Human VEGFR2/KDR/Flk-1 Antibody Summary
Ala20-Glu764
Accession # AAC16450
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
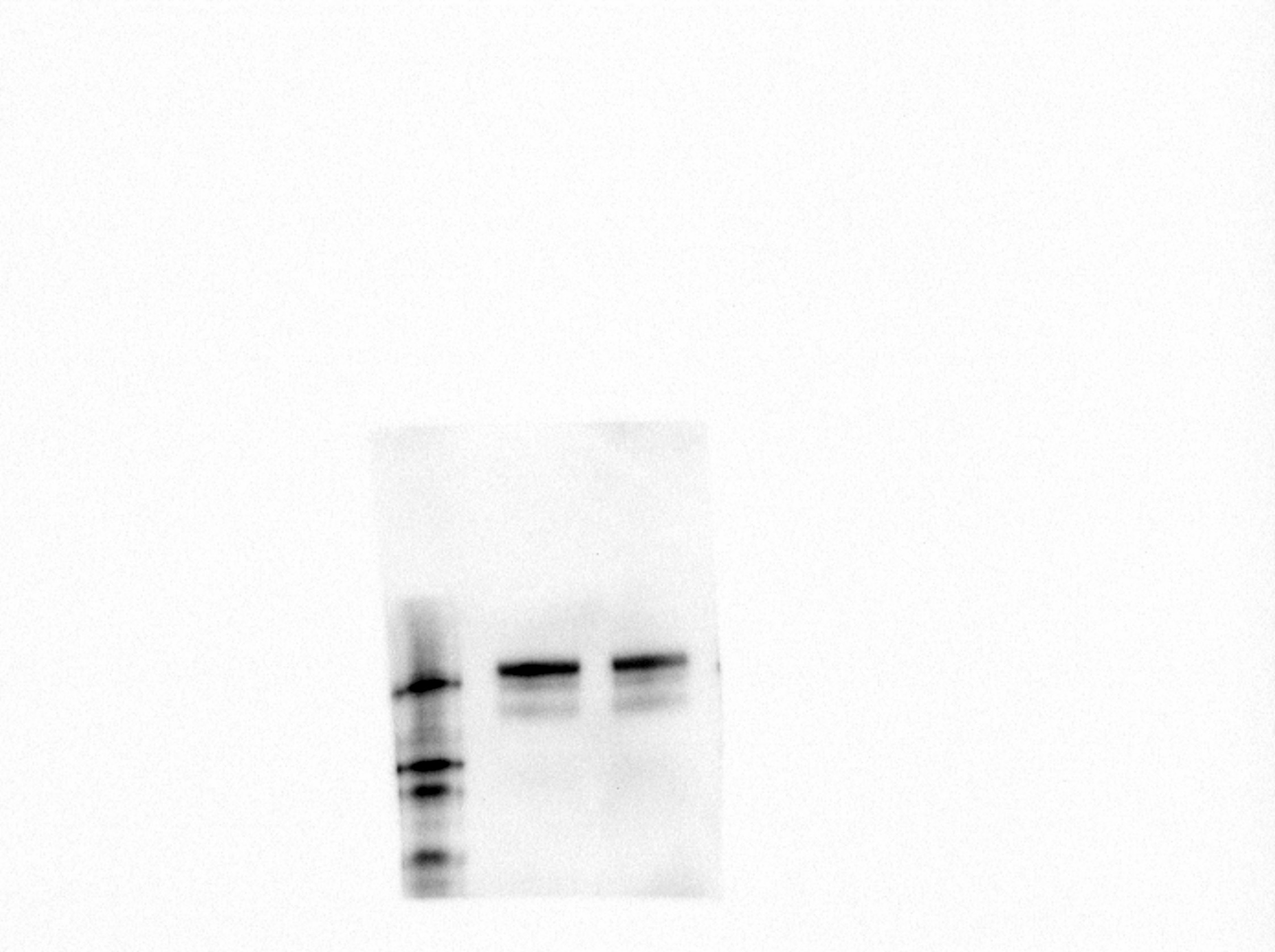
Detection of Human VEGFR2/KDR/Flk‑1 by Western Blot. Western blot shows lysate of HUVEC human umbilical vein endothelial cells. PVDF membrane was probed with 1 µg/mL of Goat Anti-Human VEGFR2/KDR/Flk-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF357) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). Specific bands were detected for VEGFR2/KDR/Flk-1 at approximately 200-250 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
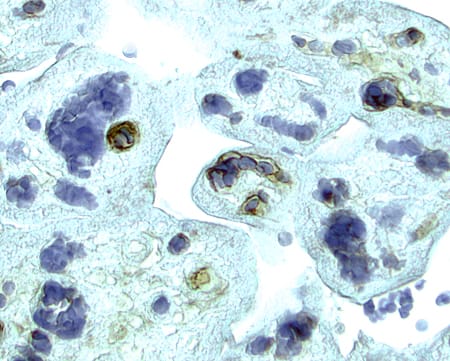
VEGFR2/KDR/Flk‑1 in Human Placenta. VEGFR2/KDR/Flk-1 was detected in immersion fixed paraffin-embedded sections of human placenta using 15 µg/mL Goat Anti-Human VEGFR2/KDR/Flk-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF357) overnight at 4 °C. Tissue was stained with the Anti-Goat HRP-AEC Cell & Tissue Staining Kit (red; Catalog # CTS009) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
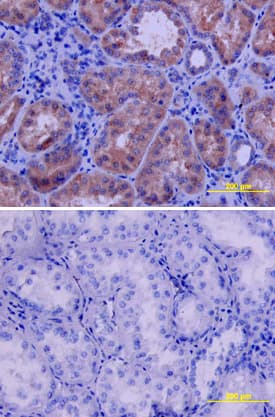
