lim, GY;Lee, H;Lee, MO;
Cancer research
Species: Human
Sample Types: Cell Culture Supernates
-
Single cell transcriptomics identifies distinct profiles in pediatric acute respiratory distress syndrome
Authors: Flerlage, T;Crawford, JC;Allen, EK;Severns, D;Tan, S;Surman, S;Ridout, G;Novak, T;Randolph, A;West, AN;Thomas, PG;
Nature communications
Species: Human
Sample Types: Tracheal Aspirate
-
Psychological stress is associated with arterial inflammation in people living with treated HIV infection
Authors: Chow, FC;Mundada, NS;Abohashem, S;La Joie, R;Iaccarino, L;Arechiga, VM;Swaminathan, S;Rabinovici, GD;Epel, ES;Tawakol, A;Hsue, PY;
Brain, behavior, and immunity
Species: Human
Sample Types: Plasma
-
Effect of stimulator of interferon genes (STING) signaling on radiation-induced chemokine expression in human osteosarcoma cells
Authors: SS Withers, CE Moeller, CN Quick, CC Liu, SM Baham, JS Looper, R Subramania, KG Kousoulas
PLoS ONE, 2023-04-20;18(4):e0284645.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased neutrophil derived chemokines (CXCL10 and CCL2) in human trichinellosis as possible serological markers of the polarization of the immune response against the parasite
Authors: F Bruschi, B Pinto, P Fallahi, SM Ferrari, A Antonelli
Cytokine, 2023-04-12;166(0):156205.
Species: Human
Sample Types: Serum
-
The Role of Plasminogen Activator Inhibitor 1 in Predicting Sepsis-Associated Liver Dysfunction: An Observational Study
Authors: E Wo?nica-Ni, P Le?nik, J Janc, M Zalewska, L ?ysenko
International Journal of Environmental Research and Public Health, 2023-03-09;20(6):.
Species: Human
Sample Types: Plasma
-
The brain reacting to COVID-19: analysis of the cerebrospinal fluid proteome, RNA and inflammation
Authors: D Reinhold, V Farztdinov, Y Yan, C Meisel, H Sadlowski, J Kühn, FH Perschel, M Endres, E Düzel, S Vielhaber, K Guttek, A Goihl, M Venø, B Teegen, W Stöcker, P Stubbemann, F Kurth, LE Sander, M Ralser, C Otto, S Streit, S Jarius, K Ruprecht, H Radbruch, J Kjems, M Mülleder, F Heppner, P Körtvelyes
Journal of Neuroinflammation, 2023-02-09;20(1):30.
Species: Human
Sample Types: CSF
-
Clinical and serological association of plasma 25-hydroxyvitamin D (25(OH)D) levels in lupus and the short-term effects of oral vitamin D supplementation
Authors: C Kavadichan, P Singh, S Maurya, S Tota, A Kiroubagar, D Kounassega, S Anand, VS Negi, A Aggarwal
Arthritis Research & Therapy, 2023-01-03;25(1):2.
Species: Human
Sample Types: Plasma
-
Effect of stimulator of interferon genes (STING) signaling on radiation-induced chemokine expression in human osteosarcoma cells
Authors: SS Withers, CE Moeller, CN Quick, CC Liu, SM Baham, JS Looper, R Subramania, KG Kousoulas
PLoS ONE, 2023;18(4):e0284645.
Species: Human
Sample Types: Cell Culture Supernates
-
Transcription factor MAFB controls type I and II interferon response-mediated host immunity in Mycobacterium tuberculosis-infected macrophages
Authors: H Hikichi, S Seto, K Wakabayash, M Hijikata, N Keicho
Frontiers in Microbiology, 2022-11-03;13(0):962306.
Species: Human
Sample Types: Cell Culture Supernates
-
MET-induced CD73 restrains STING-mediated immunogenicity of EGFR-mutant lung cancer
Authors: R Yoshida, M Saigi, T Tani, BF Springer, H Shibata, S Kitajima, NR Mahadevan, M Campisi, W Kim, Y Kobayashi, TC Thai, K Haratani, Y Yamamoto, SK Sundararam, EH Knelson, A Vajdi, I Canadas, R Uppaluri, CP Paweletz, JJ Miret, PH Lizotte, PC Gokhale, PA Janne, DA Barbie
Cancer Research, 2022-11-02;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Longitudinal Assessment of Multiple Immunological and Inflammatory Parameters during Successful DAA Therapy in HCV Monoinfected and HIV/HCV Coinfected Subjects
Authors: P Zuccalà, T Latronico, R Marocco, S Savinelli, S Vita, F Mengoni, T Tieghi, C Borgo, B Kertusha, A Carraro, G D'Ettorre, V Vullo, CM Mastroiann, GM Liuzzi, M Lichtner
International Journal of Molecular Sciences, 2022-10-08;23(19):.
Species: Human
Sample Types: Plasma
-
Dynamics of Inflammatory and Neurodegenerative Biomarkers after Autologous Hematopoietic Stem Cell Transplantation in Multiple Sclerosis
Authors: J Ruder, G Dinner, A Maceski, E Berenjeno-, AM Müller, I Jelcic, J Kuhle, R Martin
International Journal of Molecular Sciences, 2022-09-19;23(18):.
Species: Human
Sample Types: Serum
-
Transcription and splicing regulation by NLRC5 shape the interferon response in human pancreatic beta cells
Authors: F Szymczak, MI Alvelos, S Marín-Caña, Â Castela, S Demine, ML Colli, A Op de Beec, S Thomaidou, L Marselli, A Zaldumbide, P Marchetti, DL Eizirik
Science Advances, 2022-09-14;8(37):eabn5732.
Species: Human
Sample Types: Cell Culture Supernates
-
The Prostacyclin Analogue Iloprost Modulates CXCL10 in Systemic Sclerosis
Authors: T Colasanti, K Stefananto, C Fantini, C Corinaldes, M Vasile, F Marampon, L Di Luigi, C Antinozzi, P Sgrò, A Lenzi, V Riccieri, C Crescioli
International Journal of Molecular Sciences, 2022-09-05;23(17):.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum and Urine Interferon Gamma-Induced Protein 10 (IP-10) Levels in Lupus Nephritis
Authors: MP Brady, S Chava, S Tandon, MJ Rane, MT Barati, DJ Caster, DW Powell
Oncogene, 2022-06-03;11(11):.
Species: Human
Sample Types: Serum
-
Deficiency in coatomer complex I causes aberrant activation of STING signalling
Authors: A Steiner, K Hrovat-Sch, I Prigione, CH Yu, P Laohamonth, CR Harapas, RRJ Low, D De Nardo, LF Dagley, MJ Mlodzianos, KL Rogers, T Zillinger, G Hartmann, MP Gantier, M Gattorno, M Geyer, S Volpi, S Davidson, SL Masters
Nature Communications, 2022-04-28;13(1):2321.
