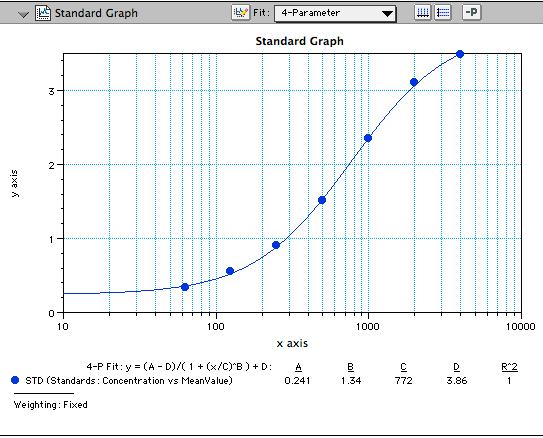
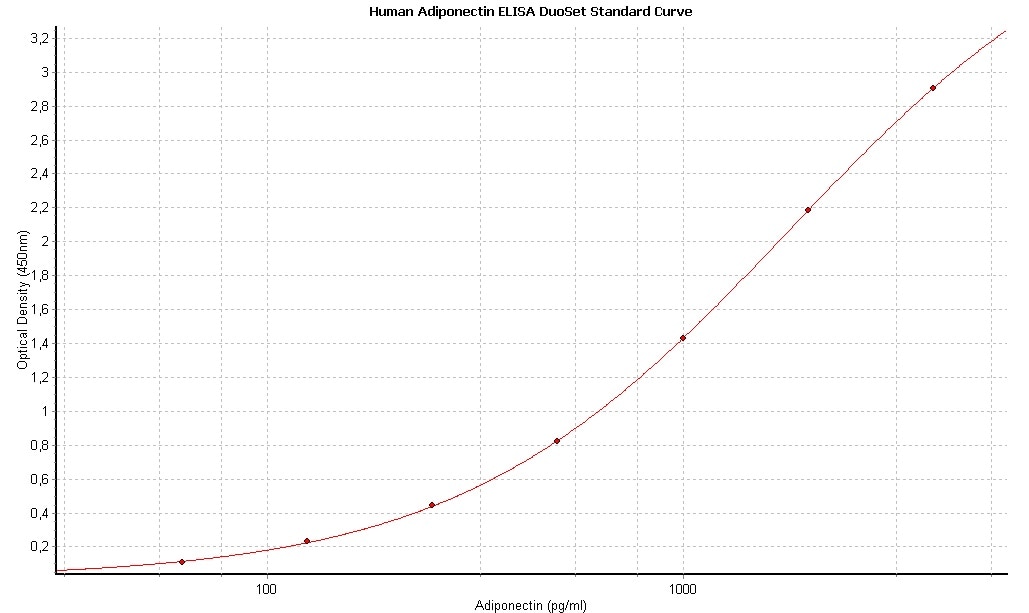
Human Adiponectin/Acrp30 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or equivalent
Reagent Diluent: 1% BSA in PBS, pH 7.2 - 7.4, 0.2 m filtered (R&D Systems Catalog # DY995). Quality of BSA is critical (see Technical Hints).
Blocking Buffer: 1% BSA in PBS, pH 7.2 - 7.4, 0.2 m filtered (R&D Systems Catalog # DY995). Quality of BSA is critical (see Technical Hints).
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990), or equivalent
Plate Sealers: ELISA Plate Sealers (Catalog # DY992), or equivalent
Scientific Data
Product Datasheets
Preparation and Storage
Background: Adiponectin/Acrp30
Adiponectin (also known as Adipocyte complement-related protein of 30 kDa, Acrp30) is an adipocyte-derived protein with wide ranging paracrine and endocrine effects on metabolism and inflammation. It promotes adipocyte differentiation, fatty acid catabolism, and insulin sensitivity and is negatively correlated with obesity, type 2 diabetes, and atherogenesis. Adiponectin associates into 70 kDa noncovalently-linked trimers that are disulfide-linked into medium molecular weight hexamers and then into >300 kDa high molecular weight oligomers.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody (to the working concentration stated in the product datasheet ) in PBS without carrier protein. Immediately coat a 96-well microplate with 100 µL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 µL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block each well of the microplate as recommended in the product datasheet. Incubate at room temperature for a minimum of 1 hour.
