Human SPARC Antibody Summary
Ala18-Ile303
Accession # P09486
Applications
This antibody functions as an ELISA detection antibody when paired with Mouse Anti-Human SPARC Monoclonal Antibody (Catalog # MAB941).
This product is intended for assay development on various assay platforms requiring antibody pairs. We recommend the Human SPARC DuoSet ELISA Kit (Catalog # DY941-05) for convenient development of a sandwich ELISA or the Human SPARC Quantikine ELISA Kit (Catalog # DSP00) for a complete optimized ELISA.
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human SPARC by Western Blot. Western blot shows lysates of MG-63 human osteosarcoma cell line. PVDF membrane was probed with 2 µg/mL of Goat Anti-Human SPARC Antigen Affinity-purified Polyclonal Antibody (Catalog # AF941) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF019). A specific band was detected for SPARC at approximately 43 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
SPARC/Osteonectin in Human Ovary. SPARC/Osteonectin was detected in immersion fixed paraffin-embedded sections of human ovary using Goat Anti-Human SPARC/Osteonectin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF941) at 3 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; CTS008) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
Detection of Human SPARC by Simple WesternTM. Simple Western lane view shows lysates of MG-63 human osteosarcoma cell line, loaded at 0.2 mg/mL. A specific band was detected for SPARC at approximately 57 kDa (as indicated) using 20 µg/mL of Goat Anti-Human SPARC Antigen Affinity-purified Polyclonal Antibody (Catalog # AF941) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF109). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
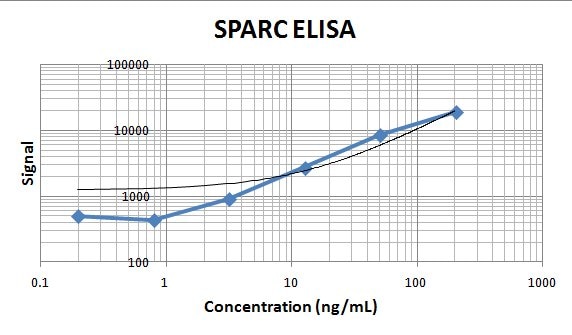
Human SPARC ELISA Standard Curve. Recombinant Human SPARC protein was serially diluted 2-fold and captured by Mouse Anti-Human SPARC Monoclonal Antibody (Catalog # MAB941) coated on a Clear Polystyrene Microplate (DY990). Goat Anti-Human SPARC Antigen Affinity-purified Polyclonal Antibody (Catalog # AF941) was biotinylated and incubated with the protein captured on the plate. Detection of the standard curve was achieved by incubating Streptavidin-HRP (DY998) followed by Substrate Solution (DY999) and stopping the enzymatic reaction with Stop Solution (DY994).
