Human/Primate IL-6 Antibody Summary
Applications
Human/Primate IL-6 Sandwich Immunoassay
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Recombinant Human IL‑6 by Western Blot. Western blot shows 25 ng of Recombinant Human IL-6 (Catalog # 206-IL), Recombinant Mouse IL-6 (Catalog # 406-ML) and Recombinant Rat IL-6 (Catalog # 506-RL). PVDF Membrane was probed with 1 µg/mL of Mouse Anti-Human/ Primate IL-6 Monoclonal Antibody (Catalog # MAB206) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF007). A specific band was detected for IL-6 at approximately 18 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 3.
 View Larger
View Larger
IL‑6 in Human Skin. IL-6 was detected in immersion fixed frozen sections of hyperplastic human skin using Mouse Anti-Human/Primate IL-6 Monoclonal Antibody (Catalog # MAB206) at 8 µg/mL overnight at 4 °C. Tissue was stained using the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (red; Catalog # NL007) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
Cell Proliferation Induced by IL‑6 and Neutralization by Human IL‑6 Antibody. Recombinant Human IL-6 (Catalog # 206-IL) stimulates proliferation in the T1165.85.2.1 mouse plasmacytoma cell line in a dose-dependent manner (orange line). Proliferation elicited by Recombinant Human IL-6 (2.5 ng/mL) is neutralized (green line) by increasing concentrations of Mouse Anti-Human/Primate IL-6 Monoclonal Antibody (Catalog # MAB206). The ND50 is typically 8.00 - 80.0 ng/mL.
 View Larger
View Larger
Detection of Human IL-6 by Immunohistochemistry The role of IL‐6 signaling in the upregulation of PD‐L1 and downregulation of NKG2D ligands in CRPC cells. (A) PD‐L1 level in C4‐2siIL‐6/sc and CWRsiIL‐6/sc cell lines (left panel, mRNA level; right panel, protein level). (B) PD‐L1 IHC staining of tumor tissues. Error bars and significance values were obtained by counting positively stained cells in one randomly chosen phase of slides of three different stains. Magnification, 20× (inlet, 100×). (C) Blocking of IL‐6 Ab by neutralizing Ab of IL‐6 and the effect on PD‐L1 level in C4‐2sc and CWRsc cells. Cells were treated with either IL‐6 Ab or control IgG, total RNA extracted, cDNA converted, and the expression of PD‐L1 was compared in qPCR analyses. (D) PD‐L1 level in parental C4‐2 and CWR22Rv1 cells upon the addition of rhIL‐6. Parental cells (C4‐2P and CWR22Rv1P) were treated with rhIL‐6 (20 ng·mL−1) and PD‐L1 mRNA level was analyzed. (E) IHC staining of CRPC patient tumor samples. Two sets of adjacent tumor tissues (both samples, CRPC stage, Gleason score 8, patient age 70, Ningbo hospital in China) were stained with IL‐6 and PD‐L1. Arrows indicate the area showing positive staining of two molecules. (F) NKG2D ligand levels in IL‐6‐expressing cells and in IL‐6‐knockdown cells. Levels of five NKG2D ligands in C4‐2siIL‐6/sc and CWRsiIL‐6/sc cells were analyzed in qPCR analyses. (G) NKG2D ligand levels in parental C4‐2 and CWR22Rv1 cells upon the addition of rhIL‐6. Parental cells (C4‐2P and CWR22Rv1P) were treated with rhIL‐6 (20 ng·mL−1) and the NKG2D ligand levels (mRNA) were analyzed. (H) Flow cytometric analyses of NKG2D and PD‐1 on NK cells. Left two panels, primary NK cells were stained with PE‐NKG2D or APC‐PD‐1 and positive staining was analyzed. Right two panels, flow cytometric analyses of PD‐1 on NK cells, after coculture with tumor cells (6 h of incubation). Primary NK cells were added into tumor cells (1 : 1 ratio, tumor cells/NK cells) and collected after 6 h of incubation. PD‐1 levels in the collected NK cells were analyzed in flow cytometric analysis (using APC‐PD‐1 Ab). *P < 0.05, **P < 0.01, ***P < 0.001. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28865178), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human IL-6 by Western Blot Autocrine IL-6 activates STAT3 signalling in lung cancer cell-induced epidural ADSCs. a Epidural ADSCs were pre-treated with CM from lung cancer cells for 48 h, and pSTAT3 and STAT3 expression levels were detected by western blotting. Epidural ADSCs cultured in untreated medium served as a control. b The effects of lung cancer cell CM on epidural ADSC proliferation were evaluated using the CCK-8 assay. Epidural ADSCs were treated with CM from one of four lung cancer cell lines, and the optical density of both groups at 450 nm was analysed. Data from three separate experiments are shown. c Western blot analysis of pSTAT3 and STAT3 in epidural ADSCs treated with either 10 ng/mL recombinant IL-6, 10 ng/mL recombinant IL-11 or 50 ng/mL recombinant LIF in the presence or absence of either neutralizing antibodies or isotype controls. Loading control, actin. d Western blot analysis of pSTAT3 and alpha -SMA expression in epidural ADSCs treated with lung cancer cell CM in the presence or absence of neutralizing antibodies against IL-6, IL-11 or LIF. Loading control, actin. *P < 0.05; **P < 0.01; ***P < 0.001 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31196220), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human IL-6 by Western Blot Autocrine IL-6 activates STAT3 signalling in lung cancer cell-induced epidural ADSCs. a Epidural ADSCs were pre-treated with CM from lung cancer cells for 48 h, and pSTAT3 and STAT3 expression levels were detected by western blotting. Epidural ADSCs cultured in untreated medium served as a control. b The effects of lung cancer cell CM on epidural ADSC proliferation were evaluated using the CCK-8 assay. Epidural ADSCs were treated with CM from one of four lung cancer cell lines, and the optical density of both groups at 450 nm was analysed. Data from three separate experiments are shown. c Western blot analysis of pSTAT3 and STAT3 in epidural ADSCs treated with either 10 ng/mL recombinant IL-6, 10 ng/mL recombinant IL-11 or 50 ng/mL recombinant LIF in the presence or absence of either neutralizing antibodies or isotype controls. Loading control, actin. d Western blot analysis of pSTAT3 and alpha -SMA expression in epidural ADSCs treated with lung cancer cell CM in the presence or absence of neutralizing antibodies against IL-6, IL-11 or LIF. Loading control, actin. *P < 0.05; **P < 0.01; ***P < 0.001 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31196220), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human IL-6 by Western Blot Activated ADSCs trigger the proliferation and invasion of lung cancer cells by regulating MMP2/9 expression and EMT. a The effects of activated ADSCs on lung cancer cell proliferation were evaluated using the CCK-8 assay. Four lung cancer cell lines were cultured in the presence of ADSC-CM or aADSC-CM, and the optical density at 450 nm was analysed. The cancer cells cultured alone in normal growth medium were used as negative controls. Data from three separate experiments are shown. b The number of lung cancer cells that migrated through 8-μm Transwell membrane pores was counted to determine the changes in the invasive capabilities in response to CM from epidural ADSCs or aADSCs. c MMP2/9, E-cadherin and vimentin expression levels in four lung cancer cell lines treated with ADSC-CM or aADSC-CM were examined by western blotting. Lung cancer cells cultured in an untreated medium served as controls. d Lung cancer cells were treated with neutralizing antibodies against IL-6, isotype controls and aADSC-CM. Lung cancer cell invasion was analysed using a Transwell assay. Lung cancer cells cultured with untreated medium served as negative controls. e Lung cancer cells were treated with neutralizing antibodies against IL-6, isotype controls and aADSC-CM. MMP2/9, E-cadherin and vimentin expression levels in four lung cancer cell lines were analysed using western blotting. Lung cancer cells cultured with untreated medium served as negative controls. ***P < 0.001 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31196220), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Human/Primate IL-6 Antibody by Western Blot Musashi-1 mitigated DDP-induced apoptosis via the IL-6/AKT regulatory loopA. Cells were pretreated with 50 μM of LY294002 or vehicle for 3 hours, followed by 50 μM DDP treatment for 24 hours. The culture media were collected and the concentrations of IL-6 was determined by ELISA. B. 05MG-FlagMSI1 cells were treated with/without 50 μM DDP for 24 hours in the absence or presence of recombinant IL-6 (10 ng/ml) or anti-IL-6 neutralizing antibody (0.1 μg/ml). The cell lysates were analyzed by Western blot. C-D. The bar diagrams represent the quantified ratio of p-AKT-308/AKT and p-AKT-473/AKT obtained by the Western blot in B. E. 05MG cells were treated with control (serum free medium, S.F.) or condition media from 05MG-FlagMSI1 cells (C.M.) in the presence or absence of 50 μM DDP, IL-6 (10 ng/ml), and anti-IL-6 antibody (0.1 μg/ml) for 24 hours. The cell lysates were analyzed by Western blot to assess the levels of p-AKT-308, p-AKT-473, and total AKT. F-G. The bar diagrams represent the quantified ratio of p-AKT-308/AKT and p-AKT-473/AKT obtained in E. H. 05MG cells were treated as described in E and analyzed by Western blot. I-J. The bar diagrams represent the quantified ratio of cleavage-caspase-3/actin and cleaved-PARP/actin obtained in H. Data represent the mean ± S.D. of two independent experiments performed in triplicate. * P<0.05 vs AKT or Actin. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27285760), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
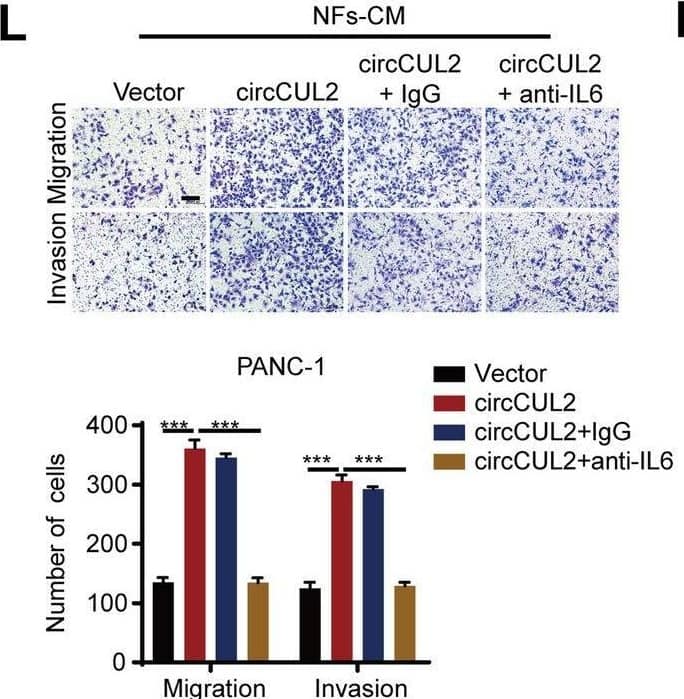
Detection of IL-6 by Immunohistochemistry circCUL2 regulates the phenotypic plasticity of CAFs and promote PDAC progression by IL6. A Gene Set Enrichment Analysis (GSEA) of affected signatures in fibroblasts (circCUL2 vs. control). B GSEA plots for inflammatory CAF (iCAF) signatures in circCUL2 overexpression NFs from control. C-D qRT–PCR analysis of iCAF markers (IL6, TNF-alpha and IL1 alpha ) and myCAF marker (Acta2 and Axin2) expression in circCUL2 overexpression NFs. E Flow cytometric analysis of PDGFR alpha and alpha -SMA expression in circCUL2 overexpression NFs. F-H. Representative cytokine arrays for circCUL2-overexpression NFs and control (n = 3). arrows indicate the cytokines with significant changes, which were further confirmed by qRT–PCR and ELISA. I-L EdU assay (I), colony formation (J), Scratch wound healing assays (K) and transwell assays (L) of PANC-1 cells treated with conditioned medium circCUL2-overexpression NFs or anti-IL6. Scale bar, 100 μm. M western blot analysis of STAT3 and p-STAT3 in PANC-1 cells. Data are expressed as the mean ± SD of three independent experiments. **p < 0.01 and ***p < 0.001 (two-tailed Student t-tests) Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35189958), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
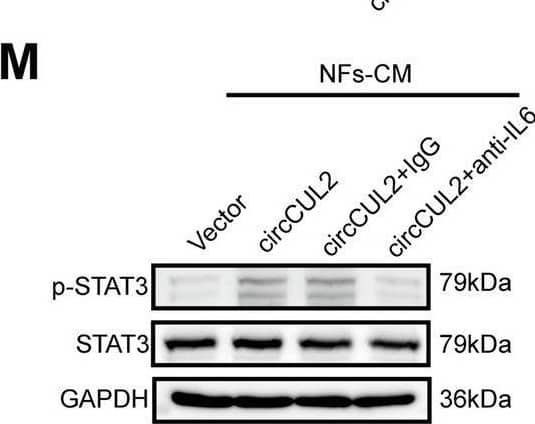
Detection of IL-6 by Western Blot circCUL2 regulates the phenotypic plasticity of CAFs and promote PDAC progression by IL6. A Gene Set Enrichment Analysis (GSEA) of affected signatures in fibroblasts (circCUL2 vs. control). B GSEA plots for inflammatory CAF (iCAF) signatures in circCUL2 overexpression NFs from control. C-D qRT–PCR analysis of iCAF markers (IL6, TNF-alpha and IL1 alpha ) and myCAF marker (Acta2 and Axin2) expression in circCUL2 overexpression NFs. E Flow cytometric analysis of PDGFR alpha and alpha -SMA expression in circCUL2 overexpression NFs. F-H. Representative cytokine arrays for circCUL2-overexpression NFs and control (n = 3). arrows indicate the cytokines with significant changes, which were further confirmed by qRT–PCR and ELISA. I-L EdU assay (I), colony formation (J), Scratch wound healing assays (K) and transwell assays (L) of PANC-1 cells treated with conditioned medium circCUL2-overexpression NFs or anti-IL6. Scale bar, 100 μm. M western blot analysis of STAT3 and p-STAT3 in PANC-1 cells. Data are expressed as the mean ± SD of three independent experiments. **p < 0.01 and ***p < 0.001 (two-tailed Student t-tests) Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35189958), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