Note: The recommended Reagent Diluent typically contains 1% BSA. Some DuoSet Development Kits require alternative blocking agents, or for plates to be blocked overnight with a higher percentage of BSA, please see the product datasheet for details.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
PRECAUTION
The Stop Solution suggested for use with this kit is an acid solution. Wear eye, hand, face and clothing protection when using this material.
Assay Procedure
- Add 100 µL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 µL of the Detection Antibody, diluted in Reagent Diluent (as recommended in the product datasheet), to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 µL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 µL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 µL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human Adiponectin/Acrp30 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
69
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Serum Adiponectin Predicts COVID-19 Severity
Authors: Pavel, V;Räth, U;Schmid, S;Krautbauer, S;Keller, D;Amend, P;Müller, M;Mester, P;Buechler, C;
Biomedicines
Species: Human
Sample Types: Serum
-
Cognitive function is associated with performance in time up and go test and with leptin blood levels in community-dwelling older women
Authors: da Costa Teixeira, LA;Soares, LA;Lima, LP;Avelar, NCP;de Moura, JA;Leopoldino, AAO;Figueiredo, PHS;Parentoni, AN;Mendonça, VA;Lacerda, ACR;
Scientific reports
Species: Human
Sample Types: Plasma
-
Trauma-associated extracellular histones mediate inflammation via a MYD88-IRAK1-ERK signaling axis and induce lytic cell death in human adipocytes
Authors: Roos, J;Zinngrebe, J;Huber-Lang, M;Lupu, L;Schmidt, MA;Strobel, H;Westhoff, MA;Stifel, U;Gebhard, F;Wabitsch, M;Mollnes, TE;Debatin, KM;Halbgebauer, R;Fischer-Posovszky, P;
Cell death & disease
Species: Human
Sample Types: Cell Culture Supernates
-
The strong inverse association between plasma concentrations of soluble tumor necrosis factor receptors type 1 with adiponectin/leptin ratio in older women
Authors: Augusto da Costa Teixeira, L;Rocha-Vieira, E;Aparecida Soares, L;Mota de Oliveira, F;Aparecida Oliveira Leopoldino, A;Netto Parentoni, A;Amaral Mendonça, V;Cristina Rodrigues Lacerda, A;
Cytokine
Species: Human
Sample Types: Plasma
-
Circulating Adipocytokines and Insulin Like-Growth Factors and Their Modulation in Obesity-Associated Endometrial Cancer
Authors: Ray, I;Möller-Levet, CS;Michael, A;Butler-Manuel, S;Chatterjee, J;Tailor, A;Ellis, PE;Meira, LB;
Cancers
Species: Human
Sample Types: Plasma
-
Relationships between Circulating Biomarkers and Body Composition Parameters in Patients with Metabolic Syndrome: A Community-Based Study
Authors: Tarabeih, N;Kalinkovich, A;Ashkenazi, S;Cherny, SS;Shalata, A;Livshits, G;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Extracellular vesicles are carriers of adiponectin with insulin-sensitizing and anti-inflammatory properties
Authors: Blandin, A;Amosse, J;Froger, J;Hilairet, G;Durcin, M;Fizanne, L;Ghesquière, V;Prieur, X;Chaigneau, J;Vergori, L;Dray, C;Pradère, JP;Blandin, S;Dupont, J;Ducluzeau, PH;Dubois, S;Boursier, J;Cariou, B;Le Lay, S;
Cell reports
Species: Human
Sample Types: Plasma
-
Dysregulation of adipokines levels among healthy first-degree relatives of type 2 diabetes patients
Authors: Purnamasari, D;Simanjuntak, CK;Tricaesario, C;Tahapary, DL;Harbuwono, DS;Yunir, E;
Heliyon
Species: Human
Sample Types: Serum
-