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokine response over the course of COVID-19 infection in pregnant women
Authors: DB Rosen, EA Murphy, RS Gejman, A Capili, RL Friedlande, S Rand, KA Cagino, SM Glynn, KC Matthews, JM Kubiak, J Yee, M Prabhu, LE Riley, YJ Yang
Cytokine, 2022-04-25;154(0):155894.
Species: Human
Sample Types: Serum
-
Systemic and mucosal immune profiling in asymptomatic and symptomatic SARS-CoV-2-infected individuals reveal unlinked immune signatures
Authors: S Ravichandr, G Grubbs, J Tang, Y Lee, C Huang, H Golding, S Khurana
Science Advances, 2021-10-13;7(42):eabi6533.
Species: Human
Sample Types: Nasal Lavage Fluid
-
TNF-alpha Increases IP-10 Expression in MCF-7 Breast Cancer Cells via Activation of the JNK/c-Jun Pathways
Authors: S Kochumon, A Al-Sayyar, T Jacob, A Hasan, F Al-Mulla, S Sindhu, R Ahmad
Biomolecules, 2021-09-13;11(9):.
Species: Human
Sample Types: Cell Culture Supernates
-
Interferon-gamma-Inducible Chemokines as Prognostic Markers for Lung Cancer
Authors: KS Lee, WY Chung, JE Park, YJ Jung, JH Park, SS Sheen, KJ Park
International Journal of Environmental Research and Public Health, 2021-09-04;18(17):.
Species: Human
Sample Types: Serum
-
Exploratory analysis to identify the best antigen and the best immune biomarkers to study SARS-CoV-2 infection
Authors: E Petrucciol, S Najafi Far, A Navarra, L Petrone, V Vanini, G Cuzzi, G Gualano, L Pierelli, A Bertoletti, E Nicastri, F Palmieri, G Ippolito, D Goletti
Journal of Translational Medicine, 2021-06-26;19(1):272.
Species: Human
Sample Types: Plasma
-
Cytokine expression patterns in hospitalized children with Bordetella pertussis, Rhinovirus or co-infection
Authors: E Pandolfi, N Panera, A Alisi, E Carloni, L Russo, I Campagna, C Rizzo, C Concato, G Linardos, L Piccioni, S Jackson, A Villani, F Midulla, AE Tozzi
Scientific Reports, 2021-05-26;11(1):10948.
Species: Human
Sample Types: Sputum
-
Murlentamab, a Low Fucosylated Anti-M�llerian Hormone Type II Receptor (AMHRII) Antibody, Exhibits Anti-Tumor Activity through Tumor-Associated Macrophage Reprogrammation and T Cell Activation
Authors: M Prat, M Salon, T Allain, O Dubreuil, G Noël, L Preisser, B Jean, L Cassard, F Lemée, I Tabah-Fish, B Pipy, P Jeannin, JF Prost, JM Barret, A Coste
Cancers, 2021-04-13;13(8):.
Species: Human
Sample Types: Cell Lysates
-
Metabolic modulation by CDK4/6 inhibitor promotes chemokine-mediated recruitment of T�cells into mammary tumors
Authors: RV Uzhachenko, V Bharti, Z Ouyang, A Blevins, S Mont, N Saleh, HA Lawrence, C Shen, SC Chen, GD Ayers, DG DeNardo, C Arteaga, A Richmond, AE Vilgelm
Cell Reports, 2021-04-06;35(1):108944.
Species: Human
Sample Types: Cell Culture Supernates
-
NF&kapppaB-Activated COX2/PGE2/EP4 Axis Controls the Magnitude and Selectivity of BCG-Induced Inflammation in Human Bladder Cancer Tissues
Authors: OM Ibrahim, PH Basse, W Jiang, K Guru, G Chatta, P Kalinski
Cancers, 2021-03-16;13(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
Cerebrospinal fluid type I interferon and cytokine profiles in enteroviral meningitis according to the presence or absence of pleocytosis
Authors: KY Lee, JH Seol, CH Yi, WH Lee
Pediatrics and neonatology, 2021-02-23;0(0):.
Species: Human
Sample Types: CSF
-
Fecal microbiota transplantation in HIV: A pilot placebo-controlled study
Authors: S Serrano-Vi, A Talavera-R, MJ Gosalbes, N Madrid, JA Pérez-Moli, RJ Elliott, B Navia, VF Lanza, A Vallejo, M Osman, F Dronda, S Budree, J Zamora, C Gutiérrez, M Manzano, MJ Vivancos, R Ron, J Martínez-S, S Herrera, U Ansa, A Moya, S Moreno
Nature Communications, 2021-02-18;12(1):1139.
Species: Human
Sample Types: Plasma
-
Role of epidermal growth factor receptor inhibitor-induced interferon pathway signaling in the head and neck squamous cell carcinoma therapeutic response
Authors: SP Korpela, TK Hinz, A Oweida, J Kim, J Calhoun, R Ferris, RA Nemenoff, SD Karam, ET Clambey, LE Heasley
Journal of Translational Medicine, 2021-01-23;19(1):43.
Species: Human, Mouse
Sample Types: Cell Culture Supernates
-
Targeted Profiling of Immunological Genes during Norovirus Replication in Human Intestinal Enteroids
Authors: JCM Chan, KN Mohammad, LY Zhang, SH Wong, MC Chan
Viruses, 2021-01-21;13(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Prospective pilot trial of calcipotriene as a novel topical treatment for acute skin graft versus host disease
Authors: G Wallace, P Khandelwal, KC Myers, EMR Perentesis, A Lane, A Teusink-Cr, K Smiley, SM Davies, S Jodele
Bone marrow transplantation, 2021-01-08;0(0):.
Species: Human
Sample Types: Plasma
-
Visfatin Enhances Breast Cancer Progression through CXCL1 Induction in Tumor-Associated Macrophages
Authors: YY Wang, HD Chen, S Lo, YK Chen, YC Huang, SC Hu, YC Hsieh, AC Hung, MF Hou, SF Yuan
Cancers, 2020-11-26;12(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Nanoparticle-Mediated Delivery of 2-Deoxy-D-Glucose Induces Antitumor Immunity and Cytotoxicity in Liver Tumors in Mice
Authors: K Sasaki, S Nishina, A Yamauchi, K Fukuda, Y Hara, M Yamamura, K Egashira, K Hino
Cell Mol Gastroenterol Hepatol, 2020-10-24;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
In vitro model of perimenopausal depression implicates steroid metabolic and proinflammatory genes
Authors: S Rudzinskas, JF Hoffman, P Martinez, DR Rubinow, PJ Schmidt, D Goldman
Mol. Psychiatry, 2020-08-12;0(0):.
Species: Human
Sample Types: Plasma
-
Serum and CSF cytokine levels mirror different neuroimmunological mechanisms in patients with LGI1 and Caspr2 encephalitis
Authors: P Körtvelyes, A Goihl, K Guttek, B Schraven, H Prüss, D Reinhold
Cytokine, 2020-08-12;135(0):155226.