VEGFR2/KDR/Flk‑1 in Human Kidney. VEGFR2/KDR/Flk-1 was detected in immersion fixed paraffin-embedded sections of human kidney using Goat Anti-Human VEGFR2/KDR/Flk-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF357) at 10 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Lower panel shows a lack of labeling if primary antibodies are omitted and tissue is stained only with secondary antibody followed by incubation with detection reagents. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
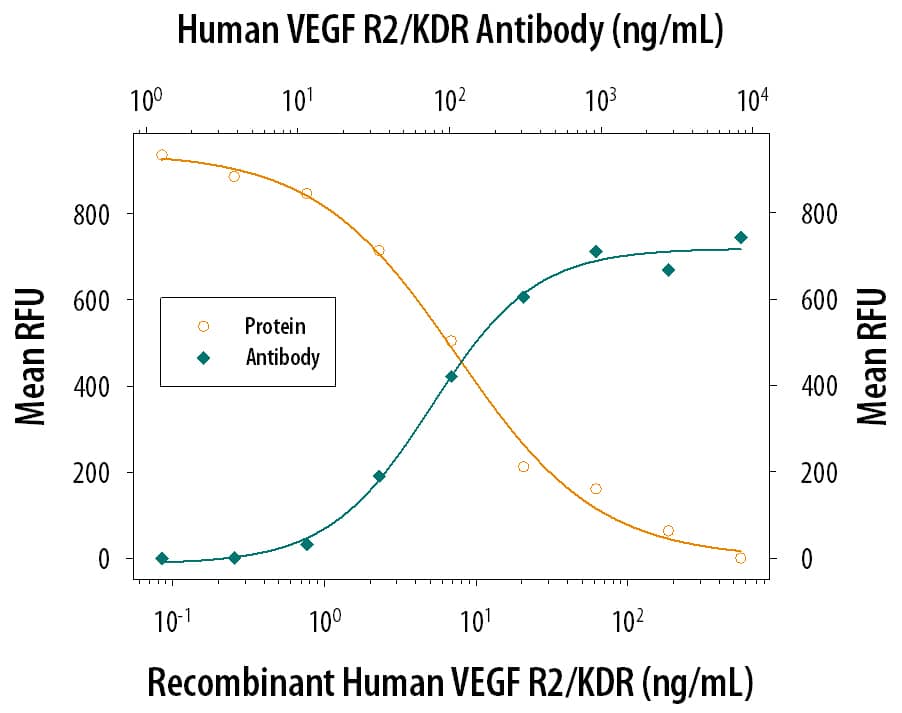
VEGFR2/KDR/Flk‑1 Inhibi-tion of VEGF-dependent Cell Proliferation and Neutral-ization by Human VEGFR2/KDR/Flk‑1 Anti-body. Recombi-nant Human VEGFR2/KDR/Flk-1 Fc Chi-mera (Catalog # 357-KD) inhibits Recombinant Human VEGF165(Catalog # 293-VE) induced proliferation in HUVEC human umbilical vein endothelial cells in a dose-dependent manner (orange line). Inhibition of Recombinant Human VEGF165(5 ng/mL) activity elicited by Recombinant Human VEGFR2/KDR/Flk-1 Fc Chi-mera (30 ng/mL) is neutralized (green line) by increasing concentrations of Goat Anti-Human VEGFR2/KDR/Flk-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF357). The ND50 is typically 0.05-0.25 µg/mL.
 View Larger
View Larger
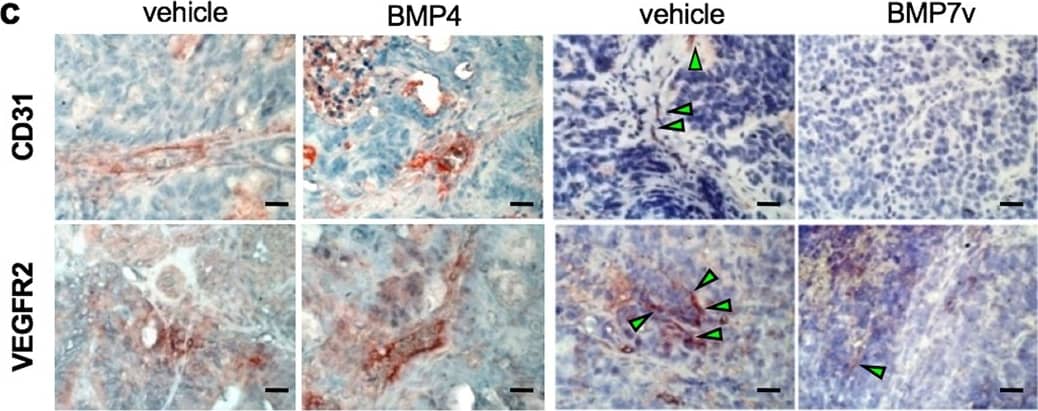
Detection of Human VEGFR2/KDR/Flk-1 by Immunohistochemistry BMP7v exerts antiangiogenic effects and sensitizes chemoresistant CSCs to standard therapy. a Azan-Mallory staining on paraffin-embedded sections of xenografts derived from the injection of CRC sphere cells and treated for 4 weeks (6–9 weeks) with PBS (vehicle) or BMP7v. Data are representative of three independent experiments using different CRC sphere cell lines (CSC#2, 7, and 18). b Percentage of necrosis evaluated on paraffin-embedded sections of xenografts treated as in a. Data are shown as mean ± SD of three independent experiments. c Immunohistochemical analysis of CD31 and VEGFR2 (red staining) on paraffin-embedded sections of xenografts generated by the injection of CRC sphere cell lines and treated with PBS (vehicle), BMP4, or BMP7v. Green arrowheads indicate microvessels expressing CD31 or VEGFR2. Images are representative of three independent experiments using cells as in a. Nuclei were revealed by hematoxylin staining (blue). The scale bar represents 20 µm. d Number of microvessels positive for CD31 (left panel) and VEGFR2 (right panel) expression, evaluated on paraffin-embedded sections of xenografts treated as in c. Data are shown as mean ± SD of cells. MVD = microvessel density. e Fold change of viable cells in 35 CR-CSC lines treated with oxaliplatin/5-FU for 24 h. Dotted line indicates the threshold between chemoresistant (red) and sensitive CR-CSCs (green). f Cell viability percentage in chemoresistant CR-CSCs (R1-R4) pretreated with BMP7 for 3 days and with oxaliplatin/5-FU (oxa/5-FU) for additional 24 h as indicated. Data are shown as mean ± SD of three different experiments performed in the indicated R-CSCs. g Colony forming efficiency of CR-CSCs treated as in f and evaluated at 21 days. Representative soft-agar analyses are reported in the lower part of the graph. Bars show the mean ± SD of seven different CRC sphere cell lines (CSC#1–3, 5, 7, 10, and 18). h Tumor size of subcutaneous growth of the indicated CR-CSCs. Mice were treated for 4 weeks (6–9 weeks) with vehicle, oxaliplatin/5-FU (oxa/5-FU) and BMP7v alone or in combination. Error bars show the mean ± SD of tumor size measured in six mice/group. Black arrowheads indicate days of treatment. i Immunohistochemical analysis of CD44v6, beta -catenin, Ki67, and CK20 (red color) in paraffin-embedded sections of CSC#7 xenografts treated as in h. Nuclei were counterstained by aqueous hematoxylin (blue color). The scale bar represents 20 µm (left panels). Percentage of CD44v6, beta -catenin, Ki67, and CK20 positive cells in paraffin-embedded sections of tumor xenografts treated with vehicle (V), BMP7v (B), oxaliplatin/5-FU (O/F), alone or in combination (B/O/F) for 72 h. Error bars are mean ± SD of positive cell counts in three serial embedded-paraffin sections of six tumor xenografts per group derived from the injection of three different CRC sphere cells (CSC#1, 2, and 7) (right panels) Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31591478), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
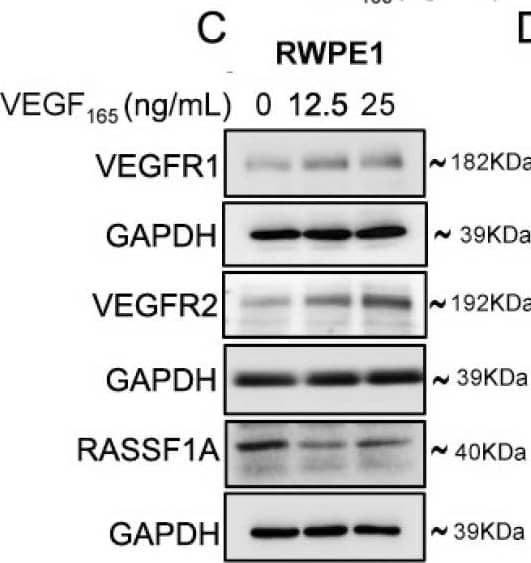
Detection of Human VEGFR2/KDR/Flk-1 by Western Blot Exogenous VEGF165 suppresses RASSF1A expression in normal epithelial and endothelial cells. Metastatic colon cancer cells (mCRC, (A) T84 and (B) Colo 205) were stimulated with VEGF165 as indicated before and cell metabolism was performed by MTT assay for 6 h (black dash), 24 h (green) and 48 h (pink). Immortalized benign protate ((C) RWPE1), human bronchial epithelial cells ((D) HBEpc), immortalized human breast epithelial cells ((E) MCF-10A), endothelial cells (F, EC) were stimulated with or without VEGF165 (12.5 and 25 ng/mL) for 24 h. Cell lysates were monitored by Western blot for p-VEGFR1, p-VEGFR2, RASSF1A, and GAPDH as indicated. Immunoblot was quantified by scanning densitometry and normalized against GAPDH expression for RWPE1 (G), HBEpc (H), MCF 10A (I) and EC (lower panel of (F)). Results are from three independent experiments and statistical significance was determined using one way-ANOVA followed Bonferroni test. (* p < 0.05, ** p < 0.01, *** p < 0.001). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30744076), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
