Human IFN-beta DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
Customers also Viewed
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
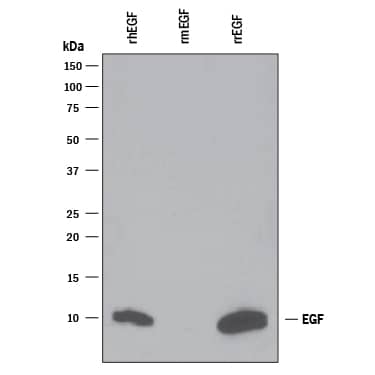
Scientific Data
Preparation and Storage
Background: IFN-beta
Interferon-beta (IFN-beta) is a cytokine that is produced by fibroblasts and pathogen-exposed dendritic cells, macrophages, and endothelial cells. It signals through the heterodimeric IFN-alpha/beta Receptor. IFN-beta-deficient mice show increased susceptibility to experimental autoimmune encephalomyelitis (EAE), a disease model of human multiple sclerosis (MS). Furthermore, IFN-beta has been shown to suppress the Th17 cell response in both MS and EAE and has commonly been used as a treatment for MS.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL of Block Buffer to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human IFN-beta DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
44
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Complete substitution with modified nucleotides in self-amplifying RNA suppresses the interferon response and increases potency
Authors: McGee, JE;Kirsch, JR;Kenney, D;Cerbo, F;Chavez, EC;Shih, TY;Douam, F;Wong, WW;Grinstaff, MW;
Nature biotechnology
Species: Human
Sample Types: Cell Culture Supernates
-
Innate lymphoid cells are activated in HFRS, and their function can be modulated by hantavirus-induced type I interferons
Authors: García, M;Carrasco García, A;Weigel, W;Christ, W;Lira-Junior, R;Wirth, L;Tauriainen, J;Maleki, K;Vanoni, G;Vaheri, A;Mäkelä, S;Mustonen, J;Nordgren, J;Smed-Sörensen, A;Strandin, T;Mjösberg, J;Klingström, J;
PLoS pathogens
Species: Human
Sample Types: Cell Culture Supernates
-
Broad-spectrum RNA antiviral inspired by ISG15 -/- deficiency
Authors: Akalu, YT;Patel, RS;Taft, J;Canas-Arranz, R;Richardson, A;Buta, S;Martin-Fernandez, M;Sazeides, C;Pearl, RL;Mainkar, G;Kurland, AP;Geltman, R;Rosberger, H;Kang, DD;Kurian, AA;Kaur, K;Altman, J;Dong, Y;Johnson, JR;Zhangi, L;Lim, JK;Albrecht, RA;García-Sastre, A;Rosenberg, BR;Bogunovic, D;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
Amyloid precursor protein induces reactive astrogliosis
Authors: Jauregui, GV;Vuki?, D;Onyango, IG;Arias, C;Novotný, JS;Texlová, K;Wang, S;Kova?ovicova, KL;Polakova, N;Zelinkova, J;?arna, M;Strail, VL;Head, BP;Havas, D;Mistrik, M;Zorec, R;Verkhratsky, A;Keegan, L;O'Connel, M;Rissman, R;Stokin, GB;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Lysates
-
Rotavirus capping enzyme VP3 inhibits interferon expression by inducing MAVS degradation during viral replication
Authors: Dai, J;Agbemabiese, CA;Griffin, AN;Patton, JT;
mBio
Species: Human
Sample Types: Cell Culture Supernates
-
Serum interleukin-6, procalcitonin, and C-reactive protein at hospital admission can identify patients at low risk for severe COVID-19 progression
Authors: Zobel, CM;Wenzel, W;Krüger, JP;Baumgarten, U;Wagelöhner, T;Neumann, N;Foroutan, B;Müller, R;Müller, A;Rauschning, D;Schü beta ler, M;Scheit, L;Weinreich, F;Oltmanns, K;Keidel, F;Koch, M;Spethmann, S;Schreiner, M;
Frontiers in microbiology
Species: Human
Sample Types: Serum
-
Met receptor is essential for MAVS-mediated antiviral innate immunity in epithelial cells independent of its kinase activity
Authors: Imamura, R;Sato, H;Chih-Cheng Voon, D;Shirasaki, T;Honda, M;Kurachi, M;Sakai, K;Matsumoto, K;
Proceedings of the National Academy of Sciences of the United States of America
Species: Human
Sample Types: Cell Culture Supernates
-
Development of cyclopeptide inhibitors of cGAS targeting protein-DNA interaction and phase separation
Authors: Wang, X;Wang, Y;Cao, A;Luo, Q;Chen, D;Zhao, W;Xu, J;Li, Q;Bu, X;Quan, J;
Nature communications
Species: Human
Sample Types: Cell Culture Supernates
-
Development of LB244, an Irreversible STING Antagonist
Authors: Barasa, L;Chaudhuri, S;Zhou, JY;Jiang, Z;Choudhary, S;Green, RM;Wiggin, E;Cameron, M;Humphries, F;Fitzgerald, KA;Thompson, PR;
Journal of the American Chemical Society
Species: Human
Sample Types: Cell Culture Supernates
-
Complete substitution with modified nucleotides suppresses the early interferon response and increases the potency of self-amplifying RNA
Authors: McGee, JE;Kirsch, JR;Kenney, D;Chavez, E;Shih, TY;Douam, F;Wong, WW;Grinstaff, MW;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Cell Culture Supernates
-
ZIKV induction of tristetraprolin in endothelial and Sertoli cells post-transcriptionally inhibits IFN?/? expression and promotes ZIKV persistence
Authors: Schutt, WR;Conde, JN;Mladinich, MC;Himmler, GE;Mackow, ER;
mBio
Species: Human
Sample Types: Cell Culture Supernates
-
TNF? induced by DNA-sensing in macrophage compromises retinal pigment epithelial (RPE) barrier function
Authors: Twarog, M;Schustak, J;Xu, Y;Coble, M;Dolan, K;Esterberg, R;Huang, Q;Saint-Geniez, M;Bao, Y;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates, Cell Lysates
-
Adipokine levels and their association with clinical disease severity in patients with dengue
Authors: Kuruppu, H;Wickramanayake, WPRH;Jeewandara, C;Peranantharajah, D;Colambage, HS;Perera, L;Gomes, L;Wijewickrama, A;Ogg, GS;Malavige, GN;
PLoS neglected tropical diseases
Species: Human
Sample Types: Serum
-
Flow-Based CL-SMIA for the Quantification of Protein Biomarkers from Nasal Secretions in Comparison with Sandwich ELISA
Authors: Neumair, J;Kröger, M;Stütz, E;Jerin, C;Chaker, AM;Schmidt-Weber, CB;Seidel, M;
Biosensors
Species: Human
Sample Types: Nasal Lining Fluid
-
SARS-CoV-2 Nsp2 Contributes to Inflammation by Activating NF-kappaB
Authors: É Lacasse, L Gudimard, I Dubuc, A Gravel, I Allaeys, É Boilard, L Flamand
Viruses, 2023-01-24;15(2):.
Species: Human
Sample Types: Cell Culture Supernates
-
Blood leukocyte transcriptional modules and differentially expressed genes associated with disease severity and age in COVID-19 patients
Authors: SY Bando, FB Bertonha, SE Vieira, DBL de Oliveir, VN Chalup, EL Durigon, P Palmeira, ACP Curi, CS Faria, L Antonangel, GDP Lauterbach, FA Regalio, RM Cesar, CA Moreira-Fi
Scientific Reports, 2023-01-17;13(1):898.
Species: Human
Sample Types: Plasma
-
Association between the expression of Toll-like receptors, cytokines, and homeostatic chemokines in SARS-CoV-2 infection and COVID-19 severity
Authors: W Alturaiki, H Alkadi, S Alamri, ME Awadalla, A Alfaez, A Mubarak, MA Alanazi, FQ Alenzi, BF Flanagan, B Alosaimi
Heliyon, 2022-12-24;0(0):e12653.
Species: Human
Sample Types: Serum
-
The Golgi-resident protein ACBD3 concentrates STING at ER-Golgi contact sites to drive export from the ER
Authors: K Motani, N Saito-Tara, K Nishino, S Yamauchi, N Minakawa, H Kosako
Cell Reports, 2022-12-20;41(12):111868.
Species: Human
Sample Types: Cell Culture Supernates
-
PRC2-mediated epigenetic suppression of type I IFN-STAT2 signaling impairs antitumor immunity in luminal breast cancer
Authors: J Hong, JH Lee, Z Zhang, Y Wu, M Yang, Y Liao, R de la Rosa, J Scheirer, D Pechacek, N Zhang, Z Xu, T Curiel, X Tan, TH Huang, K Xu
Cancer Research, 2022-12-16;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Structure-activity relationship study of amidobenzimidazole derivatives as stimulator of interferon genes (STING) agonists
Authors: X Liu, M Wang, M Yang, H Sun, B Wang, X Pan, X Chen, J Jin, X Wang
European Journal of Medicinal Chemistry, 2022-11-24;246(0):114943.