Species: Human
Sample Types: Serum
-
TLR7 dosage polymorphism shapes interferogenesis and HIV-1 acute viremia in women
Authors: P Azar, JE Mejía, C Cenac, A Shaiykova, A Youness, S Laffont, A Essat, J Izopet, C Passaes, M Müller-Tru, P Delobel, L Meyer, JC Guéry
JCI Insight, 2020-06-18;5(12):.
Species: Human
Sample Types: Plasma
-
DNA hypomethylation promotes transposable element expression and activation of immune signaling in renal cell cancer
Authors: AA de Cubas, W Dunker, A Zaninovich, RA Hongo, A Bhatia, A Panda, KE Beckermann, G Bhanot, S Ganesan, J Karijolich, WK Rathmell
JCI Insight, 2020-06-04;5(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Diminished hepatic IFN response following HCV clearance triggers HBV reactivation in coinfection
Authors: X Cheng, T Uchida, Y Xia, R Umarova, CJ Liu, PJ Chen, A Gaggar, V Suri, MM Mücke, J Vermehren, S Zeuzem, Y Teraoka, M Osawa, H Aikata, K Tsuji, N Mori, S Hige, Y Karino, M Imamura, K Chayama, TJ Liang
J. Clin. Invest., 2020-06-01;0(0):.
Species: Mouse
Sample Types: Serum
-
De novo design of functional zwitterionic biomimetic material for immunomodulation
Authors: B Li, Z Yuan, P Jain, HC Hung, Y He, X Lin, P McMullen, S Jiang
Sci Adv, 2020-05-29;6(22):eaba0754.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Gaps in Study Design for Immune Parameter Research for Latent Tuberculosis Infection: A Systematic Review
Authors: M Herrera, C Vera, Y Keynan, ZV Rueda
J Immunol Res, 2020-04-21;2020(0):8074183.
Species: Human
Sample Types: Plasma
-
Metastasis-initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs
Authors: M Pein, J Insua-Rodr, T Hongu, A Riedel, J Meier, L Wiedmann, K Decker, MAG Essers, HP Sinn, S Spaich, M Sütterlin, A Schneeweis, A Trumpp, T Oskarsson
Nat Commun, 2020-03-20;11(1):1494.
Species: Human
Sample Types: Cell culture supernate
-
Defining the landscape of ATP-competitive inhibitor resistance residues in protein kinases
Authors: NS Persky, D Hernandez, M Do Carmo, L Brenan, O Cohen, S Kitajima, U Nayar, A Walker, S Pantel, Y Lee, J Cordova, M Sathappa, C Zhu, TK Hayes, P Ram, P Pancholi, TS Mikkelsen, DA Barbie, X Yang, R Haq, F Piccioni, DE Root, CM Johannesse
Nat. Struct. Mol. Biol., 2020-01-10;27(1):92-104.
Species: Human
Sample Types: Cell Culture Supernates
-
Simian Immunodeficiency Virus Infection of Rhesus Macaques Results in Delayed Zika Virus Clearance
Authors: CL Vinton, SJ Magaziner, KA Dowd, SJ Robertson, E Amaro-Cara, EP Karmele, AM Ortiz, CE Starke, JC Mudd, SS Whitehead, SM Best, TC Pierson, HD Hickman, JM Brenchley
MBio, 2019-12-03;10(6):.
Species: Primate - Macacca Mulatta (Rhesus Macaque)
Sample Types: Plasma
-
Hydroxytyrosol Modulates Adipocyte Gene and miRNA Expression Under Inflammatory Condition
Authors: E Scoditti, S Carpi, M Massaro, M Pellegrino, B Polini, MA Carluccio, M Wabitsch, T Verri, P Nieri, R De Caterin
Nutrients, 2019-10-17;11(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
Fetal T Cell Activation in the Amniotic Cavity during Preterm Labor: A Potential Mechanism for a Subset of Idiopathic Preterm Birth
Authors: N Gomez-Lope, R Romero, Y Xu, D Miller, M Arenas-Her, V Garcia-Flo, B Panaitescu, J Galaz, CD Hsu, R Para, SM Berry
J. Immunol., 2019-09-06;0(0):.
Species: Human
Sample Types: Amniotic Fluid
-
Plasma levels of CRP, neopterin and IP-10 in HIV-infected individuals with and without pulmonary tuberculosis
Authors: F Ciccacci, M Floridia, R Bernardini, Z Sidumo, RJ Mugunhe, M Andreotti, A Passanduca, NA Magid, S Orlando, M Mattei, M Giuliano, S Mancinelli, MC Marazzi, L Palombi
J Clin Tuberc Other Mycobact Dis, 2019-06-05;16(0):100107.
Species: Human
Sample Types: Plasma
-
TLR9 polymorphism correlates with immune activation, CD4 decline and plasma IP10 levels in HIV patients
Authors: A Joshi, EB Punke, T Mehmetoglu, DP Peralta, H Garg
BMC Infect. Dis., 2019-01-16;19(1):56.
Species: Human
Sample Types: Plasma
-
Suppression of STING associated with LKB1 loss in KRAS-driven lung cancer
Authors: S Kitajima, E Ivanova, S Guo, R Yoshida, M Campisi, SK Sundararam, S Tange, Y Mitsuishi, TC Thai, S Masuda, BP Piel, LM Sholl, PT Kirschmeie, CP Paweletz, H Watanabe, M Yajima, DA Barbie
Cancer Discov, 2018-10-08;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Longitudinal transcriptomic characterization of the immune response to acute hepatitis C virus infection in patients with spontaneous viral clearance
Authors: BR Rosenberg, M Depla, CA Freije, D Gaucher, S Mazouz, M Boisvert, N Bédard, J Bruneau, CM Rice, NH Shoukry
PLoS Pathog., 2018-09-17;14(9):e1007290.
Species: Human
Sample Types: Plasma
-
HIV-1 proviral landscapes distinguish posttreatment controllers from noncontrollers
Authors: R Sharaf, GQ Lee, X Sun, B Etemad, LM Aboukhater, Z Hu, ZL Brumme, E Aga, RJ Bosch, Y Wen, G Namazi, C Gao, EP Acosta, RT Gandhi, JM Jacobson, D Skiest, DM Margolis, R Mitsuyasu, P Volberding, E Connick, DR Kuritzkes, MM Lederman, XG Yu, M Lichterfel, JZ Li
J. Clin. Invest., 2018-08-20;0(0):.
Species: Human
Sample Types: Plasma
-
Tumor innate immunity primed by specific interferon-stimulated endogenous retroviruses
Authors: I Cañadas, R Thummalapa, JW Kim, S Kitajima, RW Jenkins, CL Christense, M Campisi, Y Kuang, Y Zhang, E Gjini, G Zhang, T Tian, DR Sen, D Miao, Y Imamura, T Thai, B Piel, H Terai, AR Aref, T Hagan, S Koyama, M Watanabe, H Baba, AE Adeni, CA Lydon, P Tamayo, Z Wei, M Herlyn, TU Barbie, R Uppaluri, LM Sholl, E Sicinska, J Sands, S Rodig, KK Wong, CP Paweletz, H Watanabe, DA Barbie
Nat. Med., 2018-07-23;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Systemic DPP4 activity is reduced during primary HIV-1 infection and is associated with intestinal RORC+ CD4+ cell levels: a surrogate marker candidate of HIV-induced intestinal damage
Authors: MJ Ploquin, A Casrouge, Y Madec, N Noël, B Jacquelin, N Huot, D Duffy, SP Jochems, L Micci, C Lécuroux, F Boufassa, T Booiman, T Garcia-Tel, M Ghislain, RL Grand, O Lambotte, N Kootstra, L Meyer, C Goujard, M Paiardini, ML Albert, M Müller-Tru
J Int AIDS Soc, 2018-07-01;21(7):e25144.