 View Larger
View Larger
Detection of Human SPARC by Immunocytochemistry/ Immunofluorescence Expression of SPARC in human ovarian tissues using antibody AF941 and bs-1133R.(A) Normal human ovarian tissue using antibody AF941, (B) Benign ovarian tumor using antibody AF941, (C) High differentiation of ovarian carcinoma using antibody AF941, (D) Medium differentiation of ovarian carcinoma using antibody AF941, (E) Low differentiation of ovarian carcinoma using antibody AF941, (F) Normal human ovarian tissue using antibody bs-1133R, (G) Benign ovarian tumor using antibody bs-1133R, (H) High differentiation of ovarian carcinoma using antibody bs-1133R, (I) Medium differentiation of ovarian carcinoma using antibody bs-1133R, (J) Low differentiation of ovarian carcinoma using antibody bs-1133R (Magnification ×200). Black arrows indicate cell cytoplasm stained, red arrows indicate stroma stained. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/22879971), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human SPARC by Immunocytochemistry/ Immunofluorescence Expression of SPARC in human ovarian tissues using antibody AF941 and bs-1133R.(A) Normal human ovarian tissue using antibody AF941, (B) Benign ovarian tumor using antibody AF941, (C) High differentiation of ovarian carcinoma using antibody AF941, (D) Medium differentiation of ovarian carcinoma using antibody AF941, (E) Low differentiation of ovarian carcinoma using antibody AF941, (F) Normal human ovarian tissue using antibody bs-1133R, (G) Benign ovarian tumor using antibody bs-1133R, (H) High differentiation of ovarian carcinoma using antibody bs-1133R, (I) Medium differentiation of ovarian carcinoma using antibody bs-1133R, (J) Low differentiation of ovarian carcinoma using antibody bs-1133R (Magnification ×200). Black arrows indicate cell cytoplasm stained, red arrows indicate stroma stained. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/22879971), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human SPARC by Immunocytochemistry/ Immunofluorescence Expression of SPARC in human ovarian tissues using antibody AF941 and bs-1133R.(A) Normal human ovarian tissue using antibody AF941, (B) Benign ovarian tumor using antibody AF941, (C) High differentiation of ovarian carcinoma using antibody AF941, (D) Medium differentiation of ovarian carcinoma using antibody AF941, (E) Low differentiation of ovarian carcinoma using antibody AF941, (F) Normal human ovarian tissue using antibody bs-1133R, (G) Benign ovarian tumor using antibody bs-1133R, (H) High differentiation of ovarian carcinoma using antibody bs-1133R, (I) Medium differentiation of ovarian carcinoma using antibody bs-1133R, (J) Low differentiation of ovarian carcinoma using antibody bs-1133R (Magnification ×200). Black arrows indicate cell cytoplasm stained, red arrows indicate stroma stained. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/22879971), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human SPARC by Immunocytochemistry/ Immunofluorescence Expression of SPARC in human ovarian tissues using antibody AF941 and bs-1133R.(A) Normal human ovarian tissue using antibody AF941, (B) Benign ovarian tumor using antibody AF941, (C) High differentiation of ovarian carcinoma using antibody AF941, (D) Medium differentiation of ovarian carcinoma using antibody AF941, (E) Low differentiation of ovarian carcinoma using antibody AF941, (F) Normal human ovarian tissue using antibody bs-1133R, (G) Benign ovarian tumor using antibody bs-1133R, (H) High differentiation of ovarian carcinoma using antibody bs-1133R, (I) Medium differentiation of ovarian carcinoma using antibody bs-1133R, (J) Low differentiation of ovarian carcinoma using antibody bs-1133R (Magnification ×200). Black arrows indicate cell cytoplasm stained, red arrows indicate stroma stained. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/22879971), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
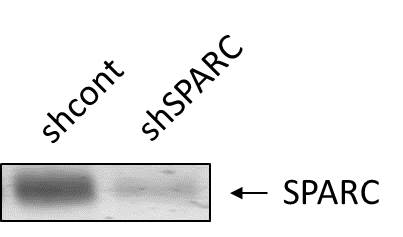
Detection of Human SPARC by Western Blot Verification of knockdown of SPARC expression by lentivirus-mediated RNA interference. (A) GFP expression images showed shRNA delivery efficiency. (Magnification × 200). (B) SPARC protein expressions of SPARC shRNA infected cells, control shRNA infected cells and non-infected cells as measured by Western blot. (C) SPARC mRNA expressions of SPARC shRNA infected cells, control shRNA infected cells and non-infected cells as measured by q-RT-PCR. (D) SPARC protein expressions of SPARC shRNA infected cells, control shRNA infected cells and non-infected cells as measured by ICC staining (Magnification ×200). *P<0.05 versus control. Image collected and cropped by CiteAb from the following