Human ADAMTS4 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human A Disintegrin and Metalloproteinase with Thrombospondin Motifs 4 (ADAMTS4). The suggested diluent is suitable for the analysis of most cell culture supernate, serum, and plasma samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
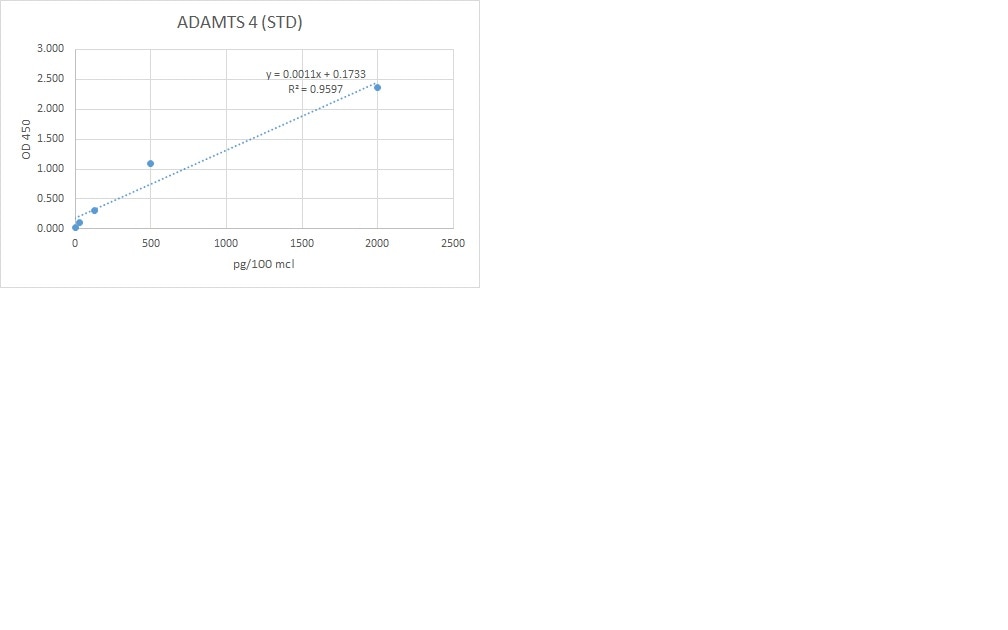
Scientific Data
Product Datasheets
Preparation and Storage
Background: ADAMTS4
Members of the ADAMTS family contain pro, catalytic, disintegrin-like, cysteine-rich, spacer and a variable number of TS type 1 motifs. In contrast to membrane-anchored ADAMs, ADAMTSs are secreted and are all active proteases. They are essential for aggrecan turnover and procollagen processing, regulation of which is important in many physiological and pathological processes such as angiogenesis and arthritis.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human ADAMTS4 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
11
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Catabolic mediators from TLR2-mediated proteoglycan aggrecan peptide-stimulated chondrocytes are reduced by Lactobacillus-conditioned media
Authors: Sengprasert, P;Waitayangkoon, P;Kamenkit, O;Sawatpanich, A;Chaichana, T;Wongphoom, J;Ngarmukos, S;Taweevisit, M;Lotinun, S;Tumwasorn, S;Tanavalee, A;Reantragoon, R;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates, Synovial Fluid
-
SARS-CoV-2 infection results in immune responses in the respiratory tract and peripheral blood that suggest mechanisms of disease severity
Authors: W Zhang, BY Chua, KJ Selva, L Kedzierski, TM Ashhurst, ER Haycroft, SK Shoffner-B, L Hensen, DF Boyd, F James, E Mouhtouris, JC Kwong, KYL Chua, G Drewett, A Copaescu, JE Dobson, LC Rowntree, JR Habel, LF Allen, HF Koay, JA Neil, MJ Gartner, CY Lee, P Andersson, SF Khan, L Blakeway, J Wisniewski, JH McMahon, EE Vine, AL Cunningham, J Audsley, I Thevarajan, T Seemann, NL Sherry, F Amanat, F Krammer, SL Londrigan, LM Wakim, NJC King, DI Godfrey, LK Mackay, PG Thomas, S Nicholson, KB Arnold, AW Chung, NE Holmes, OC Smibert, JA Trubiano, CL Gordon, THO Nguyen, K Kedzierska
Nature Communications, 2022-05-19;13(1):2774.