Effects of an Intervention with Selenium and Coenzyme Q10 on Five Selected Age-Related Biomarkers in Elderly Swedes Low in Selenium: Results That Point to an Anti-Ageing Effect-A Sub-Analysis of a Previous Prospective Double-Blind Placebo-Controlled Randomised Clinical Trial
Authors: Alehagen, U;Alexander, J;Aaseth, JO;Larsson, A;Svensson, E;Opstad, TB;
Cells
Species: Human
Sample Types: Plasma
-
Inflammatory biomarkers at different stages of Sarcopenia in older women
Authors: da Costa Teixeira, LA;Avelar, NCP;Peixoto, MFD;Parentoni, AN;Santos, JMD;Pereira, FSM;Danielewicz, AL;Leopoldino, AAO;Costa, SP;Arrieiro, AN;Soares, LA;da Silva Lage, VK;Prates, ACN;Taiar, R;de Carvalho Bastone, A;Oliveira, VC;Oliveira, MX;Costa, HS;Nobre, JNP;Brant, FP;Duarte, TC;Figueiredo, PHS;Mendonça, VA;Lacerda, ACR;
Scientific reports
Species: Human
Sample Types: Plasma
-
Increased plasma lipopolysaccharide-binding protein and altered inflammatory mediators in overweight women suggest a state of subclinical endotoxemia
Authors: Metz, CN;Xue, X;Chatterjee, PK;Adelson, R;Brines, M;Tracey, KJ;Gregersen, PK;Pavlov, VA;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Plasma
-
High Glucose Promotes Inflammation and Weakens Placental Defenses against E. coli and S. agalactiae Infection: Protective Role of Insulin and Metformin
Authors: R Jiménez-Es, D Vargas-Alc, P Flores-Esp, AC Helguera-R, O Villavicen, I Mancilla-H, C Irles, YD Torres-Ram, MY Valdespino, P Velázquez-, R Zamora-Esc, M Islas-Lópe, C Carranco-S, L Díaz, V Zaga-Clave, A Olmos-Orti
International Journal of Molecular Sciences, 2023-03-09;24(6):.
Species: Human
Sample Types: Placental Supernates
-
Obesity promotes radioresistance through SERPINE1-mediated aggressiveness and DNA repair of triple-negative breast cancer
Authors: YH Su, YZ Wu, DK Ann, JL Chen, CY Kuo
Cell Death & Disease, 2023-01-21;14(1):53.
Species: Human
Sample Types: Cell Culture Supernates
-
Adiponectin Is a Contributing Factor of Low Appendicular Lean Mass in Older Community-Dwelling Women: A Cross-Sectional Study
Authors: LAC Teixeira, JM Dos Santos, AN Parentoni, LP Lima, TC Duarte, FP Brant, CDC Neves, FSM Pereira, NCP Avelar, AL Danielewic, AAO Leopoldino, SP Costa, AN Arrieiro, LA Soares, ACN Prates, JNP Nobre, A de Carvalh, VC de Oliveir, MX Oliveira, PH Scheidt Fi, HS Costa, V Amaral Men, R Taiar, AC Rodrigues
Journal of Clinical Medicine, 2022-12-02;11(23):.
Species: Human
Sample Types: Plasma
-
Blood orange juice intake modulates plasma and PBMC microRNA expression in overweight and insulin resistance women: impact on MAPK and NFkappaB signaling pathways
Authors: VC Capetini, BJ Quintanilh, DC de Oliveir, AH Nishioka, LA de Matos, LRP Ferreira, FM Ferreira, GR Sampaio, NMA Hassimotto, FM Lajolo, RA Fock, MM Rogero
The Journal of nutritional biochemistry, 2022-11-26;0(0):109240.
Species: Human
Sample Types: Plasma
-
Exercise training-induced changes in immunometabolic markers in youth badminton athletes
Authors: FE Rossi, AJ Maldonado, JM Cholewa, SLG Ribeiro, CA de Araújo, C Figueiredo, T Reichel, K Krüger, FS Lira, LG Minuzzi
Scientific Reports, 2022-09-15;12(1):15539.
Species: Human
Sample Types: Plasma
-
Combined resistance and aerobic training improves lung function and mechanics and fibrotic biomarkers in overweight and obese women
Authors: A Silva-Reis, MA Rodrigues, R Moraes-Fer, TG Gonçalves-, VH Souza-Palm, HC Aquino-San, ALL Bachi, LVF de Oliveir, RÁB Lopes-Mart, I Oliveira-S, R Albertini, CR Frison, RP Vieira
Frontiers in Physiology, 2022-09-07;13(0):946402.