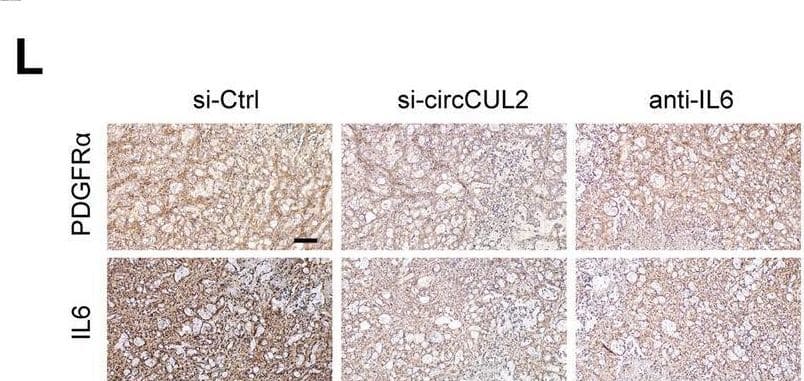
Detection of IL-6 by Immunohistochemistry circCUL2-overexpression NFs promote PDAC progression in vivo. A Representative Bioluminescence images, lung and HE staining of lung tissue of mice 4 weeks after tail vein injection of luc-PANC-1 cell treated with conditioned medium as indicated (n = 8 per group). Scale bar, 100 μm. B Relative luminescence intensity in each group. C Histogram analysis of the metastatic nodules number in per lung. D lung metastasis rate of each group (Chi-square test). E-F Representative bioluminescence images and histogram analysis of luminescence intensity in each at day 30 are shown (n = 6). G Abdominal metastasis rate was calculated for indicated group (Chi-square test). H Representative images of orthotopic model in each group on which autopsy was performed. Red arrow indicated primary tumor; S, spleen; T, primary tumor; M, metastasis. I Images of PDX from 2 patients in 5 mice. (J) Tumor growth curves of indicated group (n = 5). K qRT–PCR analysis of circCUL2 levels in PDX of mice before and after treatment. L Representative images of IHC for PDGFR alpha and IL6. Scale bar, 100 μm. Data are expressed as the mean ± SD. **p < 0.01 and ***p < 0.001 (two-tailed Student t-tests) Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35189958), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
Background: IL-6
Interleukin-6 (IL-6) is a pleiotropic, alpha -helical, phosphorylated and variably glycosylated cytokine that plays important roles in the acute phase reaction, inflammation, hematopoiesis, bone metabolism, and cancer progression. Mature human IL-6 is 183 amino acids (aa) in length expressed as a 22-28 kDA molecular weight protein. IL-6 shares 39% aa sequence identity with mouse and rat IL-6. Alternative splicing generates several isoforms with internal deletions, some of which exhibit antagonistic properties. IL-6 induces signaling through a cell surface heterodimeric receptor complex composed of a ligand binding subunit (IL-6 R alpha) and a signal transducing subunit (gp130). IL-6 binds to IL-6 R alpha, triggering IL-6 R alpha association with gp130 and gp130 dimerization. gp130 is also a component of the receptors for CLC, CNTF, CT-1, IL-11, IL-27, LIF, and OSM. Soluble forms of IL-6 R alpha are generated by both alternative splicing and proteolytic cleavage. In a mechanism known as trans-signaling, complexes of soluble IL-6 and IL-6 R alpha elicit responses from gp130-expressing cells that lack cell surface IL-6 R alpha. Trans-signaling enables a wider range of cell types to respond to IL-6, as the expression of gp130 is ubiquitous, while that of IL-6 R alpha is predominantly restricted to hepatocytes, monocytes, and resting lymphocytes. Soluble splice forms of gp130 block trans-signaling from IL-6/IL-6 R alpha but not from other cytokines that use gp130 as a co-receptor. IL-6, along with TNF-alpha and IL-1, function to drive the acute inflammatory response and the transition from acute inflammation to either acquired immunity or chronic inflammatory disease. When dysregulated, it contributes to chronic inflammation in obesity, insulin resistance, inflammatory bowel disease, arthritis, sepsis, and atherosclerosis. IL-6 can also function as an anti-inflammatory molecule, as in skeletal muscle where it is secreted in response to exercise. In addition, it enhances hematopoietic stem cell proliferation and the differentiation of Th17 cells, memory B cells, and plasma cells.
Product Datasheets
Citations for Human/Primate IL-6 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
113
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Machine learning identifies molecular regulators and therapeutics for targeting SARS‐CoV2‐induced cytokine release
Authors: Marina Chan, Siddharth Vijay, John McNevin, M Juliana McElrath, Eric C Holland, Taranjit S Gujral
Molecular Systems Biology
-
Hypothermic Ex Situ Perfusion of Human Limbs With Acellular Solution for 24 Hours
Authors: Valentin Haug, Branislav Kollar, Sotirios Tasigiorgos, Yori Endo, Martin Kauke, Ali-Farid Safi et al.
Transplantation
-
Multiplexed protein analysis using encoded antibody-conjugated microbeads
Authors: Nora Theilacker, Eric E. Roller, Kristopher D. Barbee, Matthias Franzreb, Xiaohua Huang
Journal of The Royal Society Interface
-
TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer
Authors: Zhan Yao, Silvia Fenoglio, Ding Cheng Gao, Matthew Camiolo, Brendon Stiles, Trine Lindsted et al.
Proceedings of the National Academy of Sciences
-
ID1-induced p16/IL6 axis activation contributes to the resistant of hepatocellular carcinoma cells to sorafenib
Authors: LL Niu, CL Cheng, MY Li, SL Yang, BG Hu, CCN Chong, SL Chan, J Ren, GG Chen, PBS Lai
Cell Death Dis, 2018-08-28;9(9):852.
-
Simultaneous detection of small molecules, proteins and microRNAs using single molecule arrays†
Authors: Xu Wang, David R. Walt
Chemical Science
-
Palmitate Has Proapoptotic and Proinflammatory Effects on Articular Cartilage and Synergizes With Interleukin-1
Authors: Oscar Alvarez-Garcia, Nicole H. Rogers, Roy G. Smith, Martin K. Lotz
Arthritis & Rheumatology
-
Odor Enrichment Attenuates the Anesthesia/Surgery-induced Cognitive Impairment
Authors: Ce Zhang, Yuan Han, Xiaojun Liu, Hong Tan, Yuanlin Dong, Yiying Zhang et al.
Annals of Surgery
-
Tumor‐associated macrophages promote the metastasis and growth of non‐small‐cell lung cancer cells through NF‐ kappa B/PP2Ac‐positive feedback loop
Authors: Zhan‐Wen Liang, Xin‐Xin Ge, Meng‐Dan Xu, Hualong Qin, Meng‐Yao Wu, Meng Shen et al.
Cancer Science
-
Integrated pipeline for ultrasensitive protein detection in cancer nanomedicine†
Authors: Chi-An Cheng, Li-Chiao Chiang, Yu-Syuan Chu
RSC Advances
-
Annexin A1‑mediated inhibition of inflammatory cytokines may facilitate the resolution of inflammation in acute radiation‑induced lung injury
Authors: Gaohua Han, Kaijin Lu, Wansong Xu, Sihui Zhang, Junxing Huang, Chunlei Dai et al.
Oncology Letters
-
Increased infiltration of macrophages to radioresistant lung cancer cells contributes to the development of the additional resistance of tumor cells to the cytotoxic effects of NK cells
Authors: Mingjing Shen, Yongbing Chen, Lijun Xu, Rongying Zhu, Xiang Xue, Ying Tsai et al.
International Journal of Oncology
-
Muscle-derived interleukin 6 increases exercise capacity by signaling in osteoblasts
Authors: Subrata Chowdhury, Logan Schulz, Biagio Palmisano, Parminder Singh, Julian M. Berger, Vijay K. Yadav et al.
Journal of Clinical Investigation
-
CD28 Individual Signaling Up-regulates Human IL-17A Expression by Promoting the Recruitment of RelA/NF-kappa B and STAT3 Transcription Factors on the Proximal Promoter
Authors: Martina Kunkl, Marta Mastrogiovanni, Nicla Porciello, Silvana Caristi, Emanuele Monteleone, Stefano Arcieri et al.
Frontiers in Immunology
-
Tumor-derived mesenchymal-stem-cell-secreted IL-6 enhances resistance to cisplatin via the STAT3 pathway in breast cancer
Authors: H Xu, Y Zhou, W Li, B Zhang, H Zhang, S Zhao, P Zheng, H Wu, J Yang
Oncol Lett, 2018-04-11;15(6):9142-9150.
-
IL-6 Trans–Signaling Links Inflammation with Angiogenesis in the Peritoneal Membrane
Authors: Rusan Catar, Janusz Witowski, Nan Zhu, Christian Lücht, Alicia Derrac Soria, Javier Uceda Fernandez et al.
Journal of the American Society of Nephrology
-
A high-resolution real-time quantification of astrocyte cytokine secretion under shear stress for investigating hydrocephalus shunt failure
Authors: Fatemeh Khodadadei, Allen P. Liu, Carolyn A. Harris
Communications Biology
-
Stromal cells positively and negatively modulate the growth of cancer cells: stimulation via the PGE2-TNFalpha-IL-6 pathway and inhibition via secreted GAPDH-E-cadherin interaction.
Authors: Kawada M, Inoue H, Ohba S, Yoshida J, Masuda T, Yamasaki M, Usami I, Sakamoto S, Abe H, Watanabe T, Yamori T, Shibasaki M, Nomoto A
PLoS ONE, 2015-03-18;10(3):e0119415.
-
CAFs enhance paclitaxel resistance by inducing EMT through the IL‑6/JAK2/STAT3 pathway
Authors: Linlin Wang, Fang Zhang, Jian‑Ying Cui, Liang Chen, Yue‑Ting Chen, Bo‑Wen Liu
Oncology Reports
-
Characterization of Innate Immunity in an Extended Whole Blood Model of Human Islet Allotransplantation
Authors: Maria Hårdstedt, Susanne Lindblom, Alex Karlsson-Parra, Bo Nilsson, Olle Korsgren
Cell Transplantation
-
T-2 Toxin-Induced Hepatotoxicity in HepG2 Cells Involves the Inflammatory and Nrf2/HO-1 Pathways
Authors: Taroncher, M;Franco-Campos, F;Rodríguez-Carrasco, Y;Ruiz, MJ;
Toxins
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Nanoparticles of Lactiplantibacillus plantarum K8 Reduce Staphylococcus aureus Respiratory Infection and Tumor Necrosis Factor Alpha- and Interferon Gamma-Induced Lung Inflammation
Authors: Hong, J;Son, M;Sin, J;Kim, H;Chung, DK;
Nutrients
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Capture -
Integrated pipeline for ultrasensitive protein detection in cancer nanomedicine†
Authors: Chi-An Cheng, Li-Chiao Chiang, Yu-Syuan Chu
RSC Advances
Species: Human
Sample Types: Cell Culture Supernates, Cell Lysates
Applications: Capture Assay -
Maternal pre-eclampsia serum increases neurite growth and mitochondrial function through a potential IL-6-dependent mechanism in differentiated SH-SY5Y cells
Authors: A Barron, S Manna, CJ McElwain, A Musumeci, FP McCarthy, GW O'Keeffe, CM McCarthy
Frontiers in Physiology, 2023-01-12;13(0):1043481.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
A portable analog front-end system for label-free sensing of proteins using nanowell array impedance sensors
Authors: M Tayyab, P Xie, MA Sami, H Raji, Z Lin, Z Meng, SR Mahmoodi, M Javanmard
Scientific Reports, 2022-11-22;12(1):20119.
Species: Human
Sample Types: Recombinant Protein
Applications: ELISA Capture -
IFNgamma-primed periodontal ligament cells regulate T-cell responses via IFNgamma-inducible mediators and ICAM-1-mediated direct cell contact
Authors: W Singhatana, S Kitpakorns, M Toso, P Pavasant
Royal Society open science, 2022-07-27;9(7):220056.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Treg tissue stability depends on lymphotoxin beta-receptor- and adenosine-receptor-driven lymphatic endothelial cell responses
Authors: V Saxena, W Piao, L Li, C Paluskievi, Y Xiong, T Simon, R Lakhan, CC Brinkman, S Walden, KL Hippen, M WillsonShi, YS Lee, C Wagner, BR Blazar, JS Bromberg
Cell Reports, 2022-04-19;39(3):110727.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Colorectal cancer-associated fibroblasts promote metastasis by up-regulating LRG1 through stromal IL-6/STAT3 signaling
Authors: B Zhong, B Cheng, X Huang, Q Xiao, Z Niu, YF Chen, Q Yu, W Wang, XJ Wu
Cell Death & Disease, 2021-12-20;13(1):16.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Dynamic interplay of two molecular switches enabled by the MEK1/2-ERK1/2 and IL-6-STAT3 signaling axes controls epithelial cell migration in response to growth factors
Authors: L Qin, X Cao, T Kaneko, C Voss, X Liu, G Wang, SS Li
The Journal of Biological Chemistry, 2021-09-02;0(0):101161.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Functional inhibition of cancer stemness-related protein DPP4 rescues tyrosine kinase inhibitor resistance in renal cell carcinoma
Authors: S Kamada, T Namekawa, K Ikeda, T Suzuki, M Kagawa, H Takeshita, A Yano, K Okamoto, T Ichikawa, K Horie-Inou, S Kawakami, S Inoue
Oncogene, 2021-05-10;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Interleukin-6 participates in human pancreatic stellate cell activation and collagen I production via TGF-&beta1/Smad pathway
Authors: M Zheng, H Li, L Sun, DR Brigstock, R Gao
Cytokine, 2021-04-21;0(0):155536.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Interleukin-6 as an enhancer of anti-angiogenic therapy for ovarian clear cell carcinoma
Authors: T Seki, N Yanaihara, JS Shapiro, M Saito, J Tabata, R Yokomizo, D Noguchi, T Kuroda, A Kawabata, J Suzuki, K Takahashi, H Matsuzawa, M Miyake, M Takenaka, Y Iida, S Yanagida, A Okamoto
Scientific Reports, 2021-04-08;11(1):7689.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Interleukin-1&alpha Is a Critical Mediator of the Response of Human Bronchial Fibroblasts to Eosinophilic Inflammation
Authors: K Bernau, JP Leet, H Floerke, EM Bruhn, AL Noll, IS McDermott, S Esnault, NN Jarjour, N Sandbo
Cells, 2021-03-02;10(3):.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Capture -
The vitamin D analogue calcipotriol promotes an anti-tumorigenic phenotype of human pancreatic CAFs but reduces T cell mediated immunity
Authors: L Gorchs, S Ahmed, C Mayer, A Knauf, C Fernández, M Svensson, R Heuchel, E Rangelova, P Bergman, H Kaipe
Sci Rep, 2020-10-15;10(1):17444.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Capture -
Suppressive myeloid cells are expanded by biliary tract cancer-derived cytokines in vitro and associate with aggressive disease
Authors: MB Ware, MY Zaidi, J Yang, MK Turgeon, A Krasinskas, TA Mace, K Keenan, MR Farren, AN Ruggieri, Y Li, C Zhang, Z Chen, GS Young, O Elnaggar, Z Che, SK Maithel, T Bekaii-Saa, B El-Rayes, GB Lesinski
Br. J. Cancer, 2020-08-04;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Shikonin blocks human lung adenocarcinoma cell migration and invasion in the inflammatory microenvironment via the IL?6/STAT3 signaling pathway
Authors: T Pan, F Zhang, F Li, X Gao, Z Li, X Li, X Ren
Oncol. Rep., 2020-07-09;44(3):1049-1063.
Species: Human
Sample Types: Cells, Whole Cells
Applications: B/N, Neutralization -
Mycobacterium tuberculosis Lipoarabinomannan Activates Human Neutrophils via a TLR2/1 Mechanism Distinct from Pam3CSK4
Authors: JS Hook, M Cao, K Weng, N Kinnare, JG Moreland
J. Immunol., 2019-12-23;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Capture -
Tumor suppressor HIC1 is synergistically compromised by cancer-associated fibroblasts and tumor cells through the IL-6/pSTAT3 axis in breast cancer
Authors: X Sun, Q Qu, Y Lao, M Zhang, X Yin, H Zhu, Y Wang, J Yang, J Yi, M Hao
BMC Cancer, 2019-12-03;19(1):1180.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Capture -
Hydrogel based protein biochip for parallel detection of biomarkers for diagnosis of a Systemic Inflammatory Response Syndrome (SIRS) in human serum
Authors: A Stumpf, T Brandstett, J Hübner, J Rühe
PLoS ONE, 2019-12-02;14(12):e0225525.
Species: Human
Sample Types: Serum
Applications: ELISA Capture -
Early loss of Histone H2B monoubiquitylation alters chromatin accessibility and activates key immune pathways that facilitate progression of ovarian cancer
Authors: J Hooda, M Novak, MP Salomon, C Matsuba, RI Ramos, E MacDuffie, M Song, MS Hirsch, J Lester, V Parkash, BY Karlan, M Oren, DS Hoon, R Drapkin
Cancer Res., 2018-12-18;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Exposure to wild-type AAV drives distinct capsid immunity profiles in humans
Authors: K Kuranda, P Jean-Alpho, C Leborgne, R Hardet, F Collaud, S Marmier, H Costa Verd, G Ronzitti, P Veron, F Mingozzi
J. Clin. Invest., 2018-10-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy
Authors: SE Logue, EP McGrath, P Cleary, S Greene, K Mnich, A Almanza, E Chevet, RM Dwyer, A Oommen, P Legembre, F Godey, EC Madden, B Leuzzi, J Obacz, Q Zeng, JB Patterson, R Jäger, AM Gorman, A Samali
Nat Commun, 2018-08-15;9(1):3267.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
The PD-L1- and IL6-mediated dampening of the IL27/STAT1 anticancer responses are prevented by ?-PD-L1 or ?-IL6 antibodies
Authors: C Rolvering, AD Zimmer, A Ginolhac, C Margue, M Kirchmeyer, F Servais, HM Hermanns, S Hergovits, PV Nazarov, N Nicot, S Kreis, S Haan, I Behrmann, C Haan
J. Leukoc. Biol., 2018-07-24;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Reactive astrocytic S1P3 signaling modulates the blood-tumor barrier in brain metastases
Authors: B Gril, AN Paranjape, S Woditschka, E Hua, EL Dolan, J Hanson, X Wu, W Kloc, E Izycka-Swi, R Duchnowska, R P?ksa, W Biernat, J Jassem, N Nayyar, PK Brastianos, OM Hall, CJ Peer, WD Figg, GT Pauly, C Robinson, S Difilippan, E Bialecki, P Metellus, JP Schneider, PS Steeg
Psychoneuroendocrinology, 2018-07-13;9(1):2705.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Human central nervous system astrocytes support survival and activation of B cells: implications for MS pathogenesis
Authors: H Touil, A Kobert, N Lebeurrier, A Rieger, P Saikali, C Lambert, L Fawaz, CS Moore, A Prat, J Gommerman, JP Antel, Y Itoyama, I Nakashima, A Bar-Or
J Neuroinflammation, 2018-04-19;15(1):114.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Age-Related Changes to Human Tear Composition
Authors: A Micera, A Di Zazzo, G Esposito, R Longo, W Foulsham, R Sacco, R Sgrulletta, S Bonini
Invest. Ophthalmol. Vis. Sci., 2018-04-01;59(5):2024-2031.
Species: Human
Sample Types: Tears
-
Diabetes mellitus secondary to pancreatic diseases (type 3c): The effect of smoking on the exocrine-endocrine interactions of the pancreas
Authors: M ?liwi?ska-, S Milnerowic, H Milnerowic
Diab Vasc Dis Res, 2018-03-21;0(0):1479164118764.
Species: Human
Sample Types: Whole Cells
Applications: IHC -
Tumor-Derived Mesenchymal Stem Cells Use Distinct Mechanisms to Block the Activity of Natural Killer Cell Subsets
Authors: S Galland, J Vuille, P Martin, I Letovanec, A Caignard, G Fregni, I Stamenkovi
Cell Rep, 2017-09-19;20(12):2891-2905.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
IL-33 Attenuates Sepsis by Inhibiting IL-17 Receptor Signaling through Upregulation of SOCS3
Authors: R Lv, J Zhao, M Lei, D Xiao, Y Yu, J Xie
Cell. Physiol. Biochem., 2017-08-09;42(5):1961-1972.
Species: Human
Sample Types: Serum
Applications: ELISA Development (Capture) -
Sensitization of EGFR wild-type non-small cell lung cancer cells to EGFR-tyrosine kinase inhibitor erlotinib
Authors: J Raimbourg, MP Joalland, M Cabart, L de Plater, F Bouquet, A Savina, D Decaudin, J Bennouna, FM Vallette, L Lalier
Mol. Cancer Ther., 2017-05-18;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line
Authors: P Ortiz-Mont, A Londoño-Va, JP Vernot
Cell Commun. Signal, 2017-05-04;15(1):17.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Interleukin?6 induces an epithelial?mesenchymal transition phenotype in human adamantinomatous craniopharyngioma cells and promotes tumor cell migration
Authors: J Zhou, C Zhang, J Pan, L Chen, ST Qi
Mol Med Rep, 2017-05-02;15(6):4123-4131.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Adipogenic differentiation of MSC alters their immunomodulatory properties in a tissue-specific manner
Authors: H Munir, LS Ward, L Sheriff, S Kemble, S Nayar, F Barone, GB Nash, HM McGettrick
Stem Cells, 2017-04-24;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Maslinic acid suppresses the growth of human gastric cells by inducing apoptosis via inhibition of the interleukin-6 mediated Janus kinase/signal transducer and activator of transcription 3 signaling pathway
Authors: D Wang, S Tang, Q Zhang
Oncol Lett, 2017-04-21;13(6):4875-4881.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Interleukin-10 and prostaglandin E2 have complementary but distinct suppressive effects on Toll-like receptor-mediated dendritic cell activation in ovarian carcinoma
Authors: E Brencicova, AL Jagger, HG Evans, M Georgouli, A Laios, S Attard Mon, G Mehra, J Spencer, AA Ahmed, S Raju-Kanki, LS Taams, SS Diebold
PLoS ONE, 2017-04-14;12(4):e0175712.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development (Capture) -
Epstein-Barr virus-induced gene 3 (EBI3) can mediate IL-6 trans-signaling
Authors: S Chehboun, J Labrecque-, S Pasquin, Y Meliani, B Meddah, W Ferlin, M Sharma, A Tormo, JF Masson, JF Gauchat
J. Biol. Chem, 2017-03-09;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory FADDosome Complex upon TRAIL Stimulation
Authors: CM Henry, SJ Martin
Mol. Cell, 2017-02-16;65(4):715-729.e5.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Monocyte Subsets Coregulate Inflammatory Responses by Integrated Signaling through TNF and IL-6 at the Endothelial Cell Interface
Authors: M Chimen, CM Yates, HM McGettrick, LS Ward, MJ Harrison, B Apta, LH Dib, BA Imhof, P Harrison, GB Nash, GE Rainger
J. Immunol, 2017-02-13;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Cytosolic DNA Promotes Signal Transducer and Activator of Transcription 3 (STAT3) Phosphorylation by TANK-binding kinase 1 (TBK1) to Restrain STAT3 Activity
Authors: HC Hsia, JE Hutti, AS Baldwin
J. Biol. Chem, 2017-02-10;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Bone metastasis of prostate cancer can be therapeutically targeted at the TBX2-WNT signaling axis
Authors: S Nandana, M Tripathi, P Duan, CY Chu, R Mishra, C Liu, R Jin, H Yamashita, M Zayzafoon, NA Bhowmick, HE Zhau, RJ Matusik, LW Chung
Cancer Res, 2017-01-20;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
IL-6 trans-signaling is another pathway to upregulate Osteopontin
Cytokine, 2016-11-15;90(0):88-95.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Comparative Ability of Mesenchymal Stromal Cells from Different Tissues to Limit Neutrophil Recruitment to Inflamed Endothelium
PLoS ONE, 2016-05-12;11(5):e0155161.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Characterization of Innate Immunity in an Extended Whole Blood Model of Human Islet Allotransplantation
Authors: Maria Hårdstedt, Susanne Lindblom, Alex Karlsson-Parra, Bo Nilsson, Olle Korsgren
Cell Transplantation
Species: Human
Sample Types: Plasma
Applications: ELISA Detection -
Interleukin-1 Receptor Type 2 Acts with c-Fos to Enhance the Expression of Interleukin-6 and Vascular Endothelial Growth Factor A in Colon Cancer Cells and Induce Angiogenesis.
Authors: Mar A, Chu C, Lee H, Chien C, Cheng J, Yang S, Jiang J, Lee T
J Biol Chem, 2015-07-24;290(36):22212-24.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
CXCL8 and CCL20 Enhance Osteoclastogenesis via Modulation of Cytokine Production by Human Primary Osteoblasts.
Authors: Pathak J, Bakker A, Verschueren P, Lems W, Luyten F, Klein-Nulend J, Bravenboer N
PLoS ONE, 2015-06-23;10(6):e0131041.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Chemotherapy-Derived Inflammatory Responses Accelerate the Formation of Immunosuppressive Myeloid Cells in the Tissue Microenvironment of Human Pancreatic Cancer.
Authors: Takeuchi S, Baghdadi M, Tsuchikawa T, Wada H, Nakamura T, Abe H, Nakanishi S, Usui Y, Higuchi K, Takahashi M, Inoko K, Sato S, Takano H, Shichinohe T, Seino K, Hirano S
Cancer Res, 2015-05-07;75(13):2629-40.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Neutralization -
Secreted ectodomain of sialic acid-binding Ig-like lectin-9 and monocyte chemoattractant protein-1 promote recovery after rat spinal cord injury by altering macrophage polarity.
Authors: Matsubara K, Matsushita Y, Sakai K, Kano F, Kondo M, Noda M, Hashimoto N, Imagama S, Ishiguro N, Suzumura A, Ueda M, Furukawa K, Yamamoto A
J Neurosci, 2015-02-11;35(6):2452-64.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
Urocortin 2 role in placental and myometrial inflammatory mechanisms at parturition.
Authors: Voltolini C, Battersby S, Novembri R, Torricelli M, Severi F, Petraglia F, Norman J
Endocrinology, 2014-11-26;156(2):670-9.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development (Capture) -
Podocytes regulate neutrophil recruitment by glomerular endothelial cells via IL-6-mediated crosstalk.
Authors: Kuravi S, McGettrick H, Satchell S, Saleem M, Harper L, Williams J, Rainger G, Savage C
J Immunol, 2014-05-28;193(1):234-43.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Epstein-Barr virus infection induces indoleamine 2,3-dioxygenase expression in human monocyte-derived macrophages through p38/mitogen-activated protein kinase and NF-kappaB pathways: impairment in T cell functions.
Authors: Liu W, Lin Y, Xiao H, Xing S, Chen H, Chi P, Zhang G
J Virol, 2014-04-02;88(12):6660-71.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Digital microfluidic assay for protein detection.
Authors: Mok J, Mindrinos M, Davis R, Javanmard M
Proc Natl Acad Sci U S A, 2014-01-21;111(6):2110-5.
Species: N/A
Sample Types: Beads, Recombinant Protein
Applications: Immunoassay Development -
Reference range and short- and long-term biological variation of interleukin (IL)-6, IL-17A and tissue necrosis factor-alpha using high sensitivity assays.
Authors: Todd J, Simpson P, Estis J, Torres V, Wub A
Cytokine, 2013-10-12;64(3):660-5.
Species: Human
Sample Types: Plasma
Applications: ELISA Development -
Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner.
Authors: Mace T, Ameen Z, Collins A, Wojcik S, Mair M, Young G, Fuchs J, Eubank T, Frankel W, Bekaii-Saab T, Bloomston M, Lesinski G
Cancer Res, 2013-03-20;73(10):3007-18.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Monocytes Induce STAT3 Activation in Human Mesenchymal Stem Cells to Promote Osteoblast Formation.
Authors: Nicolaidou V, Wong MM, Redpath AN
PLoS ONE, 2012-07-03;7(7):e39871.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Pre-analytical effects of blood sampling and handling in quantitative immunoassays for rheumatoid arthritis.
Authors: Zhao X, Qureshi F, Eastman PS, Manning WC, Alexander C, Robinson WH, Hesterberg LK
J. Immunol. Methods, 2012-02-17;378(1):72-80.
Species: Human
Sample Types: Serum
Applications: ELISA Development -
Measurement of interleukin-33 (IL-33) and IL-33 receptors (sST2 and ST2L) in patients with rheumatoid arthritis.
Authors: Hong YS, Moon SJ, Moon S, Joo YB, Joo Y, Jeon CH, Jeon C, Cho ML, Ju JH, Oh HJ, Heo YJ, Heo Y, Park SH, Kim HY, Min JK
J Korean Med Sci, 2011-09-01;26(9):1132-9.
Species: Human
Sample Types: Serum
Applications: ELISA Development -
Protection from RNA and DNA viruses by IL-32.
Authors: Zepp JA, Nold-Petry CA, Dinarello CA, Nold MF
J. Immunol., 2011-02-23;186(7):4110-8.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Electrochemiluminescent Assay -
Phagocytosis of apoptotic cells modulates mesenchymal stem cells osteogenic differentiation to enhance IL-17 and RANKL expression on CD4+ T cells.
Authors: Tso GH, Law HK, Tu W
Stem Cells, 2010-05-01;28(5):939-54.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Resistance of human alveolar macrophages to Bacillus anthracis lethal toxin.
Authors: Wu W, Mehta H, Chakrabarty K, Booth JL, Duggan ES, Patel KB, Ballard JD, Coggeshall KM, Metcalf JP
J. Immunol., 2009-10-07;183(9):5799-806.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Noninvasive detection of acute and chronic injuries in human renal transplant by elevation of multiple cytokines/chemokines in urine.
Authors: Hu H, Kwun J, Aizenstein BD, Knechtle SJ
Transplantation, 2009-06-27;87(12):1814-20.
Species: Human
Sample Types: Urine
Applications: Antibody Array Development -
TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23.
Authors: Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M
Eur. J. Immunol., 2009-05-01;39(5):1221-30.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Human airway smooth muscle promotes human lung mast cell survival, proliferation, and constitutive activation: cooperative roles for CADM1, stem cell factor, and IL-6.
Authors: Hollins F, Kaur D, Yang W, Cruse G, Saunders R, Sutcliffe A, Berger P, Ito A, Brightling CE, Bradding P
J. Immunol., 2008-08-15;181(4):2772-80.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Maternal and cord plasma cytokine and chemokine profile in pregnancies complicated by asthma.
Authors: Osei-Kumah A, Smith R, Clifton VL
Cytokine, 2008-07-17;43(2):187-93.
Species: Human
Sample Types: Plasma
Applications: ELISA Development -
Endogenous IL-32 controls cytokine and HIV-1 production.
Authors: Nold MF, Nold-Petry CA, Pott GB, Zepp JA, Saavedra MT, Kim SH, Dinarello CA
J. Immunol., 2008-07-01;181(1):557-65.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Electrochemiluminescent Assay -
C5a and TNF-alpha up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome.
Authors: Kambas K, Markiewski MM, Pneumatikos IA, Rafail SS, Theodorou V, Konstantonis D, Kourtzelis I, Doumas MN, Magotti P, Deangelis RA, Lambris JD, Ritis KD
J. Immunol., 2008-06-01;180(11):7368-75.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Bacterial vaginosis, not HIV, is primarily responsible for increased vaginal concentrations of proinflammatory cytokines.
Authors: Mitchell CM, Balkus J, Agnew KJ, Cohn S, Luque A, Lawler R, Coombs RW, Hitti JE
AIDS Res. Hum. Retroviruses, 2008-05-01;24(5):667-71.
Species: Human
Sample Types: Vaginal Fluid
Applications: ELISA Development -
Lipopolysaccharide-squamous cell carcinoma-monocyte interactions induce cancer-supporting factors leading to rapid STAT3 activation.
Authors: Kurago ZB, Lam-ubol A, Stetsenko A
Head Neck Pathol, 2008-03-01;2(1):1-12.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling.
Authors: Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F
Stem Cells, 2007-10-25;26(1):279-89.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Tumor necrosis factor alpha as a new target for renal cell carcinoma: two sequential phase II trials of infliximab at standard and high dose.
Authors: Harrison ML, Obermueller E, Maisey NR, Hoare S, Edmonds K, Li NF, Chao D, Hall K, Lee C, Timotheadou E, Charles K, Ahern R, King DM, Eisen T, Corringham R, DeWitte M, Balkwill F, Gore M
J. Clin. Oncol., 2007-10-10;25(29):4542-9.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-Fr -
Ultrasensitive flow-based immunoassays using single-molecule counting.
Authors: Todd J, Freese B, Lu A, Held D, Morey J, Livingston R, Goix P
Clin. Chem., 2007-09-21;53(11):1990-5.
Species: Human
Sample Types: Plasma
Applications: ELISA Development -
Peroxisome proliferator-activated receptor-gamma agonists inhibit respiratory syncytial virus-induced expression of intercellular adhesion molecule-1 in human lung epithelial cells.
Authors: Arnold R, Neumann M, Konig W
Immunology, 2007-05-01;121(1):71-81.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Differential survival of leukocyte subsets mediated by synovial, bone marrow, and skin fibroblasts: site-specific versus activation-dependent survival of T cells and neutrophils.
Authors: Filer A, Parsonage G, Smith E, Osborne C, Thomas AM, Curnow SJ, Rainger GE, Raza K, Nash GB, Lord J, Salmon M, Buckley CD
Arthritis Rheum., 2006-07-01;54(7):2096-108.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Th2 cytokine response in Major Depressive Disorder patients before treatment.
Authors: Kiryushko D, Pavon L, Novitskaya V, Sandoval-Lopez G, Soroka V, Eugenia Hernandez M, Klingelhofer J, Loria F, Lukanidin E, Estrada I, Berezin V, Perez M, Bock E, Moreno J, Avila U, Leff P, Anton B, Heinze G
J. Neuroimmunol., 2006-02-02;172(1):156-65.
Species: Human
Sample Types: Serum
Applications: ELISA Development -
Interleukin-6 and interleukin-8 in cervical fluid in a population of Swedish women in preterm labor: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation, and preterm delivery.