Species: Human
Sample Types: Cell Culture Supernates
-
Signal strength of STING activation determines cytokine plasticity and cell death in human monocytes
Authors: D Kabelitz, M Zarobkiewi, M Heib, R Serrano, M Kunz, G Chitadze, D Adam, C Peters
Scientific Reports, 2022-10-24;12(1):17827.
Species: Human
Sample Types: Cell Culture Supernates
-
Pro-Inflammatory Cytokines and Interferon-Stimulated Gene Responses Induced by Seasonal Influenza A Virus with Varying Growth Capabilities in Human Lung Epithelial Cell Lines
Authors: AA Hinay, S Kakee, S Kageyama, A Tsuneki-To, WY Perdana, Y Akena, S Nishiyama, K Kanai
Vaccines, 2022-09-09;10(9):.
Species: Human
Sample Types: Cell Lysates
-
Deactylation by SIRT1 enables liquid-liquid phase separation of IRF3/IRF7 in innate antiviral immunity
Authors: Z Qin, X Fang, W Sun, Z Ma, T Dai, S Wang, Z Zong, H Huang, H Ru, H Lu, B Yang, S Lin, F Zhou, L Zhang
Nature Immunology, 2022-07-25;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Distinct airway epithelial immune responses after infection with SARS-CoV-2 compared to H1N1
Authors: H Stölting, L Baillon, R Frise, K Bonner, RJ Hewitt, PL Molyneaux, ML Gore, Breathing, WS Barclay, S Saglani, CM Lloyd
Mucosal Immunology, 2022-07-15;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Decreased leukocyte exhaustion is associated with decreased IFN-beta and increased alpha-defensin-1 levels in type-2 diabetes
Authors: S Shruthi, JM Sibi, V Mohan, S Babu, V Nirmaladev, V Aravindhan
Cytokine, 2022-05-28;156(0):155918.
Species: Human
Sample Types: Cell Culture Supernates
-
A genetic screen identifies a protective type III interferon response to Cryptosporidium that requires TLR3 dependent recognition
Authors: Alexis R. Gibson, Adam Sateriale, Jennifer E. Dumaine, Julie B. Engiles, Ryan D. Pardy, Jodi A. Gullicksrud et al.
PLOS Pathogens
Species: Mouse
Sample Types: Cell Culture Supernates
-
Green and Roasted Coffee Extracts Inhibit Interferon-beta Release in LPS-Stimulated Human Macrophages
Authors: V Artusa, C Ciaramelli, A D'Aloia, FA Facchini, N Gotri, A Bruno, B Costa, A Palmioli, C Airoldi, F Peri
Frontiers in Pharmacology, 2022-05-05;13(0):806010.
Species: Human
Sample Types: Cell Culture Supernates
-
Translocated Legionella pneumophila small RNAs mimic eukaryotic microRNAs targeting the host immune response
Authors: T Sahr, P Escoll, C Rusniok, S Bui, G Pehau-Arna, G Lavieu, C Buchrieser
Nature Communications, 2022-02-09;13(1):762.
Species: Human
Sample Types: Cell Culture Supernates
-
Development of Small-Molecule STING Activators for Cancer Immunotherapy
Authors: HR Jung, S Jo, MJ Jeon, H Lee, Y Chu, J Lee, E Kim, GY Song, C Jung, H Kim, S Lee
Biomedicines, 2021-12-24;10(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Downregulation of calcium-regulated heat stable protein 1 expression by low-temperature stimulation causes reduction of interferon-beta expression and sensitivity to influenza viral infection
Authors: K Nishioka, T Daidoji, T Nakaya
Virus research, 2021-12-17;0(0):198659.
Species: Human
Sample Types: Cell Culture Supernates
-
Cross-species analysis of viral nucleic acid interacting proteins identifies TAOKs as innate immune regulators
Authors: FL Pennemann, A Mussabekov, C Urban, A Stukalov, LL Andersen, V Grass, TM Lavacca, C Holze, L Oubraham, Y Benamrouch, E Girardi, RE Boulos, R Hartmann, G Superti-Fu, M Habjan, JL Imler, C Meignin, A Pichlmair
Nature Communications, 2021-12-01;12(1):7009.