Species: Human
Sample Types: Plasma
-
Regulation of Adipose Progenitor Cell Expansion in a Novel Micro-Physiological Model of Human Adipose Tissue Mimicking Fibrotic and Pro-Inflammatory Microenvironments
Authors: V Dani, S Bruni-Favi, B Chignon-Si, A Loubat, A Doglio, C Dani
Cells, 2022-09-07;11(18):.
Species: Human
Sample Types: Cell Culture Supernates
-
Di-(2-ethylhexyl) phthalate substitutes accelerate human adipogenesis through PPARgamma activation and cause oxidative stress and impaired metabolic homeostasis in mature adipocytes
Authors: A Schaffert, I Karkossa, E Ueberham, R Schlichtin, K Walter, J Arnold, M Blüher, JT Heiker, J Lehmann, M Wabitsch, BI Escher, M von Bergen, K Schubert
Environment international, 2022-05-06;164(0):107279.
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of transdermal gender-affirming hormone therapy on markers of inflammation and hemostasis
Authors: MH Schutte, R Kleemann, NM Nota, CM Wiepjes, JM Snabel, G T'Sjoen, A Thijs, M den Heijer
PLoS ONE, 2022-03-15;17(3):e0261312.
Species: Human
Sample Types: Plasma
-
Transcriptional and DNA Methylation Signatures of Subcutaneous Adipose Tissue and Adipose-Derived Stem Cells in PCOS Women
Authors: A Divoux, E Erdos, K Whytock, TF Osborne, SR Smith
Cells, 2022-03-01;11(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Pre-Transplant Serum Leptin Levels and Relapse of Acute Myeloid Leukemia after Allogeneic Transplantation
Authors: MA Schwarzbic, H Dai, L Kordelas, DW Beelen, A Radujkovic, C Müller-Tid, P Dreger, T Luft
International Journal of Molecular Sciences, 2022-02-20;23(4):.
Species: Human
Sample Types: Serum
-
Transient Agarose Spot (TAS) Assay: A New Method to Investigate Cell Migration
Authors: A Veres-Szék, D Pap, B Szebeni, L ?rfi, C Szász, C Pajtók, E Lévai, AJ Szabó, Á Vannay
International Journal of Molecular Sciences, 2022-02-14;23(4):.
Species: Human
Sample Types: Serum
-
Circulating Interleukin-6 and CD16 positive monocytes increase following angioplasty of an arteriovenous fistula
Authors: S Hakki, EJ Robinson, MG Robson
Scientific Reports, 2022-01-26;12(1):1427.
Species: Human
Sample Types: Plasma
-
Adiponectin and Stnfr2 peripheral levels are associated with cardiovascular risk in patients with schizophrenia
Authors: ICS Dias, SM de Campos-, ELM Vieira, APL Mota, PS Azevedo, VTDS Anício, FC Guimarães, LM Mantovani, BF Cruz, AL Teixeira, JV Salgado
Journal of psychiatric research, 2021-11-09;0(0):.
Species: Human
Sample Types: Serum
-
Gestational Weight Gain Influences the Adipokine-Oxidative Stress Association during Pregnancy
Authors: JM Solis Pare, O Perichart, A Montoya Es, E Reyes Muño, S Espino Y S, V Ortega Cas, D Medina Bas, M Tolentino, M Sanchez Ma, S Nava Salaz, G Estrada Gu
Obesity facts, 2021-09-14;0(0):1-9.
Species: Human
Sample Types: Serum
-
Altered cardiometabolic profile in girls with central precocious puberty and adipokines: A propensity score matching analysis
Authors: JN Zurita-Cru, MA Villasís-K, L Manuel-Apo, L Damasio-Sa, A Gutierrez-, G Wakida-Kus, M Padilla-Ro, C Maldonado-, E Garrido-Ma, AJ Rivera-Her, E Nishimura-
Cytokine, 2021-07-30;0(0):155660.