Species: Human
Sample Types:
-
Novel Insights into Staphylococcus aureus Deep Bone Infections: the Involvement of Osteocytes
Authors: D Yang, AR Wijenayaka, LB Solomon, SM Pederson, DM Findlay, SP Kidd, GJ Atkins
MBio, 2018-04-24;9(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Disruption of XIAP-RIP2 Association Blocks NOD2-Mediated Inflammatory Signaling
Authors: T Goncharov, S Hedayati, MM Mulvihill, A Izrael-Tom, K Zobel, S Jeet, AV Fedorova, C Eidenschen, J deVoss, K Yu, AS Shaw, DS Kirkpatric, WJ Fairbrothe, K Deshayes, D Vucic
Mol. Cell, 2018-02-15;69(4):551-565.e7.
Species: Human
Sample Types: Cell Culture Supernates
-
Interferon-alpha treatment rapidly clears Hepatitis E virus infection in humanized mice
Authors: MDB van de Gar, SD Pas, GW van Oord, L Gama, Y Choi, RA de Man, A Boonstra, T Vanwollegh
Sci Rep, 2017-08-15;7(1):8267.
Species: Mouse
Sample Types: Serum
-
Impact of hepatitis B vaccination on HBsAg kinetics, interferon-inducible protein 10 level and recurrence of viremia
Authors: A Shaaban Ha
Cytokine, 2017-08-10;99(0):99-105.
Species: Human
Sample Types: Serum
-
Impact of early cART on HIV blood and semen compartments at the time of primary infection
Authors: A Chéret, C Durier, A Mélard, M Ploquin, J Heitzmann, C Lécuroux, V Avettand-F, L David, G Pialoux, JM Chennebaul, M Müller-Tru, C Goujard, C Rouzioux, L Meyer
PLoS ONE, 2017-07-14;12(7):e0180191.
Species: Human
Sample Types: Plasma
-
Changes in inflammatory biomarkers in HCV-infected patients undergoing direct acting antiviral-containing regimens with or without interferon
Authors: C Mascia, S Vita, P Zuccalà, R Marocco, T Tieghi, S Savinelli, R Rossi, M Iannetta, I Pozzetto, C Furlan, F Mengoni, CM Mastroiann, V Vullo, M Lichtner
PLoS ONE, 2017-06-21;12(6):e0179400.
Species: Human
Sample Types: Plasma
-
Development of a novel site-specific pegylated interferon beta for antiviral therapy for chronic hepatitis B
Authors: M Tsuge, T Uchida, N Hiraga, H Kan, GN Makokha, H Abe-Chayam, D Miki, M Imamura, H Ochi, CN Hayes, R Shimozono, T Iwamura, H Narumi, T Suzuki, M Kainoh, T Taniguchi, K Chayama
Antimicrob. Agents Chemother., 2017-05-24;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Plasma IP-10 Concentrations Correlate Positively with Viraemia and Inversely with CD4 Counts in Untreated HIV Infection
Authors: K Mhandire, T Mlambo, LS Zijenah, K Duri, K Mateveke, M Tshabalala, DZ Mhandire, C Musarurwa, PT Wekare, LR Mazengera, HT Matarira, B Stray-Pede
Open AIDS J, 2017-04-26;11(0):24-31.
Species: Human
Sample Types: Plasma
-
Salt suppresses IFN? inducible chemokines through the IFN?-JAK1-STAT1 signaling pathway in proximal tubular cells
Authors: Y Arai, D Takahashi, K Asano, M Tanaka, M Oda, SBH Ko, MSH Ko, S Mandai, N Nomura, T Rai, S Uchida, E Sohara
Sci Rep, 2017-04-20;7(0):46580.
Species: Human
Sample Types: Cell Culture Supernates
-
Thyroid function in children and adolescents with Hashimoto's thyroiditis after L-thyroxine discontinuation
Authors: G Radetti, M Salerno, C Guzzetti, M Cappa, A Corrias, A Cassio, G Cesaretti, R Gastaldi, M Rotondi, F Lupi, A Fanolla, G Weber, S Loche
Endocr Connect, 2017-03-27;0(0):.
Species: Human
Sample Types: Serum
-
The therapeutic HIV Env C5/gp41 vaccine candidate Vacc-C5 induces specific T cell regulation in a phase I/II clinical study
Authors: K Brekke, M Sommerfelt, M Ökvist, AM Dyrhol-Rii, D Kvale
BMC Infect. Dis, 2017-03-24;17(1):228.
Species: Human
Sample Types: Serum
-
Case-control, exploratory study of cerebrospinal fluid chemokines/cytokines and lymphocyte subsets in childhood Tourette syndrome with positive streptococcal markers
Authors: MR Pranzatell, ED Tate, TJ Allison
Cytokine, 2017-03-10;96(0):49-53.
Species: Human
Sample Types: CSF
-
Predictive Value of Serum IFN-? inducible Protein-10 and IFN-?/IL-4 Ratio for Liver Fibrosis Progression in CHB Patients
Authors: Y Wang, W Yu, C Shen, W Wang, L Zhang, F Liu, H Sun, Y Zhao, H Che, C Zhao
Sci Rep, 2017-01-09;7(0):40404.
Species: Human
Sample Types: Serum
-
Transcript-activated collagen matrix as sustained mRNA delivery system for bone regeneration
J Control Release, 2016-08-30;239(0):137-148.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated Basal Pre-infection CXCL10 in Plasma and in the Small Intestine after Infection Are Associated with More Rapid HIV/SIV Disease Onset
PLoS Pathog, 2016-08-10;12(8):e1005774.
Species: Human
Sample Types: Plasma
-
Randomized Trial Evaluating the Impact of Ribavirin Mono-Therapy and Double Dosing on Viral Kinetics, Ribavirin Pharmacokinetics and Anemia in Hepatitis C Virus Genotype 1 Infection
PLoS ONE, 2016-05-11;11(5):e0155142.
Species: Human
Sample Types: Plasma
-
Extracellular Histones Induce Chemokine Production in Whole Blood Ex Vivo and Leukocyte Recruitment In Vivo.
Authors: Westman J, Papareddy P, Dahlgren M, Chakrakodi B, Norrby-Teglund A, Smeds E, Linder A, Morgelin M, Johansson-Lindbom B, Egesten A, Herwald H
PLoS Pathog, 2015-12-08;11(12):e1005319.