Species: Human
Sample Types: Cell Culture Supernates
-
The Endogenous RIG-I Ligand Is Generated in Influenza A-Virus Infected Cells
Authors: J Steinberg, T Wadenpohl, S Jung
Viruses, 2021-08-07;13(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Genetic and chemical inhibition of IRF5 suppresses pre-existing mouse lupus-like disease
Authors: T Ban, M Kikuchi, GR Sato, A Manabe, N Tagata, K Harita, A Nishiyama, K Nishimura, R Yoshimi, Y Kirino, H Yanai, Y Matsumoto, S Suzuki, H Hihara, M Ito, K Tsukahara, K Yoshimatsu, T Yamamoto, T Taniguchi, H Nakajima, S Ito, T Tamura
Nature Communications, 2021-07-19;12(1):4379.
Species: Human
Sample Types: Cell Culture Supernates
-
Paracrine IFN Response Limits ZIKV Infection in Human Sertoli Cells
Authors: DP Strange, B Jiyarom, H Sadri-Arde, LH Cazares, TA Kenny, MD Ward, S Verma
Frontiers in Microbiology, 2021-05-17;12(0):667146.
Species: Human
Sample Types: Cell Culture Supernates
-
Extracellular vesicles promote transkingdom nutrient transfer during viral-bacterial co-infection
Authors: MR Hendricks, S Lane, JA Melvin, Y Ouyang, DB Stolz, JV Williams, Y Sadovsky, JM Bomberger
Cell Reports, 2021-01-26;34(4):108672.
Species: Human
Sample Types: Cell Culture Supernates
-
PPP6C Negatively Regulates STING-Dependent Innate Immune Responses
Authors: G Ni, Z Ma, JP Wong, Z Zhang, E Cousins, MB Major, B Damania
MBio, 2020-08-04;11(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Major 5'terminally deleted enterovirus populations modulate type I IFN response in acute myocarditis patients and in human cultured cardiomyocytes
Authors: M Glenet, Y N'Guyen, A Mirand, C Henquell, AL Lebreil, F Berri, F Bani-Sadr, B Lina, I Schuffenec, L Andreolett
Sci Rep, 2020-07-20;10(1):11947.
Species: Human
Sample Types: Cell Culture Supernates
-
MG53 suppresses interferon-&beta and inflammation via regulation of ryanodine receptor-mediated intracellular calcium signaling
Authors: M Sermershei, AD Kenney, PH Lin, TM McMichael, C Cai, K Gumpper, TMA Adesanya, H Li, X Zhou, KH Park, JS Yount, J Ma
Nat Commun, 2020-07-17;11(1):3624.
Species: Human
Sample Types: Cell Culture Supernates
-
Axl Promotes Zika Virus Entry and Modulates the Antiviral State of Human Sertoli Cells
Authors: DP Strange, B Jiyarom, N Pourhabibi, X Xie, C Baker, H Sadri-Arde, PY Shi, S Verma
MBio, 2019-07-16;10(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
MicroRNA-122 supports robust innate immunity in hepatocytes by targeting the RTKs/STAT3 signaling pathway
Authors: H Xu, SJ Xu, SJ Xie, Y Zhang, JH Yang, WQ Zhang, MN Zheng, H Zhou, LH Qu
Elife, 2019-02-08;8(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Human polyomavirus BKV infection of endothelial cells results in interferon pathway induction and persistence
Authors: P An, MT Sáenz Robl, AM Duray, PG Cantalupo, JM Pipas
PLoS Pathog., 2019-01-08;15(1):e1007505.
Species: Human
Sample Types: Cell Culture Supernates
-
An Anti-Inflammatory Azaphenothiazine Inhibits Interferon ? Expression and CXCL10 Production in KERTr Cells
Authors: L Strzadala, A Fiedorowic, E Wysokinska, E Ziolo, M Grudzie?, M Jelen, K Pluta, B Morak-Mlod, M Zimecki, W Kalas
Molecules, 2018-09-24;23(10):.
Species: Human
Sample Types: Cell Culture Supernates
-
The effects of CD14 and IL-27 on induction of endotoxin tolerance in human monocytes and macrophages
Authors: C Petes, V Mintsopoul, RL Finnen, BW Banfield, K Gee
J. Biol. Chem., 2018-09-21;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Zika Virus Persistently Infects and Is Basolaterally Released from Primary Human Brain Microvascular Endothelial Cells
Authors: MC Mladinich, J Schwedes, ER Mackow
MBio, 2017-07-11;8(4):.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsDuoSet Ancillary Reagent Kits
Supplemental ELISA Products
Reviews for Human IFN-beta DuoSet ELISA
Average Rating: 4.4 (Based on 10 Reviews)
Have you used Human IFN-beta DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
THP-1 cells were stimulated with cytosolic nucleic acids for 18 hours at 1microgram/ml.
We used this kit for the quantification of INF b in human serum and plasma. Works very well and well-described protocol.