Species: Human
Sample Types: Serum
-
The dynamics of human bone marrow adipose tissue in response to feeding and fasting
Authors: PK Fazeli, MA Bredella, OG Pachon-Peñ, W Zhao, X Zhang, AT Faje, M Resulaj, SP Polineni, TM Holmes, H Lee, EK O'Donnell, OA MacDougald, MC Horowitz, CJ Rosen, A Klibanski
JCI Insight, 2021-06-22;0(0):.
Species: Human
Sample Types: Serum
-
Predictors of Early-Recurrence Atrial Fibrillation after Catheter Ablation in Women and Men with Abnormal Body Weight
Authors: J Budzianows, J Hiczkiewic, K ?ojewska, E Kawka, R Rutkowski, K Korybalska
Journal of Clinical Medicine, 2021-06-18;10(12):.
Species: Human
Sample Types: Serum
-
Impact of the Uncoupling Protein 1 on Cardiovascular Risk in Patients with Rheumatoid Arthritis
Authors: LI Lyngfelt, MC Erlandsson, M Nadali, S Hedjazifar, R Pullerits, KM Andersson, P Brembeck, ST Silfverswä, U Smith, MI Bokarewa
Cells, 2021-05-07;10(5):.
Species: Human
Sample Types: Serum
-
Bioprinting and Differentiation of Adipose-Derived Stromal Cell Spheroids for a 3D Breast Cancer-Adipose Tissue Model
Authors: H Horder, M Guaza Lash, N Grummel, A Nadernezha, J Herbig, S Ergün, J Te beta mar, J Groll, B Fabry, P Bauer-Krei, T Blunk
Cells, 2021-04-03;10(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of Gestational Diabetes Mellitus on the fetal compartment
Authors: TPB De Luccia, E Ono, R Menon, AU Borbely, R Mattar, L Richardson, ALM da Silva, RM Botelho, MLTLF da Rocha, S Daher
Journal of reproductive immunology, 2021-03-24;145(0):103314.
Species: Human
Sample Types: Serum
-
Reduced concentrations of the B cell cytokine Interleukin 38 are associated with cardiovascular disease risk in overweight subjects
Authors: DM de Graaf, M Jaeger, ICL van den Mu, RT Horst, K Schraa, J Zwaag, M Kox, M Fujita, T Yamauchi, L Mercurio, S Madonna, JHW Rutten, J de Graaf, NP Riksen, FL van de Vee, MG Netea, LAB Joosten, CA Dinarello
Eur J Immunol, 2020-11-19;0(0):.
Species: Human
Sample Types: Plasma
-
First trimester secreted Frizzled-Related Protein 4 and other adipokine serum concentrations in women developing gestational diabetes mellitus
Authors: JHN Schuitemak, RHJ Beernink, A Franx, TIFH Cremers, MPH Koster
PLoS ONE, 2020-11-18;15(11):e0242423.
Species: Human
Sample Types: Serum
-
The Associations between Dairy Product Consumption and Biomarkers of Inflammation, Adipocytokines, and Oxidative Stress in Children: A Cross-Sectional Study
Authors: H Aslam, FN Jacka, W Marx, K Karatzi, C Mavrogiann, E Karaglani, M Mohebbi, JA Pasco, A O'Neil, M Berk, T Nomikos, S Kanellakis, O Androutsos, Y Manios, G Moschonis
Nutrients, 2020-10-06;12(10):.
Species: Human
Sample Types: Plasma
-
BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner
Authors: VACM Koeken, LCJ de Bree, VP Mourits, SJ Moorlag, J Walk, B Cirovic, RJ Arts, M Jaeger, H Dijkstra, H Lemmers, LA Joosten, CS Benn, R van Crevel, M Netea
J. Clin. Invest., 2020-10-01;0(0):.
Species: Human
Sample Types: Plasma
-
Elafin inhibits obesity, hyperglycemia, and liver steatosis in high-fat diet-treated male mice
Authors: J Wang, C Ortiz, L Fontenot, R Mukhopadhy, Y Xie, IKM Law, DQ Shih, SA Mattai, Z Li, HW Koon
Sci Rep, 2020-07-30;10(1):12785.
Species: Human
Sample Types: Serum
-
Maternal Anthropometric Factors and Circulating Adipokines as Predictors of Birth Weight and Length
Authors: D Mazurek, M Bronkowska
Int J Environ Res Public Health, 2020-07-03;17(13):.