Authors: Holst RM, Mattsby-Baltzer I, Wennerholm UB, Hagberg H, Jacobsson B
Acta Obstet Gynecol Scand, 2005-06-01;84(6):551-7.
Species: Human
Sample Types: Amniotic Fluid
Applications: ELISA Development -
trans fatty acids and systemic inflammation in heart failure.
Authors: Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC
Am. J. Clin. Nutr., 2004-12-01;80(6):1521-5.
Species: Human
Sample Types: Plasma
Applications: ELISA Development -
Rapid analysis of inflammatory cytokines in cerebrospinal fluid using chip-based immunoaffinity electrophoresis.
Authors: Phillips TM
Electrophoresis, 2004-06-01;25(10):1652-9.
Species: Human
Sample Types: Serum
Applications: Affinity Chromatography -
Direct comparison of traditional ELISAs and membrane protein arrays for detection and quantification of human cytokines.
Authors: Copeland S, Siddiqui J, Remick D
J. Immunol. Methods, 2004-01-01;284(1):99-106.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Array Development -
Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation.
Authors: McLoughlin RM, Witowski J, Robson RL, Wilkinson TS, Hurst SM, Williams AS, Williams JD, Rose-John S, Jones SA, Topley N
J. Clin. Invest., 2003-08-01;112(4):598-607.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Secretion of oncostatin M by infiltrating neutrophils: regulation of IL-6 and chemokine expression in human mesothelial cells.
Authors: Hurst SM, McLoughlin RM, Monslow J, Owens S, Morgan L, Fuller GM, Topley N, Jones SA
J. Immunol., 2002-11-01;169(9):5244-51.
Species: Human
Sample Types: Peritoneal Fluid
Applications: ELISA Development -
The reprogrammed host: Chlamydia trachomatis-induced up-regulation of glycoprotein 130 cytokines, transcription factors, and antiapoptotic genes.
Authors: Hess S, 2019, Rheinheimer C, Tidow F, Bartling G, Kaps C, Lauber J, Buer J, Klos A
7046, 2001-10-01;44(10):2392-401.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Dual-Enhanced Plasmonic Biosensing for Point-of-Care Sepsis Detection
Authors: Lip Ket Chin, Jun-Yeong Yang, Benjamin Chousterman, Sunghoon Jung, Do-Geun Kim, Dong-Ho Kim et al.
ACS Nano
-
Combination therapy targeting the elevated interleukin‐6 level reduces invasive migration of BRAF inhibitor‐resistant melanoma cells
Authors: Purusottam Mohapatra, Chandra Prakash Prasad, Tommy Andersson
Molecular Oncology
-
Isolation of immunomodulatory triterpene acids from a standardized rose hip powder (Rosa canina L.)
Authors: Lasse Saaby, Anna Katharina Jäger, Lise Moesby, Erik Wind Hansen, Søren Brøgger Christensen
Phytotherapy Research
-
Interleukin-6 is elevated in synovial fluid of patients with focal cartilage defects and stimulates cartilage matrix production in an in vitro regeneration model
Authors: Anika I Tsuchida, Michiel Beekhuizen, Marijn Rutgers, Gerjo J V M van Osch, Joris E J Bekkers, Arjan G J Bot et al.
Arthritis Research & Therapy
-
Acute elevation of interleukin 6 and matrix metalloproteinase 9 during the onset of pituitary apoplexy in Cushing’s disease
Authors: Takako Araki, Jutarat Sangtian, Darin Ruanpeng, Ramachandra Tummala, Brent Clark, Lynn Burmeister et al.
Pituitary
-
Multi-cellular human bronchial models exposed to diesel exhaust particles: assessment of inflammation, oxidative stress and macrophage polarization
Authors: Jie Ji, Swapna Upadhyay, Xiaomiao Xiong, Maria Malmlöf, Thomas Sandström, Per Gerde et al.
Particle and Fibre Toxicology
-
Cytokine detection and simultaneous assessment of rheumatoid factor interference in human serum and synovial fluid using high-sensitivity protein arrays on plasmonic gold chips
Authors: Manfè Valentina, Fleckner Jan, Nørby Lisby Peder, Zhang Bo, Dai Hongjie, Keller Pernille
BMC Biotechnology
-
Sensitive Colorimetric Detection of Interleukin-6 via Lateral Flow Assay Incorporated Silver Amplification Method
Authors: Mohammad Rahbar, Yuling Wu, J. Anand Subramony, Guozhen Liu
Frontiers in Bioengineering and Biotechnology
-
Discovery and Characterization of a Potent Interleukin-6 Binding Peptide with Neutralizing Activity In Vivo
Authors: Sheila Ranganath, Ashok Bhandari, Nicole Avitahl-Curtis, Jaimee McMahon, Derek Wachtel, Jenny Zhang et al.
PLOS ONE
-
Proximity Extension Assay in Combination with Next-Generation Sequencing for High-throughput Proteome-wide Analysis
Authors: Lotta Wik, Niklas Nordberg, John Broberg, Johan Björkesten, Erika Assarsson, Sara Henriksson et al.
Molecular & Cellular Proteomics
-
CD28 Autonomous Signaling Orchestrates IL-22 Expression and IL-22-Regulated Epithelial Barrier Functions in Human T Lymphocytes
Authors: Martina Kunkl, Carola Amormino, Simone Frascolla, Manolo Sambucci, Marco De Bardi, Silvana Caristi et al.
Frontiers in Immunology
-
Plasmacytoid dendritic cells are inefficient in activation of human regulatory T cells.
Authors: Hubo M et al.
PLoS One
-
Probiotic therapy to men with incipient arteriosclerosis initiates increased bacterial diversity in colon: A randomized controlled trial
Authors: Caroline Karlsson, Siv Ahrné, Göran Molin, Anna Berggren, Ingrid Palmquist, Gunilla Nordin Fredrikson et al.
Atherosclerosis
FAQs
-
Which R&D Systems antibody product could be used as an isotype control for catalog # MAB206?
Catalog # MAB002 or MAB002R (Mouse IgG1 isotype control) could be used as a control for catalog # MAB206.
-
What is the light chain of Human/Primate IL-6 Antibody, Catalog # MAB206, Clone # 6708?
Catalog # MAB206 has a kappa light chain.
Reviews for Human/Primate IL-6 Antibody
Average Rating: 4.5 (Based on 14 Reviews)
Have you used Human/Primate IL-6 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
After biotinylation, used as a capture reagent according to the manufacturer’s protocol (Meso Scale Diagnostics LLC). Paired with goat PC IL-6 antibody (AF-206-NA) as a detection antibody. A standard curve with recombinant human IL-6 (Cat# 206-IL/CF) is shown (1.6-25,000 pg/ml).
Li C, Iness A, Yoon J, Grider JR, Murthy KS, Kellum JM, Kuemmerle JF. Increased autocrine IL-6 and STAT3 (Ser727) phosphorylation is profibrotic regulating TGF-β1 and Collagen I in muscle of stricturing Crohn's disease. J Immunol. 194(7):3422-31; 2015