Species: Human
Sample Types: Plasma
-
Left Ventricular Dysfunction and CXCR3 Ligands in Hypertension: From Animal Experiments to a Population-Based Pilot Study.
Authors: Altara R, Gu Y, Struijker-Boudier H, Thijs L, Staessen J, Blankesteijn W
PLoS ONE, 2015-10-27;10(10):e0141394.
Species: Human
Sample Types: Plasma
-
Small Ubiquitin-like Modifier Alters IFN Response.
Authors: Maarifi G, Maroui M, Dutrieux J, Dianoux L, Nisole S, Chelbi-Alix M
J Immunol, 2015-07-29;195(5):2312-24.
Species: Human
Sample Types: Cell Culture Supernates
-
Effective Apical Infection of Differentiated Human Bronchial Epithelial Cells and Induction of Proinflammatory Chemokines by the Highly Pneumotropic Human Adenovirus Type 14p1.
Authors: Lam E, Ramke M, Warnecke G, Schrepfer S, Kopfnagel V, Dobner T, Heim A
PLoS ONE, 2015-07-13;10(7):e0131201.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated plasma soluble CD14 and skewed CD16+ monocyte distribution persist despite normalisation of soluble CD163 and CXCL10 by effective HIV therapy: a changing paradigm for routine HIV laboratory monitoring?
Authors: Castley, Alison, Berry, Cassandr, French, Martyn, Fernandez, Sonia, Krueger, Romano, Nolan, David
PLoS ONE, 2014-12-29;9(12):e115226.
Species: Human
Sample Types: Plasma
-
Improving membrane based multiplex immunoassays for semi-quantitative detection of multiple cytokines in a single sample.
Authors: Altara, Raffaele, Manca, Marco, Hessel, Marleen, Janssen, Ben J, Struijker-Boudier, Harry H, Hermans, Rob J J, Blankesteijn, W Matthi
BMC Biotechnol, 2014-07-15;14(0):63.
Species: Human
Sample Types: Serum
-
Identification of selective small molecule inhibitors of the nucleotide-binding oligomerization domain 1 (NOD1) signaling pathway.
Authors: Rickard, David J, Sehon, Clark A, Kasparcova, Viera, Kallal, Lorena A, Haile, Pamela A, Zeng, Xin, Montoute, Monica N, Poore, Derek D, Li, Hu, Wu, Zining, Eidam, Patrick, Emery, John G, Marquis, Robert W, Gough, Peter J, Bertin, John
PLoS ONE, 2014-05-07;9(5):e96737.
Species: Human
Sample Types: Cell Culture Supernates
-
Tumor necrosis factor (TNF)-alpha induction of CXCL10 in endothelial cells requires protein arginine methyltransferase 5 (PRMT5)-mediated nuclear factor (NF)-kappaB p65 methylation.
Authors: Harris D, Bandyopadhyay S, Maxwell T, Willard B, DiCorleto P
J Biol Chem, 2014-04-21;289(22):15328-39.
Species: Human
Sample Types: Cell Culture Supernates
-
Randomized comparison of single dose of recombinant human IL-12 versus placebo for restoration of hematopoiesis and improved survival in rhesus monkeys exposed to lethal radiation.
Authors: Gluzman-Poltorak Z, Mendonca S, Vainstein V, Kha H, Basile L
J Hematol Oncol, 2014-04-06;7(0):31.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Plasma
-
Vitamin D levels vary during antiviral treatment but are unable to predict treatment outcome in HCV genotype 1 infected patients.
Authors: Grammatikos G, Lange C, Susser S, Schwendy S, Dikopoulos N, Buggisch P, Encke J, Teuber G, Goeser T, Thimme R, Klinker H, Boecher W, Schulte-Frohlinde E, Penna-Martinez M, Badenhoop K, Zeuzem S, Berg T, Sarrazin C
PLoS ONE, 2014-02-07;9(2):e87974.
Species: Human
Sample Types: Serum
-
Anti-interferon beta antibody titers strongly correlate between two bioassays and in vivo biomarker expression, and indicates that a titer of 150 TRU/mL is a biologically functional cut-point.
Authors: Hermanrud C, Ryner M, Engdahl E, Fogdell-Hahn A
J Interferon Cytokine Res, 2014-01-20;34(7):498-504.
Species: Human
Sample Types: Serum
-
CXCL10 gene promoter polymorphism -1447A>G correlates with plasma CXCL10 levels and is associated with male susceptibility to cerebral malaria.
Authors: Wilson N, Driss A, Solomon W, Dickinson-Copeland C, Salifu H, Jain V, Singh N, Stiles J
PLoS ONE, 2013-12-09;8(12):e81329.
Species: Human
Sample Types: Plasma
-
Increased CXCL9 serum levels in hepatitis C-related mixed cryoglobulinemia, with autoimmune thyroiditis, associated with high levels of CXCL10.
Authors: Antonelli A, Fallahi P, Ferrari S, Colaci M, Giuggioli D, Saraceno G, Benvenga S, Ferri C
J Interferon Cytokine Res, 2013-07-31;33(12):739-45.
Species: Human
Sample Types: Serum
-
Expression of CXCR3 and its ligands CXCL9, -10 and -11 in paediatric opsoclonus-myoclonus syndrome.
Authors: Pranzatelli M, Tate E, McGee N, Travelstead A, Verhulst S, Ransohoff R
Clin Exp Immunol, 2013-06-01;172(3):427-36.
Species: Human
Sample Types: Serum
-
BAFF/APRIL system in pediatric OMS: relation to severity, neuroinflammation, and immunotherapy.
Authors: Pranzatelli, Michael, Tate, Elizabet, McGee, Nathan R, Travelstead, Anna L, Colliver, Jerry A, Ness, Jayne M, Ransohoff, Richard
J Neuroinflammation, 2013-01-16;10(0):10.
Species: Human
Sample Types: Serum
-
Human trophectoderm apposition is regulated by interferon gamma-induced protein 10 (IP-10) during early implantation.
Authors: Sela H, Goldman-Wohl D, Haimov-Kochman R, Greenfield C, Natanson-Yaron S, Hamani Y, Revel A, Lavy Y, Singer O, Yachimovich-Cohen N, Turetsky T, Mandelboim O, Reubinoff B, Yagel S
Placenta, 2013-01-08;34(3):222-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Cleaved/associated TLR3 represents the primary form of the signaling receptor.
Authors: Toscano F, Estornes Y, Virard F, Garcia-Cattaneo A, Pierrot A, Vanbervliet B, Bonnin M, Ciancanelli M, Zhang S, Funami K, Seya T, Matsumoto M, Pin J, Casanova J, Renno T, Lebecque S
J Immunol, 2012-12-19;190(2):764-73.
Species: Human
Sample Types: Cell Culture Supernates
-
Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis.
Authors: Chaturvedi, Pallavi, Gilkes, Daniele, Wong, Carmen C, Luo, Weibo, Zhang, Huafeng, Wei, Hong, Takano, Naoharu, Schito, Luana, Levchenko, Andre, Semenza, Gregg L
J Clin Invest, 2012-12-17;123(1):189-205.