Species: Human
Sample Types: Plasma
-
Immune responses in COVID-19 respiratory tract and blood reveal mechanisms of disease severity
Authors: W Zhang, B Chua, K Selva, L Kedzierski, T Ashhurst, E Haycroft, S Shoffner, L Hensen, D Boyd, F James, E Mouhtouris, J Kwong, K Chua, G Drewett, A Copaescu, J Dobson, L Rowntree, J Habel, L Allen, HF Koay, J Neil, M Gartner, C Lee, P Andersson, T Seemann, N Sherry, F Amanat, F Krammer, S Londrigan, L Wakim, N King, D Godfrey, L Mackay, P Thomas, S Nicholson, K Arnold, A Chung, N Holmes, O Smibert, J Trubiano, C Gordon, T Nguyen, K Kedzierska
Research square, 2021-08-26;0(0):.
Species: Human
Sample Types: Plasma
-
Exuberant fibroblast activity compromises lung function via ADAMTS4
Authors: DF Boyd, EK Allen, AG Randolph, XJ Guo, Y Weng, CJ Sanders, R Bajrachary, NK Lee, CS Guy, P Vogel, W Guan, Y Li, X Liu, T Novak, MM Newhams, TP Fabrizio, N Wohlgemuth, PM Mourani, PALISI Ped, TN Wight, S Schultz-Ch, SA Cormier, K Shaw-Salib, A Pekosz, RE Rothman, KF Chen, Z Yang, RJ Webby, N Zhong, JC Crawford, PG Thomas
Nature, 2020-10-28;0(0):.
Species: Mouse
Sample Types: BALF
-
Concerted actions by MMPs, ADAMTS and serine proteases during remodeling of the cartilage callus into bone during osseointegration of hip implants
Authors: J Cassuto, A Folestad, J Göthlin, H Malchau, J Kärrholm
Bone Rep, 2020-09-11;13(0):100715.
Species: Human
Sample Types: Plasma
-
Lentinan Inhibits AGE-Induced Inflammation and the Expression of Matrix-Degrading Enzymes in Human Chondrocytes
Authors: Z Zhang, Z Zha, Z Zhao, W Liu, W Li
Drug Des Devel Ther, 2020-07-17;14(0):2819-2829.
Species: Human
Sample Types: Cell Culture Supernates
-
Differentiation of adipose-derived stem cells into functional chondrocytes by a small molecule that induces Sox9
Authors: J Lee, CY Lee, JH Park, HH Seo, S Shin, BW Song, IK Kim, SW Kim, S Lee, JC Park, S Lim, KC Hwang
Exp. Mol. Med., 2020-04-21;0(0):.
Species: Human
Sample Types: Cell Culture Superantes
-
Knockdown of TRIM8 Attenuates IL-1beta-induced Inflammatory Response in Osteoarthritis Chondrocytes Through the Inactivation of NF-kappaB Pathway
Authors: R Liu, H Wu, H Song
Cell Transplant, 2020-01-01;29(0):9636897209436.
Species: Human
Sample Types: Cell Culture Supernates
-
Photobiomodulation of extracellular matrix enzymes in human nucleus pulposus cells as a potential treatment for intervertebral disk degeneration
Authors: MH Hwang, HG Son, JW Lee, CM Yoo, JH Shin, HG Nam, HJ Lim, SM Baek, JH Park, JH Kim, H Choi
Sci Rep, 2018-08-03;8(1):11654.
Species: Human
Sample Types: Cell Culture Supernates
-
Promotion of Tumor Growth by ADAMTS4 in Colorectal Cancer: Focused on Macrophages
Authors: J Chen, Y Luo, Y Zhou, S Qin, Y Qiu, R Cui, M Yu, J Qin, M Zhong
Cell. Physiol. Biochem., 2018-04-20;46(4):1693-1703.
Species: Human, Mouse
Sample Types: Cell Culture Supernates
-
Protein Array-Based Detection of Proteins in Kidney Tissues from Patients with Membranous Nephropathy
Authors: S Wang, Y Lu, Q Hong, X Geng, X Wang, W Zheng, C Song, C Liu, M Fan, Y Xi, M Guo, D Wu
Biomed Res Int, 2017-02-27;2017(0):7843584.
Species: Human
Sample Types: Tissue Homogenates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human ADAMTS4 DuoSet ELISA
Average Rating: 3 (Based on 1 Review)
Have you used Human ADAMTS4 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: