Human VCAM-1/CD106 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human VCAM-1. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
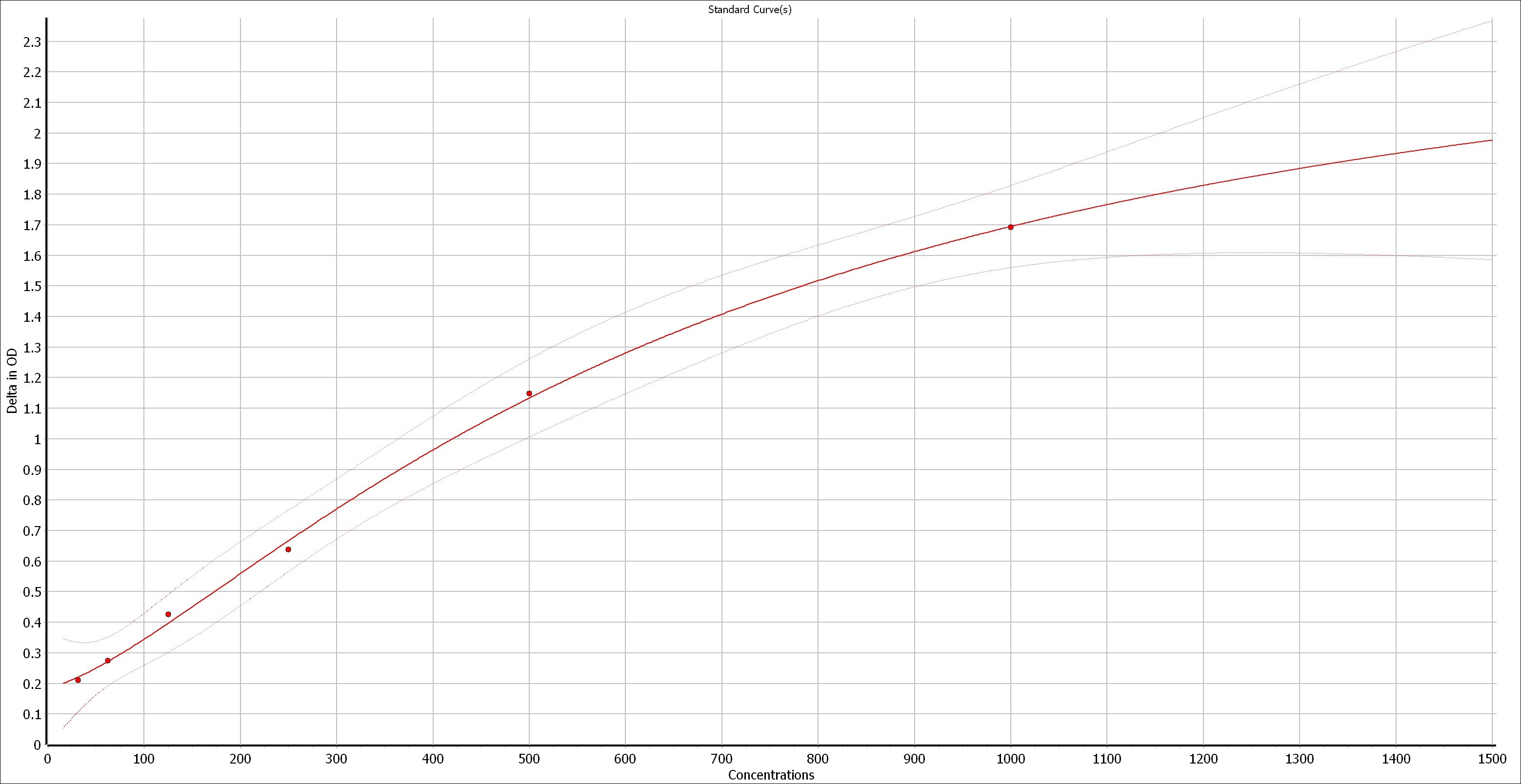
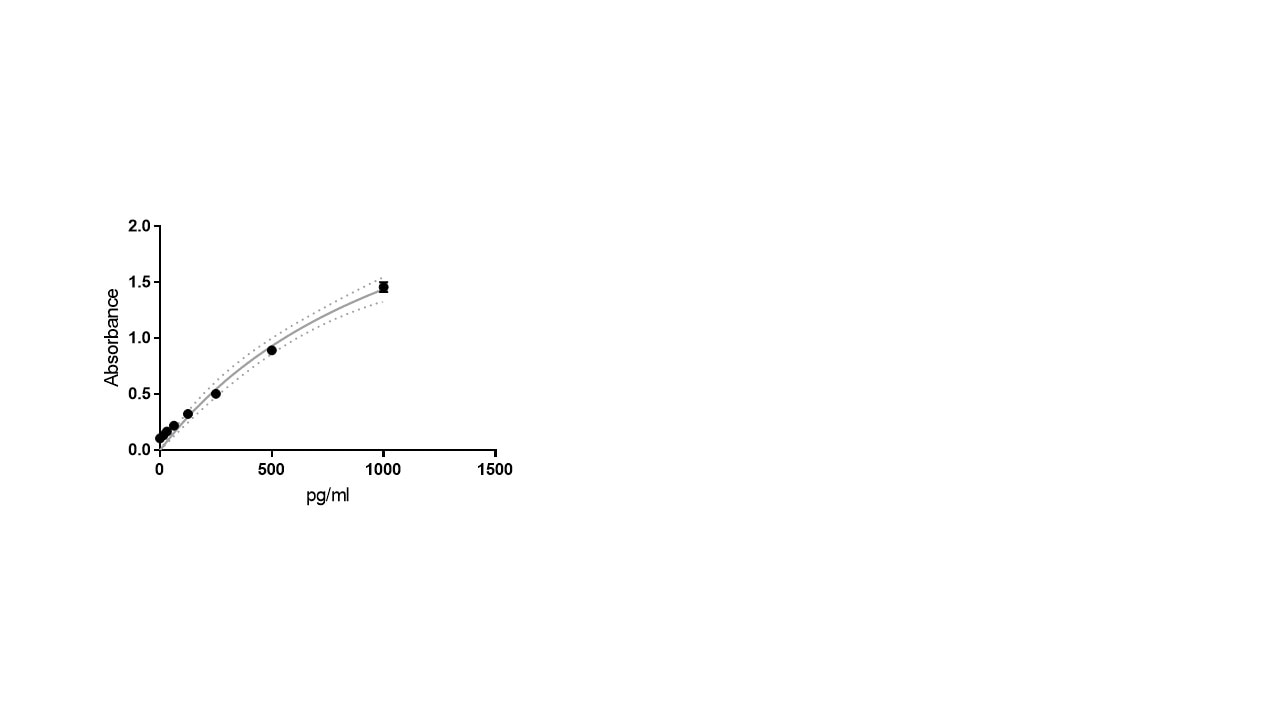
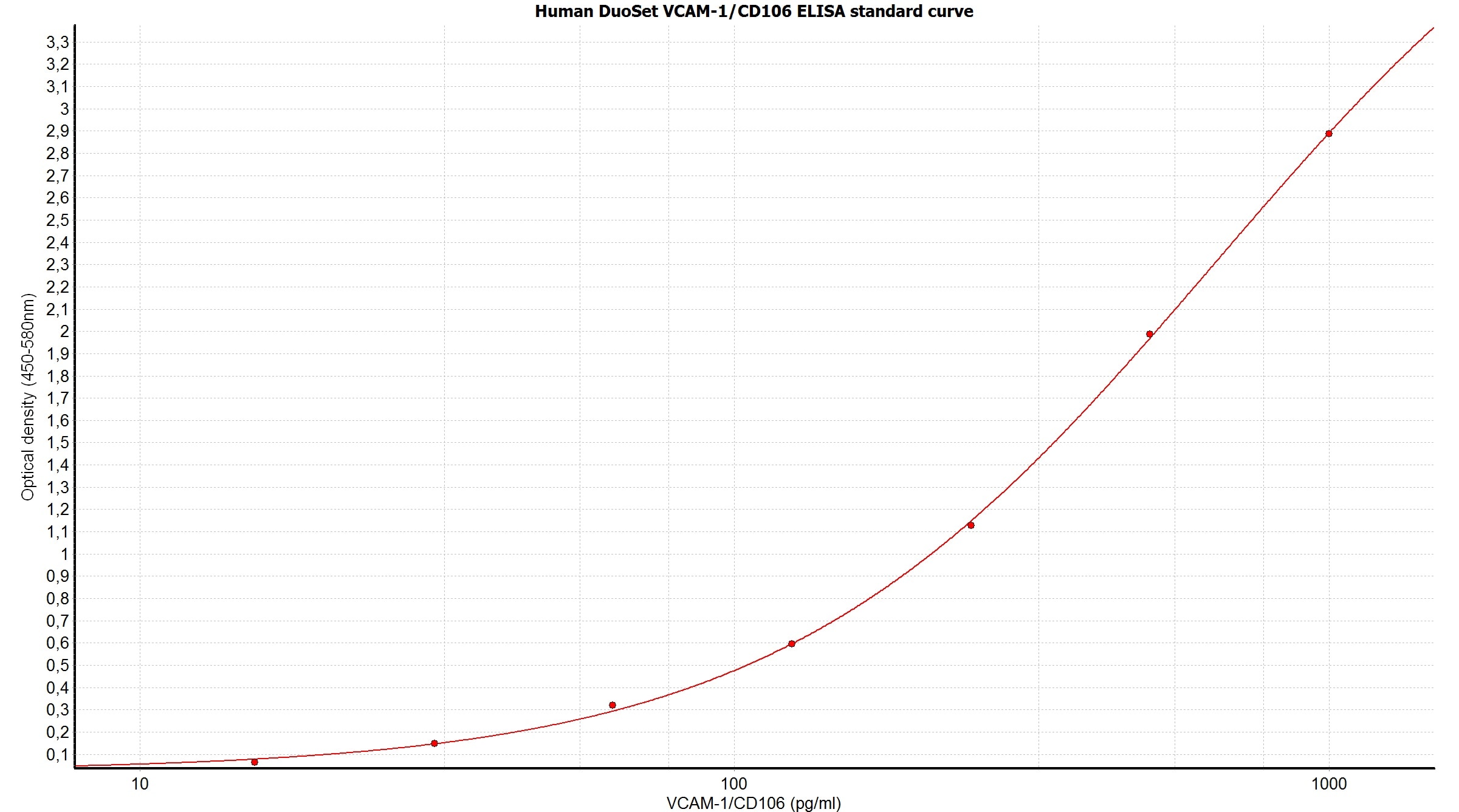
Scientific Data
Product Datasheets
Preparation and Storage
Background: VCAM-1/CD106
VCAM-1 (Vascular Cell Adhesion Molecule-1; CD106) is a transmembrane molecule that mediates the adhesion of immune cells to the vascular endothelium during inflammation. It binds to several integrins including alpha4/beta1 (VLA-4), alpha4/beta7, alpha9/beta1, and alphaD/beta2. It is expressed constitutively in the bone marrow where it regulates T cell, B cell, and hematopoietic progenitor cell migration. A soluble form VCAM-1 can promote monocyte chemotaxis.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human VCAM-1/CD106 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
27
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
TNF Induces Laminin-332-Encoding Genes in Endothelial Cells and Laminin-332 Promotes an Atherogenic Endothelial Phenotype
Authors: Hayderi, A;Zegeye, MM;Meydan, S;Sirsjö, A;Kumawat, AK;Ljungberg, LU;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates, Cell Lysates
-
Are soluble E-selectin, ICAM-1, and VCAM-1 potential predictors for the development of diabetic retinopathy in young adults, 15-34 years of age? A Swedish prospective cohort study
Authors: Ekelund, C;Dereke, J;Nilsson, C;Landin-Olsson, M;
PloS one
Species: Human
Sample Types: Plasma
-
Inhibitory Effects of Urolithins, Bioactive Gut Metabolites from Natural Polyphenols, against Glioblastoma Progression
Authors: Shen, CK;Huang, BR;Charoensaensuk, V;Yang, LY;Tsai, CF;Liu, YS;Lai, SW;Lu, DY;Yeh, WL;Lin, C;
Nutrients
Species: Human
Sample Types: Cell Culture Supernates
-
Blood orange juice intake modulates plasma and PBMC microRNA expression in overweight and insulin resistance women: impact on MAPK and NFkappaB signaling pathways
Authors: VC Capetini, BJ Quintanilh, DC de Oliveir, AH Nishioka, LA de Matos, LRP Ferreira, FM Ferreira, GR Sampaio, NMA Hassimotto, FM Lajolo, RA Fock, MM Rogero
The Journal of nutritional biochemistry, 2022-11-26;0(0):109240.
Species: Human
Sample Types: Plasma
-
Pro-cancerogenic effects of spontaneous and drug-induced senescence of ovarian cancer cells in vitro and in vivo: a comparative analysis
Authors: S Rutecki, P Szulc, M Paku?a, P Uruski, A Radziemski, E Naumowicz, R Moszy?ski, A Tykarski, J Miku?a-Pie, K Ksi??ek
Journal of ovarian research, 2022-07-26;15(1):87.
Species: Human
Sample Types: Cell Culture Supernates
-
The effect of transdermal gender-affirming hormone therapy on markers of inflammation and hemostasis
Authors: MH Schutte, R Kleemann, NM Nota, CM Wiepjes, JM Snabel, G T'Sjoen, A Thijs, M den Heijer
PLoS ONE, 2022-03-15;17(3):e0261312.
Species: Human
Sample Types: Plasma
-
The effect of moderate wine consumption on cytokine secretion by peripheral blood mononuclear cells: A randomized clinical study in coronary heart disease patients
Authors: E Fragopoulo, C Argyrou, M Detopoulou, S Tsitsou, S Seremeti, M Yannakouli, S Antonopoul, G Kolovou, P Kalogeropo
Cytokine, 2021-07-08;146(0):155629.
Species: Human
Sample Types: Plasma
-
Predictors of Early-Recurrence Atrial Fibrillation after Catheter Ablation in Women and Men with Abnormal Body Weight
Authors: J Budzianows, J Hiczkiewic, K ?ojewska, E Kawka, R Rutkowski, K Korybalska
Journal of Clinical Medicine, 2021-06-18;10(12):.
Species: Human
Sample Types: Serum
-
Complement activation is associated with poor outcome after out-of-hospital cardiac arrest
Authors: V Chaban, ER Nakstad, H Stær-Jense, C Schjalm, I Seljeflot, J Vaage, C Lundqvist, JŠ Benth, K Sunde, TE Mollnes, GØ Andersen, SE Pischke
Resuscitation, 2021-06-11;0(0):.
Species: Human
Sample Types: Plasma
-
Soluble VCAM-1 promotes gemcitabine resistance via macrophage infiltration and predicts therapeutic response in pancreatic cancer
Authors: R Takahashi, H Ijichi, M Sano, K Miyabayash, D Mohri, J Kim, G Kimura, T Nakatsuka, H Fujiwara, K Yamamoto, Y Kudo, Y Tanaka, K Tateishi, Y Nakai, Y Morishita, K Soma, N Takeda, HL Moses, H Isayama, K Koike
Scientific Reports, 2020-12-03;10(1):21194.
Species: Human
Sample Types: Plasma
-
Urinary soluble VCAM-1 is a useful biomarker of disease activity and treatment response in lupus nephritis
Authors: AA Gasparin, NPB de Andrade, V Hax, PE Palominos, M Siebert, R Marx, PG Schaefer, FV Veronese, OA Monticielo
BMC rheumatology, 2020-12-01;4(1):67.
Species: Human
Sample Types: Urine
-
Urinary proteomics links keratan sulfate degradation and lysosomal enzymes to early type 1 diabetes
Authors: JAD Van, S Clotet-Fre, AC Hauschild, I Batruch, I Jurisica, Y Elia, FH Mahmud, E Sochett, EP Diamandis, JW Scholey, A Konvalinka
PLoS ONE, 2020-05-26;15(5):e0233639.
Species: Human
Sample Types: Urine
-
The human glomerular endothelial cells are potent pro-inflammatory contributors in an in vitro model of lupus nephritis
Authors: P Dimou, RD Wright, KL Budge, A Midgley, SC Satchell, M Peak, MW Beresford
Sci Rep, 2019-06-06;9(1):8348.
Species: Human
Sample Types: Cell Culture Supernates
-
Blood inflammatory and endothelial markers in women with von Willebrand disease
Authors: I Govorov, K Bremme, A Larsson, M Holmström, E Komlichenk, R Chaireti, M Mints
PLoS ONE, 2019-01-10;14(1):e0210544.
Species: Human
Sample Types: Plasma
-
Biomarkers of endothelial dysfunction predict sepsis mortality in young infants: a matched�case-control study
Authors: JK Wright, K Hayford, V Tran, GM Al Kibria, A Baqui, A Manajjir, A Mahmud, N Begum, M Siddiquee, KC Kain, A Farzin
BMC Pediatr, 2018-03-23;18(1):118.
Species: Human
Sample Types: Plasma
-
Serum Soluble Vascular Cell Adhesion Molecule-1 Overexpression Is a Disease Marker in Patients with First-Time Diagnosed Antinuclear Antibodies: A Prospective, Observational Pilot Study
Authors: M Oleszowsky, MF Seidel
Biomed Res Int, 2018-02-01;2018(0):8286067.
Species: Human
Sample Types: Serum
-
Urinary angiostatin, CXCL4 and VCAM-1 as biomarkers of lupus nephritis
Authors: CC Mok, S Soliman, LY Ho, FA Mohamed, FI Mohamed, C Mohan
Arthritis Res. Ther., 2018-01-11;20(1):6.
Species: Human
Sample Types: Urine
-
Excess atherosclerosis in systemic lupus erythematosus,-A matter of renal involvement: Case control study of 281 SLE patients and 281 individually matched population controls
Authors: JT Gustafsson, M Herlitz Li, I Gunnarsson, S Pettersson, K Elvin, J Öhrvik, A Larsson, K Jensen-Urs, E Svenungsso
PLoS ONE, 2017-04-17;12(4):e0174572.
Species: Human
Sample Types: Tissue Homogenates
-
Serum retinol-binding protein-induced endothelial inflammation is mediated through the activation of toll-like receptor 4
Authors: M Du, A Martin, F Hays, J Johnson, RA Farjo, KM Farjo
Mol. Vis., 2017-03-31;23(0):185-197.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum Biomarkers of Myocardial Remodeling and Coronary Dysfunction in Early Stages of Hypertrophic Cardiomyopathy in the Young
Authors: E Fernlund, T Gyllenhamm, R Jablonowsk, M Carlsson, A Larsson, J Ärnlöv, P Liuba
Pediatr Cardiol, 2017-03-30;0(0):.
Species: Human
Sample Types: Serum
-
Serum from Varicose Patients Induces Senescence-Related Dysfunction of Vascular Endothelium Generating Local and Systemic Proinflammatory Conditions
Oxid Med Cell Longev, 2016-11-23;2016(0):2069290.
Species: Human
Sample Types: Serum
-
Urine VCAM-1 as a marker of renal pathology activity index in lupus nephritis.
Authors: Singh S, Wu T
Oncogene, 2012-07-13;14(4):R164.
Species: Human
Sample Types: Urine
-
Integrated proteomic analysis of human cancer cells and plasma from tumor bearing mice for ovarian cancer biomarker discovery.
Authors: Pitteri SJ, JeBailey L, Faca VM, Thorpe JD, Silva MA, Ireton RC, Horton MB, Wang H, Pruitt LC, Zhang Q, Cheng KH, Urban N, Hanash SM, Dinulescu DM
PLoS ONE, 2009-11-19;4(11):e7916.
Species: Human
Sample Types: Plasma
-
Suspension microarrays for the identification of the response patterns in hyperinflammatory diseases.
Authors: Hsu HY, Wittemann S, Schneider EM, Weiss M, Joos TO
Med Eng Phys, 2008-03-03;30(8):976-83.
Species: Human
Sample Types: Plasma
-
Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis.
Authors: Wu T, Xie C, Wang HW, Zhou XJ, Schwartz N, Calixto S, Mackay M, Aranow C, Putterman C, Mohan C
J. Immunol., 2007-11-15;179(10):7166-75.
Species: Human
Sample Types: Serum
-
Cardiovascular effects of intravenous ghrelin infusion in healthy young men.
Authors: Vestergaard ET, Andersen NH, Hansen TK, Rasmussen LM, Moller N, Sorensen KE, Sloth E, Jorgensen JO
Am. J. Physiol. Heart Circ. Physiol., 2007-09-14;293(5):H3020-6.
Species: Human
Sample Types: Serum
-
Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer.
Authors: Furstenberger G, von Moos R, Lucas R, Thurlimann B, Senn HJ, Hamacher J, Boneberg EM
Br. J. Cancer, 2006-02-27;94(4):524-31.
Species: Human
Sample Types: Serum
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human VCAM-1/CD106 DuoSet ELISA
Average Rating: 4.4 (Based on 5 Reviews)
Have you used Human VCAM-1/CD106 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Used in Serum and EDTA Plasma at 1:10 dilution, the sample may need further dilution, some times we get high %CV.
We used human serum at a dilution of 1:2000. Excellent result.