Species: Human
Sample Types: Cell Culture Supernates
-
USP18 is a key regulator of the interferon-driven gene network modulating pancreatic beta cell inflammation and apoptosis.
Authors: Santin I, Moore F, Grieco F, Marchetti P, Brancolini C, Eizirik D
Cell Death Dis, 2012-11-15;3(0):e419.
Species: Human
Sample Types: Cell Culture Supernates
-
Evaluation of IL-28B Polymorphisms and Serum IP-10 in Hepatitis C Infected Chimpanzees.
Authors: Verstrepen B, De Groot N, Groothuismink Z, Verschoor E, de Groen R, Bogers W, Janssen H, Mooij P, Bontrop R, Koopman G, Boonstra A
PLoS ONE, 2012-10-30;7(10):e46645.
Species: Human, Primate - Pan troglodytes (Chimpanzee)
Sample Types: Serum
-
A20/TNFAIP3 inhibits NF-kappaB activation induced by the Kaposi's sarcoma-associated herpesvirus vFLIP oncoprotein.
Authors: Sakakibara S, Espigol-Frigole G, Gasperini P, Uldrick T, Yarchoan R, Tosato G
Oncogene, 2012-04-23;32(10):1223-32.
Species: Human
Sample Types: Whole Cells
-
Circulating chemokine (CXC motif) ligand (CXCL)9 is increased in aggressive chronic autoimmune thyroiditis, in association with CXCL10.
Authors: Antonelli A, Ferrari SM, Frascerra S, Galetta F, Franzoni F, Corrado A, Miccoli M, Benvenga S, Paolicchi A, Ferrannini E, Fallahi P
Cytokine, 2011-05-20;55(2):288-93.
Species: Human
Sample Types: Serum
-
Human cytomegalovirus IE1 protein elicits a type II interferon-like host cell response that depends on activated STAT1 but not interferon-gamma.
Authors: Knoblach T, Grandel B, Seiler J, Nevels M, Paulus C
PLoS Pathog., 2011-04-14;7(4):e1002016.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-1beta, C-x-C motif ligand 10, and interferon-gamma serum levels in mixed cryoglobulinemia with or without autoimmune thyroiditis.
Authors: Antonelli A, Ferri C, Ferrari SM, De Marco S, Di Domenicantonio A, Centanni M, Pupilli C, Villa E, Menichetti F, Fallahi P
J. Interferon Cytokine Res., 2010-10-07;30(11):835-42.
Species: Human
Sample Types: Serum
-
Evidence for a pathophysiological role of keratinocyte-derived type III interferon (IFN-lambda) in cutaneous lupus erythematosus.
Authors: Zahn S, Rehkamper C, Kummerer BM, Ferring-Schmidt S, Bieber T, Tuting T, Wenzel J
J. Invest. Dermatol., 2010-08-19;131(1):133-40.
Species: Human
Sample Types: Cell Culture Supernates
-
Phagocytosis of apoptotic cells modulates mesenchymal stem cells osteogenic differentiation to enhance IL-17 and RANKL expression on CD4+ T cells.
Authors: Tso GH, Law HK, Tu W
Stem Cells, 2010-05-01;28(5):939-54.
Species: Human
Sample Types: Cell Culture Supernates
-
Cerebrospinal fluid in HIV-1 systemic viral controllers: absence of HIV-1 RNA and intrathecal inflammation.
Authors: Probasco JC, Deeks SG, Lee E, Hoh R, Hunt PW, Liegler T, Price RW, Spudich SS
AIDS, 2010-04-24;24(7):1001-5.
Species: Human
Sample Types: CSF
-
NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway.
Authors: Watanabe T, Asano N, Fichtner-Feigl S, Gorelick PL, Tsuji Y, Matsumoto Y, Chiba T, Fuss IJ, Kitani A, Strober W
J. Clin. Invest., 2010-04-12;120(5):1645-62.
Species: Human
Sample Types: Cell Culture Supernates
-
Synthesis and immunological activities of novel agonists of toll-like receptor 9.
Authors: Struthers M, Bett AJ, Wisniewski T, Dubey SA, Precopio M, Jiang W, Sun Z, Wang H, Nowak I, Putta MR, Yu D, Tang JX, Kandimalla ER, Agrawal S, Casimiro DR
Cell. Immunol., 2010-03-10;263(1):105-13.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Cell Culture Supernates
-
CD14-independent responses induced by a synthetic lipid A mimetic.
Authors: Legat A, Thomas S, Hermand P, Van Mechelen M, Goldman M, De Wit D
Eur J Immunol, 2010-03-01;40(3):797-802.
Species: Human
Sample Types: Cell Culture Supernates
-
Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome.
Authors: Yoon KC, Park CS, You IC, Choi HJ, Lee KH, Im SK, Park HY, Pflugfelder SC
Invest. Ophthalmol. Vis. Sci., 2009-10-22;51(2):643-50.
Species: Human
Sample Types: Tears
-
Serum IP-10 as a biomarker of human rhinovirus infection at exacerbation of COPD.
Authors: Quint JK, Donaldson GC, Goldring JJ, Baghai-Ravary R, Hurst JR, Wedzicha JA
Chest, 2009-10-16;137(4):812-22.
Species: Human
Sample Types: Serum
-
The imbalance in serum concentration of Th-1- and Th-2-derived chemokines as one of the factors involved in pathogenesis of atopic dermatitis.
Authors: Narbutt J, Lesiak A, Sysa-Jedrzeiowska A, Zakrzewski M, Bogaczewicz J, Stelmach I, Kuna P
Mediators Inflamm., 2009-07-22;2009(0):269541.
Species: Human
Sample Types: Serum
-
Phenotype of atopic dermatitis subjects with a history of eczema herpeticum.
Authors: Beck LA, Boguniewicz M, Hata T, Schneider LC, Hanifin J, Gallo R, Paller AS, Lieff S, Reese J, Zaccaro D, Milgrom H, Barnes KC, Leung DY
J. Allergy Clin. Immunol., 2009-06-27;124(2):260-9, 269.e1.
Species: Human
Sample Types: Serum
-
CXCL10 and CCL2 chemokine serum levels in patients with hepatitis C associated with autoimmune thyroiditis.
Authors: Antonelli A, Ferri C, Fallahi P, Ferrari SM, Frascerra S, Pampana A, Panicucci E, Carpi A, Nicolini A, Ferrannini E
J. Interferon Cytokine Res., 2009-06-01;29(6):345-51.
Species: Human
Sample Types: Serum
-
The major outer membrane protein of a periodontopathogen induces IFN-beta and IFN-stimulated genes in monocytes via lipid raft and TANK-binding kinase 1/IFN regulatory factor-3.
Authors: Lee SH, Kim JS, Jun HK
J. Immunol., 2009-05-01;182(9):5823-35.
Species: Human
Sample Types: Cell Culture Supernates
-
CXCR3 ligands are augmented during the pathogenesis of pulmonary sarcoidosis.