Species: Human
Sample Types: Plasma
-
Deficiency of Urokinase Plasminogen Activator May Impair beta Cells Regeneration and Insulin Secretion in Type 2 Diabetes Mellitus
Authors: CZ Wu, SH Ou, LC Chang, YF Lin, D Pei, JS Chen
Molecules, 2019-11-20;24(23):.
Species: Human
Sample Types: Serum
-
Using a cultural dance program to increase sustainable physical activity for breast cancer survivors-A pilot study
Authors: LWM Loo, K Nishibun, L Welsh, T Makolo, CD Chong, I Pagano, H Yu, EO Bantum
Complement Ther Med, 2019-09-21;47(0):102197.
Species: Human
Sample Types: Plasma
-
Whey Protein Supplementation Compared to Collagen Increases Blood Nesfatin Concentrations and Decreases Android Fat in Overweight Women: A Randomized Double-Blind Study
Authors: BM Giglio, RM Schincagli, AS da Silva, ICS Fazani, PA Monteiro, JF Mota, JP Cunha, C Pichard, GD Pimentel
Nutrients, 2019-09-02;11(9):.
Species: Human
Sample Types: Plasma
-
Growth and differentiation factor 15 is a biomarker for low back pain-associated disability
Authors: N Tarabeih, A Shalata, S Trofimov, A Kalinkovic, G Livshits
Cytokine, 2019-02-15;117(0):8-14.
Species: Human
Sample Types: Plasma
-
Effects of the inspiratory muscle training and aerobic training on respiratory and functional parameters, inflammatory biomarkers, redox status and quality of life in hemodialysis patients: A randomized clinical trial
Authors: PHS Figueiredo, MMO Lima, HS Costa, JB Martins, OD Flecha, PF Gonçalves, FL Alves, VGB Rodrigues, EHB Maciel, VA Mendonça, ACR Lacerda, ÉLM Vieira, AL Teixeira, F de Paula, CH Balthazar
PLoS ONE, 2018-07-26;13(7):e0200727.
Species: Human
Sample Types: Plasma
-
Impact of rural-urban environment on metabolic profile and response to a 5-day high-fat diet
Authors: DL Tahapary, K de Ruiter, F Kurniawan, Y Djuardi, Y Wang, SME Nurdin, E Iskandar, D Minggu, E Yunir, B Guigas, T Supali, PCN Rensen, E Sartono, P Soewondo, DS Harbuwono, JWA Smit, M Yazdanbakh
Sci Rep, 2018-05-25;8(1):8149.
Species: Human
Sample Types: Plasma
-
Nutrients restriction upregulates adiponectin in epicardial or subcutaneous adipose tissue: impact inde novoheart failure patients
Authors: RM Agra, Á Fernández-, E Díaz-Rodrí, A Cordero, A Varela-Rom, I Gómez-Oter, JNL Canoa, ÁL Fernández, JM Martínez-C, JR González-J, S Eiras
Int J Med Sci, 2018-02-12;15(5):417-424.
Species: Human
Sample Types: Plasma
-
Using Resistin, glucose, age and BMI to predict the presence of breast cancer
Authors: M Patrício, J Pereira, J Crisóstomo, P Matafome, M Gomes, R Seiça, F Caramelo
BMC Cancer, 2018-01-04;18(1):29.
Species: Human
Sample Types: Serum
-
High Expression of STAT3 in Subcutaneous Adipose Tissue Associates with Cardiovascular Risk in Women with Rheumatoid Arthritis
Authors: M Nadali, R Pullerits, KME Andersson, ST Silfverswä, MC Erlandsson, MI Bokarewa
Int J Mol Sci, 2017-11-13;18(11):.
Species: Human
Sample Types: Serum
-
Evolution and bad prognostic value of advanced glycation end products after acute heart failure: relation with body composition
Authors: B Paradela-D, Á Fernández-, D Bou-Teen, S Eiras, R González-F, RM Agra, A Varela-Rom, AI Castro-Pai, MC Carreira, FF Casanueva, E Álvarez, JR González-J
Cardiovasc Diabetol, 2017-09-15;16(1):115.