open publication (https://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-12-464), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human SPARC by Immunocytochemistry/ Immunofluorescence Overview of Multi-dimensional Microscopic Molecular Profiling (MMMP).The overall MMMP approach is depicted using an example tissue section from normal human duodenum (sample #1.9.7). (a) Slides were subjected to repeated cycles of staining and imaging with fluorescent primary antibodies and DAPI. At the end of each cycle, fluorescent signal was removed by a chemical bleaching process, and slides were again imaged, before proceeding to the next round of this iterative procedure. After the final antibody stain (#15 Sma), slides were analyzed with a series of histochemical stains. (b) A set of tiling images spanning each tissue section was initially generated by the microscope system. The tiling images were then computationally ‘stitched’ together to produce a single image per staining cycle for each sample. (c) Image registration was performed to align images from the same tissue section across cycles. Mean intensities of the DAPI signal from all immuno-fluorescence images are shown from before (Unregistered) and after (Registered) the image registration procedure was completed. (d) Following registration, signal intensities from the relevant channels for each image (columns) in the MMMP series were extracted for each pixel (rows) within the tissue section and compiled into a large data matrix of in situ molecular profiles. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pone.0128975), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human SPARC by Immunocytochemistry/ Immunofluorescence Expression of SPARC in human ovarian tissues using antibody AF941 and bs-1133R.(A) Normal human ovarian tissue using antibody AF941, (B) Benign ovarian tumor using antibody AF941, (C) High differentiation of ovarian carcinoma using antibody AF941, (D) Medium differentiation of ovarian carcinoma using antibody AF941, (E) Low differentiation of ovarian carcinoma using antibody AF941, (F) Normal human ovarian tissue using antibody bs-1133R, (G) Benign ovarian tumor using antibody bs-1133R, (H) High differentiation of ovarian carcinoma using antibody bs-1133R, (I) Medium differentiation of ovarian carcinoma using antibody bs-1133R, (J) Low differentiation of ovarian carcinoma using antibody bs-1133R (Magnification ×200). Black arrows indicate cell cytoplasm stained, red arrows indicate stroma stained. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/22879971), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: SPARC
SPARC, an acronym for “secreted protein, acidic and rich in cysteine”, is also known as osteonectin or BM-40 (1-5). It is the founding member of a family of secreted matricellular proteins with similar domain structure. The 286 amino acid (aa), 43 kDa protein contains an N-terminal acidic region that binds calcium, a follistatin domain that contains Kazal-like sequences, and a C-terminal extracellular calcium (EC) binding domain with two EF-hand motifs (1-5). Crystal structure modeling shows that residues implicated in cell binding, inhibition of cell spreading, and disassembly of focal adhesions cluster on one face of SPARC, while a collagen binding epitope and an N-glycosylation site are opposite this face (6). SPARC is produced by fibroblasts, capillary endothelial cells, platelets and macrophages, especially in areas of tissue morphogenesis and remodeling (3, 7). SPARC shows context-specific effects, but generally inhibits adhesion, spreading and proliferation, and promotes collagen matrix formation (3-5). For endothelial cells, SPARC disrupts focal adhesions and binds and sequesters PDGF and VEGF (3-5). SPARC is abundantly expressed in bone, where it promotes osteoblast differentiation and inhibits adipogenesis (5, 8). SPARC is potentially cleaved by metalloproteinases, producing an angiogenic peptide that includes the copper-binding sequence KGHK (7). Paradoxically, SPARC is highly expressed in many tumor types undergoing an endothelial to mesenchymal transistion; its expression, however, mainly decreases the likelihood of metastasis and confers sensitivity to chemotherapy and radiation (4, 9-11). Stabilin-1, which is expressed on alternately activated macrophages, is the first SPARC receptor to be identified. It binds the SPARC EC domain and mediates endocytosis for degradation (12). Mature human SPARC shows 92%, 92%, 97%, 99%, 96%, and 85% aa identity with mouse, rat, canine, bovine, porcine, and chick SPARC, respectively.
- Lankat-Buttgereit, B. et al. (1988) FEBS Lett. 236:352.
- Sweetwyne, M.T. et al. (2004) J. Histochem. Cytochem. 52:723.
- Sage, H. et al. (1989) J. Cell Biol. 109:341.
- Framson, P.E. and E.H. Sage (2004) J. Cell. Biochem. 92:679.
- Alford, A.I. and K. D. Hankenson (2006) Bone 38:749.
- Hohenester, E et al. (1997) EMBO J. 16:3778.
- Sage, E.H. et al. (2003) J. Biol. Chem. 278:37849.
- Delany, A.M. et al. (2003) Endocrinology 144:2588.
- Robert, G. et al. (2006) Cancer Res. 66:7516.
- Koblinski, J.E. et al. (2005) Cancer Res. 65:7370.
- Tai, I.T. et al. (2005) J. Clin. Invest. 115:1492.
- Kzhyshkowska, J. et al. (2006) J. Immunol. 176:5825.
Product Datasheets
Citations for Human SPARC Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
22
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Expanding therapeutic utility of carfilzomib for breast cancer therapy by novel albumin-coated nanocrystal formulation
Authors: Ji Eun Park, Joonyoung Park, Yearin Jun, Yunseok Oh, Gongmi Ryoo, Yoo-Seong Jeong et al.
Journal of Controlled Release
-
Overexpression of SPARC correlates with poor prognosis in patients with cervical carcinoma and regulates cancer cell epithelial-mesenchymal transition
Authors: DEHUAN SHI, KAN JIANG, YING FU, RUI FANG, X I LIU, JIE CHEN
Oncology Letters
-
A non-immunological role for gamma -interferon–inducible lysosomal thiol reductase (GILT) in osteoclastic bone resorption
Authors: Benjamin W. Ewanchuk, Corey R. Arnold, Dale R. Balce, Priyatha Premnath, Tanis L. Orsetti, Amy L. Warren et al.
Science Advances
-
PAI-1 uncouples integrin-?1 from restrain by membrane-bound ?-catenin to promote collagen fibril remodeling in obesity-related neoplasms
Authors: Lin, LL;Nayak, B;Osmulski, PA;Wang, E;Wang, CP;Valente, PT;Wang, CM;Tan, X;Santanam, N;Wang, TL;Gaczynska, ME;Kost, ER;Huang, TH;Kirma, NB;
Cell reports
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Cell Competition Shapes Metastatic Latency and Relapse
Authors: Kim K, Huang H, Parida PK et al.
Cancer Discovery
-
Imaging of Indocyanine Green-Human Serum Albumin (ICG-HSA) Complex in Secreted Protein Acidic and Rich in Cysteine (SPARC)-Expressing Glioblastoma
Authors: HJ Jang, MG Song, CR Park, H Youn, YS Lee, GJ Cheon, KW Kang
International Journal of Molecular Sciences, 2023-01-03;24(1):.
Species: Mouse
Sample Types: Whole Tissue
Applications: ICC/IF -
Mesenchymal stem cells induce tumor stroma formation and epithelial?mesenchymal transition through SPARC expression in colorectal cancer
Authors: T Naito, R Yuge, Y Kitadai, H Takigawa, Y Higashi, T Kuwai, K Kuraoka, S Tanaka, K Chayama
Oncology reports, 2021-04-28;45(6):.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Accumulation of stabilin-1 positive macrophages in the early stage of gastric cancer is associated with short cumulative survival
Authors: SP Yin, Y Gao, XS Xie, DD Xu, V Riabov, WD Du
Oncol Lett, 2020-01-16;19(3):2404-2412.
Species: Human
Sample Types: Whole Tissue
Applications: IF -
Secreted protein acidic and rich in cysteine mediates active targeting of human serum albumin in U87MG xenograft mouse models
Authors: CR Park, JH Jo, MG Song, JY Park, YH Kim, H Youn, SH Paek, JK Chung, JM Jeong, YS Lee, KW Kang
Theranostics, 2019-10-11;9(24):7447-7457.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
In-depth proteomic analysis of tissue interstitial fluid for hepatocellular carcinoma serum biomarker discovery
Authors: J Zhang, N Hao, W Liu, M Lu, L Sun, N Chen, M Wu, X Zhao, B Xing, W Sun, F He
Br. J. Cancer, 2017-10-12;117(11):1676-1684.
Species: Human
Sample Types: Interstitial Fluid
Applications: Western Blot -
Stabilin-1 is expressed in human breast cancer and supports tumor growth in mammary adenocarcinoma mouse model
Oncotarget, 2016-05-24;7(21):31097-110.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Automated Analysis and Classification of Histological Tissue Features by Multi-Dimensional Microscopic Molecular Profiling.
Authors: Riordan D, Varma S, West R, Brown P
PLoS ONE, 2015-07-15;10(7):e0128975.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Collagen signaling enhances tumor progression after anti-VEGF therapy in a murine model of pancreatic ductal adenocarcinoma.
Authors: Aguilera K, Rivera L, Hur H, Carbon J, Toombs J, Goldstein C, Dellinger M, Castrillon D, Brekken R
Cancer Res, 2013-12-17;74(4):1032-44.
Species: Human
Sample Types: Recombinant Protein
Applications: ELISA Development -
Targeting SPARC by lentivirus-mediated RNA interference inhibits cervical cancer cell growth and metastasis.
BMC Cancer, 2012-10-10;12(0):464.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
SPARC is a key regulator of proliferation, apoptosis and invasion in human ovarian cancer.
Authors: Chen, Jie, Wang, Mei, Xi, Bo, Xue, Jian, He, Dan, Zhang, Jie, Zhao, Yueran
PLoS ONE, 2012-08-03;7(8):e42413.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Diverse functions of reactive cysteines facilitate unique biosynthetic processes of aggregate-prone interleukin-31.
Authors: Shen M, Siu S, Byrd S, Edelmann KH, Patel N, Ketchem RR, Mehlin C, Arnett HA, Hasegawa H
Exp. Cell Res., 2010-12-21;317(7):976-93.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
SPARC is a VCAM-1 counter-ligand that mediates leukocyte transmigration.
Authors: Kelly KA, Allport JR, Yu AM, Sinh S, Sage EH, Gerszten RE, Weissleder R
J. Leukoc. Biol., 2006-12-18;81(3):748-56.
Species: Human
Sample Types: Protein
Applications: Western Blot -
Angiogenesis regulators S100A4, SPARC and SPP1 correlate with macrophage infiltration and are prognostic biomarkers in colon and rectal cancers
Authors: Kazakova E, Rakina M, Sudarskikh T et al.
Frontiers in oncology
-
The impact of the ovarian microenvironment on the anti-tumor effect of SPARC on ovarian cancer1This article is part of Special Issue entitled Asilomar Chromatin and has undergone the Journal’s usual peer review process
Authors: James B. Greenaway, Anne Koehler, Christopher A. McCulloch, James Petrik, Theodore J. Brown, Maurice J. Ringuette
Biochemistry and Cell Biology
-
Cell Competition Shapes Metastatic Latency and Relapse
Authors: Kim K, Huang H, Parida PK et al.
Cancer Discovery
-
Enhancing Docetaxel Delivery to Multidrug-Resistant Cancer Cells with Albumin-Coated Nanocrystals
Authors: SF Gad, J Park, JE Park, GN Fetih, SS Tous, W Lee, Y Yeo
Mol. Pharm., 2018-01-29;0(0):.
-
A Magnetic Bead-Based Sensor for the Quantification of Multiple Prostate Cancer Biomarkers.
Authors: Jokerst JV, Chen Z, Xu L et al.
PLoS One
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human SPARC Antibody
Average Rating: 5 (Based on 3 Reviews)
Have you used Human SPARC Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
This antibody was used as detection along with the capture MAB941. It worked great as an ELISA to detect human serum and plasma samples.