Authors: Busuttil A, Weigt SS, Keane MP, Xue YY, Palchevskiy V, Burdick MD, Huang C, Zisman DA, Fishbein M, Lynch JP, Strieter RM, Elashoff RM, Belperio JA
Eur. Respir. J., 2009-04-22;34(3):676-86.
Species: Human
Sample Types: BALF
-
Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease.
Authors: Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, Kim JS
J. Immunol., 2009-03-15;182(6):3919-27.
Species: Human
Sample Types: Plasma
-
Evidence for a pathogenetic role of interleukin-18 in cutaneous lupus erythematosus.
Authors: Wang D, Drenker M, Eiz-Vesper B, Werfel T, Wittmann M
Arthritis Rheum., 2008-10-01;58(10):3205-15.
Species: Human
Sample Types: Cell Culture Supernates
-
Urinary fractalkine is a marker of acute rejection.
Authors: Peng W, Chen J, Jiang Y, Wu J, Shou Z, He Q, Wang Y, Chen Y, Wang H
Kidney Int., 2008-09-17;74(11):1454-60.
Species: Human
Sample Types: Urine
-
Endometrial NK cells are special immature cells that await pregnancy.
Authors: Manaster I, Mizrahi S, Goldman-Wohl D, Sela HY, Stern-Ginossar N, Lankry D, Gruda R, Hurwitz A, Bdolah Y, Haimov-Kochman R, Yagel S, Mandelboim O
J. Immunol., 2008-08-01;181(3):1869-76.
Species: Human
Sample Types: Cell Culture Supernates
-
Chemokine production and chemokine receptor expression by human glioma cells: role of CXCL10 in tumour cell proliferation.
Authors: Maru SV, Holloway KA, Flynn G, Lancashire CL, Loughlin AJ, Male DK, Romero IA
J. Neuroimmunol., 2008-06-09;199(1):35-45.
Species: Human
Sample Types: Cell Culture Supernates
-
Agonists of proteinase-activated receptor-2 enhance IFN-gamma-inducible effects on human monocytes: role in influenza A infection.
Authors: Feld M, Shpacovitch VM, Ehrhardt C, Kerkhoff C, Hollenberg MD, Vergnolle N, Ludwig S, Steinhoff M
J. Immunol., 2008-05-15;180(10):6903-10.
Species: Human
Sample Types: Cell Culture Supernates
-
In vitro induction of a dendritic cell phenotype in primary human acute myelogenous leukemia (AML) blasts alters the chemokine release profile and increases the levels of T cell chemotactic CCL17 and CCL22.
Authors: Olsnes AM, Ryningen A, Ersvaer E, Bruserud Ø
J. Interferon Cytokine Res., 2008-05-01;28(5):297-310.
Species: Human
Sample Types: Cell Culture Supernates
-
The antibacterial chemokine MIG/CXCL9 is constitutively expressed in epithelial cells of the male urogenital tract and is present in seminal plasma.
Authors: Linge HM, Collin M, Giwercman A, Malm J, Bjartell A, Egesten A
J. Interferon Cytokine Res., 2008-03-01;28(3):191-6.
Species: Human
Sample Types: Seminal Plasma
-
Proteomics analysis of Hodgkin lymphoma: identification of new players involved in the cross-talk between HRS cells and infiltrating lymphocytes.
Authors: Ma Y, Visser L, Roelofsen H, de Vries M, Diepstra A, van Imhoff G, van der Wal T, Luinge M, Alvarez-Llamas G, Vos H, Poppema S, Vonk R, Van Den Berg A
Blood, 2007-12-10;111(4):2339-46.
Species: Human
Sample Types: Cell Culture Supernates
-
First in human phase I trial of 852A, a novel systemic toll-like receptor 7 agonist, to activate innate immune responses in patients with advanced cancer.
Authors: Dudek AZ, Yunis C, Harrison LI, Kumar S, Hawkinson R, Cooley S, Vasilakos JP, Gorski KS, Miller JS
Clin. Cancer Res., 2007-12-01;13(23):7119-25.
Species: Human
Sample Types: Serum
-
Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling.
Authors: Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F
Stem Cells, 2007-10-25;26(1):279-89.
Species: Human
Sample Types: Cell Culture Supernates
-
Polymorphisms in the chemokine (C-X-C motif) ligand 10 are associated with invasive aspergillosis after allogeneic stem-cell transplantation and influence CXCL10 expression in monocyte-derived dendritic cells.
Authors: Mezger M, Steffens M, Beyer M, Manger C, Eberle J, Toliat MR, Wienker TF, Ljungman P, Hebart H, Dornbusch HJ, Einsele H, Loeffler J
Blood, 2007-10-23;111(2):534-6.
Species: Human
Sample Types: Serum
-
Functional diversity of T-cell subpopulations in subacute and chronic hypersensitivity pneumonitis.
Authors: Barrera L, Mendoza F, Zuniga J, Estrada A, Zamora AC, Melendro EI, Ramirez R, Pardo A, Selman M
Am. J. Respir. Crit. Care Med., 2007-10-18;177(1):44-55.
Species: Human
Sample Types: BALF
-
CXCR3 and CCR5 chemokines in induced sputum from patients with COPD.
Authors: Costa C, Rufino R, Traves SL, Lapa E Silva JR, Barnes PJ, Donnelly LE
Chest, 2007-10-09;133(1):26-33.
Species: Human
Sample Types: Saliva
-
The alarm anti-protease, secretory leukocyte protease inhibitor, is a proliferation and survival factor for ovarian cancer cells.
Authors: Simpkins FA, Devoogdt NM, Rasool N, Tchabo NE, Alejandro EU, Kamrava MM, Kohn EC
Carcinogenesis, 2007-10-04;29(3):466-72.
Species: Human
Sample Types: Cell Culture Supernates
-
Phase 2 study of pegylated liposomal doxorubicin in combination with interleukin-12 for AIDS-related Kaposi sarcoma.
Authors: Little RF, Aleman K, Kumar P, Wyvill KM, Pluda JM, Read-Connole E, Wang V, Pittaluga S, Catanzaro AT, Steinberg SM, Yarchoan R
Blood, 2007-09-10;110(13):4165-71.
Species: Human
Sample Types: Serum
-
Th1/Th2 cytokines reciprocally regulate in vitro pulmonary angiogenesis via CXC chemokine synthesis.
Authors: Matsuda A, Fukuda S, Matsumoto K, Saito H
Am. J. Respir. Cell Mol. Biol., 2007-08-20;38(2):168-75.
Species: Human
Sample Types: Cell Culture Supernates
-
Airway dendritic cell phenotypes in inflammatory diseases of the human lung.
Authors: Lommatzsch M, Bratke K, Bier A, Julius P, Kuepper M, Luttmann W, Virchow JC
Eur. Respir. J., 2007-07-11;30(5):878-86.
Species: Human
Sample Types: BALF
-
Serum interleukin-18 and soluble tumour necrosis factor receptor 2 are associated with disease severity in patients with paracoccidioidomycosis.
Authors: Corvino CL, Mamoni RL, Fagundes GZ, Blotta MH
Clin. Exp. Immunol., 2007-03-01;147(3):483-90.