Species: Human
Sample Types: Plasma
-
Experimental type 2 diabetes induction reduces serum vaspin, but not serum omentin, in Wistar rats
Authors: CA Castro, KA da Silva, MM Buffo, KNZ Pinto, FO Duarte, KO Nonaka, FF Aníbal, ACGO Duarte
Int J Exp Pathol, 2017-04-25;0(0):.
Species: Human
Sample Types: Serum
-
Presence of IL-17 in synovial fluid identifies a potential inflammatory osteoarthritic phenotype
Authors: SJ Snelling, S Bas, GJ Puskas, SG Dakin, D Suva, A Finckh, C Gabay, P Hoffmeyer, AJ Carr, A Lübbeke
PLoS ONE, 2017-04-11;12(4):e0175109.
Species: Human
Sample Types: Serum
-
Low adiponectin is associated with diastolic dysfunction in women: a cross-sectional study from the Troms� Study
Authors: JV Norvik, H Schirmer, K Ytrehus, TG Jenssen, SN Zykova, AE Eggen, BO Eriksen, MD Solbu
BMC Cardiovasc Disord, 2017-03-14;17(1):79.
Species: Human
Sample Types: Serum
-
Improvement of cardiometabolic markers after fish oil intervention in young Mexican adults and the role of PPAR? L162V and PPAR?2 P12A
Authors: A Binia, C Vargas-Mar, M Ancira-Mor, LM Gosoniu, I Montoliu, E Gámez-Vald, DC Soria-Cont, A Angeles-Qu, R Gonzalez-A, S Fernández, D Martínez-C, B Hernández-, M Ramírez-So, C Pérez-Orte, Y Rodríguez-, I Castan, I Rubio-Alia, F Vadillo-Or, R Márquez-Ve, R Bojalil, JC López-Alva, P Valet, M Kussmann, I Silva-Zole, ME Tejero
J. Nutr. Biochem., 2017-02-10;43(0):98-106.
Species: Human
Sample Types: Serum
-
Secretory activity of subcutaneous abdominal adipose tissue in male patients with rheumatoid arthritis and osteoarthritis - association with clinical and laboratory data
Authors: Ewa Kontny
Reumatologia, 2016-11-28;54(5):227-235.
Species: Human
Sample Types: Tissue Explants
-
Circulating periostin in relation to insulin resistance and nonalcoholic fatty liver disease among overweight and obese subjects
Sci Rep, 2016-11-25;6(0):37886.
Species: Human
Sample Types: Serum
-
Association study of arcuate nucleus neuropeptide Y neuron receptor gene variation and serum NPY levels in clozapine treated patients with schizophrenia
Authors: E Leinonen
Eur. Psychiatry, 2016-11-10;40(0):13-19.
Species: Human
Sample Types: Serum
-
The Association Between Adiponectin, Serum Uric Acid and Urinary Markers of Renal Damage in the General Population: Cross-Sectional Data from the Troms� Study
Kidney Blood Press Res, 2016-09-14;41(5):623-634.
Species: Human
Sample Types: Serum
-
Human adipose tissue conditioned media from lean subjects is protective against H2O2 induced neurotoxicity in human SH-SY5Y neuronal cells.
Authors: Wan Z, Mah D, Simtchouk S, Kluftinger A, Little J
Int J Mol Sci, 2015-01-06;16(1):1221-31.
Species: Human
Sample Types: Cell Culture Supernates
-
Expression of obesity markers and Persistent Organic Pollutants levels in adipose tissue of obese patients: reinforcing the obesogen hypothesis?
Authors: Pereira-Fernandes A, Dirinck E, Dirtu A, Malarvannan G, Covaci A, Van Gaal L, Vanparys C, Jorens P, Blust R
PLoS ONE, 2014-01-10;9(1):e84816.
Species: Human
Sample Types: Serum
-
Osteocalcin increase after bariatric surgery predicts androgen recovery in hypogonadal obese males.
Authors: Samavat J, Facchiano E, Cantini G, Di Franco A, Alpigiano G, Poli G, Seghieri G, Lucchese M, Forti G, Luconi M
Int J Obes (Lond), 2013-12-05;38(3):357-63.
Species: Human
Sample Types: Serum
-
Endothelial cells from visceral adipose tissue disrupt adipocyte functions in a three-dimensional setting: partial rescue by angiopoietin-1.
Authors: Pellegrinelli V, Rouault C, Veyrie N, Clement K, Lacasa D
Diabetes, 2013-10-15;63(2):535-49.
Species: Human
Sample Types: Cell Culture Supernates
-
Adipokine resistin predicts anti-inflammatory effect of glucocorticoids in asthma.
Authors: Leivo-Korpela S, Lehtimaki L, Vuolteenaho K, Nieminen R, Kankaanranta H, Saarelainen S, Moilanen E
J Inflamm (Lond), 2011-05-26;8(0):12.
Species: Human
Sample Types: Plasma
-
LIGHT (TNFSF14) inhibits adipose differentiation without affecting adipocyte metabolism.
Authors: Tiller G, Laumen H, Fischer-Posovszky P, Finck A, Skurk T, Keuper M, Brinkmann U, Wabitsch M, Link D, Hauner H
Int J Obes (Lond), 2010-06-15;35(2):208-16.
Species: Human
Sample Types: Cell Culture Supernates
-
Regulation of adipokine production in human adipose tissue by propionic acid.
Authors: Al-Lahham SH, Roelofsen H, Priebe M, Weening D, Dijkstra M, Hoek A, Rezaee F, Venema K, Vonk RJ
Eur. J. Clin. Invest., 2010-03-25;40(5):401-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Gene-nutrient interactions in the metabolic syndrome: single nucleotide polymorphisms in ADIPOQ and ADIPOR1 interact with plasma saturated fatty acids to modulate insulin resistance.
Authors: Ferguson JF, Phillips CM, Tierney AC, Perez-Martinez P, Defoort C, Helal O, Lairon D, Planells R, Shaw DI, Lovegrove JA, Gjelstad IM, Drevon CA, Blaak EE, Saris WH, Leszczynska-Golabek I, Kiec-Wilk B, Riserus U, Karlstrom B, Miranda JL, Roche HM
Am. J. Clin. Nutr., 2009-12-23;91(3):794-801.
Species: Human
Sample Types: Plasma
-
Adiponectin upregulates monocytic activin A but systemic levels are not altered in obesity or type 2 diabetes.
Authors: Weigert J, Neumeier M, Wanninger J, Schober F, Sporrer D, Weber M, Schramm A, Wurm S, Stogbauer F, Filarsky M, Schaffler A, Aslanidis C, Scholmerich J, Buechler C
Cytokine, 2009-01-06;45(2):86-91.
Species: Human
Sample Types: Plasma
-
Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes.
Authors: Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, Bing C
Endocrinology, 2008-12-12;150(4):1688-96.
Species: Human
Sample Types: Cell Culture Supernates
-
Reduced response to adiponectin and lower abundance of adiponectin receptor proteins in type 2 diabetic monocytes.
Authors: Weigert J, Neumeier M, Wanninger J, Wurm S, Kopp A, Schober F, Filarsky M, Schaffler A, Zeitoun M, Aslanidis C, Buechler C
FEBS Lett., 2008-04-28;582(12):1777-82.
Species: Human
Sample Types: Cell Culture Supernates
-
Isoginkgetin enhances adiponectin secretion from differentiated adiposarcoma cells via a novel pathway involving AMP-activated protein kinase.
Authors: Liu G, Grifman M, Macdonald J, Moller P, Wong-Staal F, Li QX
J. Endocrinol., 2007-09-01;194(3):569-78.
Species: Human
Sample Types: Cell Culture Supernates
-
Cardiovascular risk factors are reduced with a low dose of acarbose in obese patients with polycystic ovary syndrome.
Authors: Araujo Penna I, Canella PR, Vieira CS, Silva de Sa MF, dos Reis RM, Ferriani RA
Fertil. Steril., 2007-04-06;88(2):519-22.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human Adiponectin/Acrp30 DuoSet ELISA
Average Rating: 4.6 (Based on 7 Reviews)
Have you used Human Adiponectin/Acrp30 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
plasma diluted 1:5000
Used on EDTA Plasma at 1/6000 dilution. Signal at 450nm corrected for signal at 550nm. Very tight replicates, confident results.
Sample dilution used- 1 in 10000 in reagent diluent