Species: Human
Sample Types: Serum
-
Chemokines CXCL10 and CXCL11 in the cerebrospinal fluid of patients with tick-borne encephalitis.
Authors: Lepej SZ, Misic-Majerus L, Jeren T, Rode OD, Remenar A, Sporec V, Vince A
Acta Neurol. Scand., 2007-02-01;115(2):109-14.
Species: Human
Sample Types: Serum
-
Prolactin enhances interferon-gamma-induced production of CXC ligand 9 (CXCL9), CXCL10, and CXCL11 in human keratinocytes.
Authors: Kanda N, Watanabe S
Endocrinology, 2007-01-25;148(5):2317-25.
Species: Human
Sample Types: Cell Culture Supernates
-
IFN-gamma alters the response of Borrelia burgdorferi-activated endothelium to favor chronic inflammation.
Authors: Dame TM, Orenzoff BL, Palmer LE, Furie MB
J. Immunol., 2007-01-15;178(2):1172-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Different angiogenic activity in pulmonary sarcoidosis and idiopathic pulmonary fibrosis.
Authors: Antoniou KM, Tzouvelekis A, Alexandrakis MG, Sfiridaki K, Tsiligianni I, Rachiotis G, Tzanakis N, Bouros D, Milic-Emili J, Siafakas NM
Chest, 2006-10-01;130(4):982-8.
Species: Human
Sample Types: BALF
-
Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer.
Authors: Datta D, Flaxenburg JA, Laxmanan S, Geehan C, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S
Cancer Res., 2006-10-01;66(19):9509-18.
Species: Human
Sample Types: Cell Culture Supernates
-
Induction of dendritic cell maturation by pertussis toxin and its B subunit differentially initiate Toll-like receptor 4-dependent signal transduction pathways.
Authors: Wang ZY, Yang D, Chen Q, Leifer CA, Segal DM, Su SB, Caspi RR, Howard ZO, Oppenheim JJ
Exp. Hematol., 2006-08-01;34(8):1115-24.
Species: Human
Sample Types: Cell Culture Supernates
-
The apoptotic-cell receptor CR3, but not alphavbeta5, is a regulator of human dendritic-cell immunostimulatory function.
Authors: Skoberne M, Somersan S, Almodovar W, Truong T, Petrova K, Henson PM, Bhardwaj N
Blood, 2006-04-13;108(3):947-55.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis.
Authors: Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H
J. Immunol., 2005-11-01;175(9):6177-89.
Species: Human
Sample Types: Cell Culture Supernates
-
CpG oligonucleotides induce strong humoral but only weak CD4+ T cell responses to protein antigens in rhesus macaques in vivo.
Authors: Hartmann G, Marschner A, Viveros PR, Stahl-Hennig C, Eisenblatter M, Suh YS, Endres S, Tenner-Racz K, Uberla K, Racz P, Steinman RM, Ignatius R
Vaccine, 2005-05-09;23(25):3310-7.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Plasma
-
Deoxycytidyl-deoxyguanosine oligonucleotide classes A, B, and C induce distinct cytokine gene expression patterns in rhesus monkey peripheral blood mononuclear cells and distinct alpha interferon responses in TLR9-expressing rhesus monkey plasmacytoid dendritic cells.
Authors: Abel K, Wang Y, Fritts L, Sanchez E, Chung E, Fitzgerald-Bocarsly P, Krieg AM, Miller CJ
Clin. Diagn. Lab. Immunol., 2005-05-01;12(5):606-21.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Cell Culture Supernates
-
Increased expression of CXCR3 and CCR5 on memory CD4+ T-cells migrating into the cerebrospinal fluid of patients with neuroborreliosis: the role of CXCL10 and CXCL11.
Authors: Lepej SZ, Rode OD, Jeren T, Vince A, Remenar A, Barsic B
J. Neuroimmunol., 2005-04-19;163(1):128-34.
Species: Human
Sample Types: Serum
-
Simian human immunodeficiency virus-associated pneumonia correlates with increased expression of MCP-1, CXCL10, and viral RNA in the lungs of rhesus macaques.
Authors: Sui Y, Li S, Pinson D, Adany I, Li Z, Villinger F, Narayan O, Buch S
Am. J. Pathol., 2005-02-01;166(2):355-65.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Tissue Homogenates
-
CXCR3 and CCR5 positive T-cell recruitment in acute human renal allograft rejection.
Authors: Panzer U, Reinking RR, Steinmetz OM, Zahner G, Sudbeck U, Fehr S, Pfalzer B, Schneider A, Thaiss F, Mack M, Conrad S, Huland H, Helmchen U, Stahl RA
Transplantation, 2004-11-15;78(9):1341-50.
Species: Human
Sample Types: Urine
-
Involvement of the CXCL12/CXCR4 pathway in the advanced liver disease that is associated with hepatitis C virus or hepatitis B virus.
Authors: Wald O, Pappo O, Safadi R, Dagan-Berger M, Beider K, Wald H, Franitza S, Weiss I, Avniel S, Boaz P, Hanna J, Zamir G, Eid A, Mandelboim O, Spengler U, Galun E, Peled A
Eur. J. Immunol., 2004-04-01;34(4):1164-74.
Species: Human
Sample Types: Plasma
-
Enhanced generation of helper T type 1 and 2 chemokines in allergen-induced asthma.
Authors: Liu L, Jarjour NN, Busse WW, Kelly EA
Am. J. Respir. Crit. Care Med., 2004-03-04;169(10):1118-24.
Species: Human
Sample Types: BALF
-
Helper T-lymphocyte-related chemokines in healthy newborns.
Authors: Leung TF, Ng PC, Tam WH, Li CY, Wong E, Ma TP, Lam CW, Fok TF
Pediatr. Res., 2003-11-19;55(2):334-8.
Species: Human
Sample Types: Serum
-
Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS.
Authors: Giunti D, Borsellino G, Benelli R, Marchese M, Capello E, Valle MT, Pedemonte E, Noonan D, Albini A, Bernardi G, Mancardi GL, Battistini L, Uccelli A
J. Leukoc. Biol., 2003-05-01;73(5):584-90.
Species: Human
Sample Types: CSF
-
TNFalpha-induced macrophage chemokine secretion is more dependent on NF-kappaB expression than lipopolysaccharides-induced macrophage chemokine secretion.
Authors: Ciesielski CJ, Andreakos E, Foxwell BM, Feldmann M
Eur. J. Immunol., 2002-07-01;32(7):2037-45.
Species: Human
Sample Types: Cell Culture Supernates
-
Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation.
Authors: Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA
Immunity, 2001-06-01;14(6):705-14.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human CXCL10/IP-10 Quantikine ELISA Kit
Average Rating: 4.4 (Based on 12 Reviews)
Have you used Human CXCL10/IP-10 Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Can detect in human plasma at 10-fold and in human urine at 5-fold.
IP-10 Quantikine assay works better than in the Luminex panel
I used for the mouse samples since it had 60% similarity with human but didnot detect any protein of interest. However, the exoeriment well ok with nice standard curve