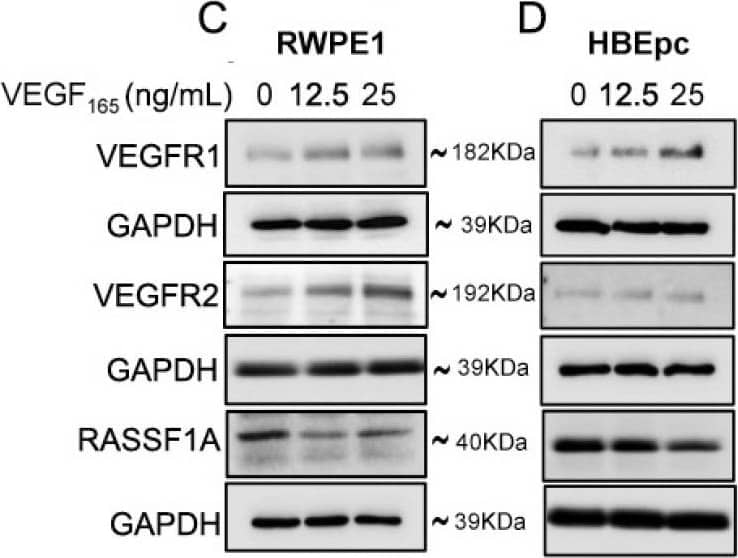
Detection of Human VEGFR2/KDR/Flk-1 by Western Blot Exogenous VEGF165 suppresses RASSF1A expression in normal epithelial and endothelial cells. Metastatic colon cancer cells (mCRC, (A) T84 and (B) Colo 205) were stimulated with VEGF165 as indicated before and cell metabolism was performed by MTT assay for 6 h (black dash), 24 h (green) and 48 h (pink). Immortalized benign protate ((C) RWPE1), human bronchial epithelial cells ((D) HBEpc), immortalized human breast epithelial cells ((E) MCF-10A), endothelial cells (F, EC) were stimulated with or without VEGF165 (12.5 and 25 ng/mL) for 24 h. Cell lysates were monitored by Western blot for p-VEGFR1, p-VEGFR2, RASSF1A, and GAPDH as indicated. Immunoblot was quantified by scanning densitometry and normalized against GAPDH expression for RWPE1 (G), HBEpc (H), MCF 10A (I) and EC (lower panel of (F)). Results are from three independent experiments and statistical significance was determined using one way-ANOVA followed Bonferroni test. (* p < 0.05, ** p < 0.01, *** p < 0.001). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30744076), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
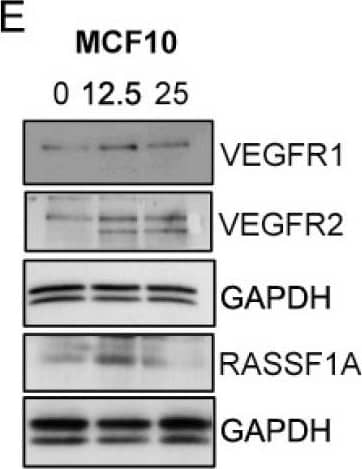
Detection of Human VEGFR2/KDR/Flk-1 by Western Blot Exogenous VEGF165 suppresses RASSF1A expression in normal epithelial and endothelial cells. Metastatic colon cancer cells (mCRC, (A) T84 and (B) Colo 205) were stimulated with VEGF165 as indicated before and cell metabolism was performed by MTT assay for 6 h (black dash), 24 h (green) and 48 h (pink). Immortalized benign protate ((C) RWPE1), human bronchial epithelial cells ((D) HBEpc), immortalized human breast epithelial cells ((E) MCF-10A), endothelial cells (F, EC) were stimulated with or without VEGF165 (12.5 and 25 ng/mL) for 24 h. Cell lysates were monitored by Western blot for p-VEGFR1, p-VEGFR2, RASSF1A, and GAPDH as indicated. Immunoblot was quantified by scanning densitometry and normalized against GAPDH expression for RWPE1 (G), HBEpc (H), MCF 10A (I) and EC (lower panel of (F)). Results are from three independent experiments and statistical significance was determined using one way-ANOVA followed Bonferroni test. (* p < 0.05, ** p < 0.01, *** p < 0.001). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30744076), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
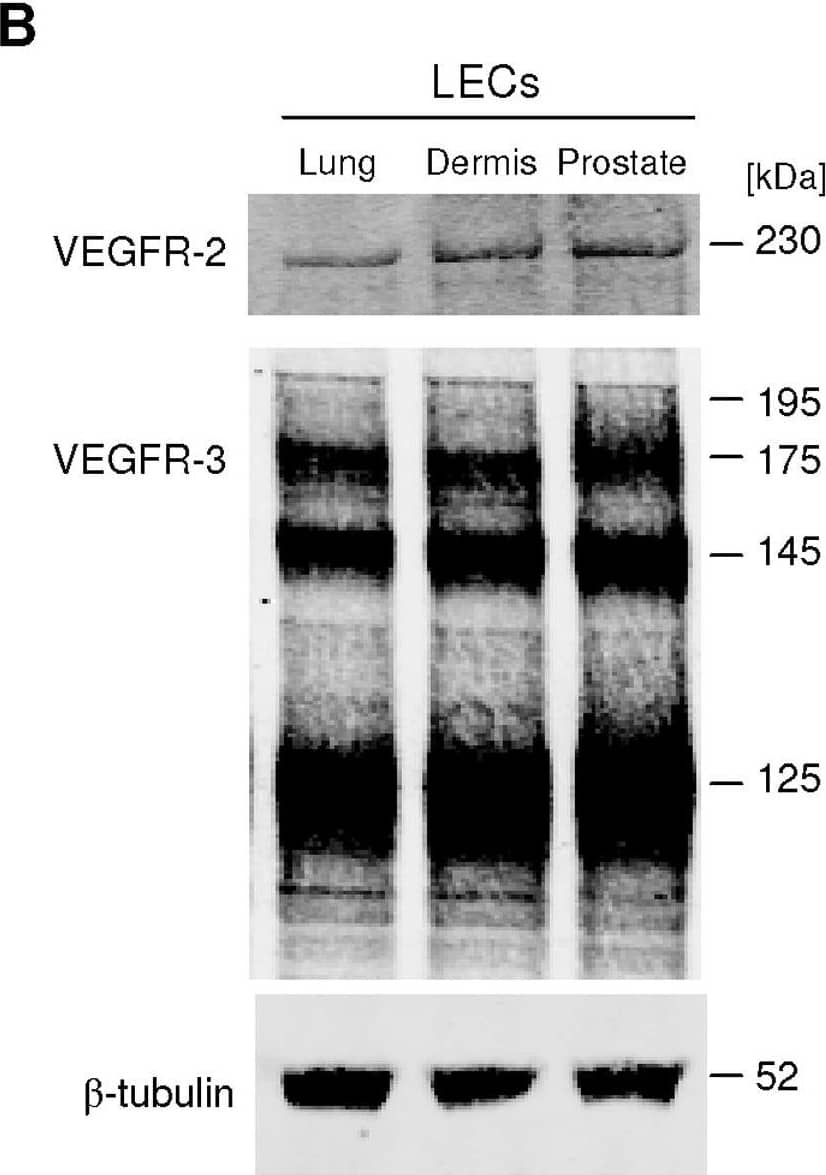
Detection of Human VEGFR2/KDR/Flk-1 by Western Blot VEGF-C induces LEC tube formation.A, Prostatic LEC tube length at 4.5 hours post VEGF-C treatment was quantified using ImageJ. VEGF-C significantly increased the number of tubes formed compared to vehicle control. Data expressed as mean±s.e.m., n = 3, ***P<0.001 using One-way ANOVA, Bonferroni post-analysis. B, Western blotting analysis of VEGFR-2 and VEGFR-3 expression in lung, neonatal dermis and prostate LECs. beta -tubulin was used as a loading control. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/22745786), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: VEGFR2/KDR/Flk-1
VEGFR2 (KDR/Flk-1), VEGFR1 (Flt-1) and VEGFR3 (Flt-4) belong to the class III subfamily of receptor tyrosine kinases (RTKs). All three receptors contain seven immunoglobulin-like repeats in their extracellular domains and kinase insert domains in their intracellular regions. The expression of VEGFR1, 2, and 3 is almost exclusively restricted to the endothelial cells. These receptors are likely to play essential roles in vasculogenesis and angiogenesis.
VEGFR2 cDNA encodes a 1356 amino acid (aa) residue precursor protein with a 19 aa residue signal peptide. Mature VEGFR2 is composed of a 745 aa residue extracellular domain, a 25 aa residue transmembrane domain and a 567 aa residue cytoplasmic domain. In contrast to VEGFR1 which binds both PlGF and VEGF with high affinity, VEGFR2 binds VEGF but not PlGF with high affinity. The recombinant soluble VEGFR2/Fc chimera binds VEGF with high affinity and is a potent VEGF antagonist.
Product Datasheets
Citations for Human VEGFR2/KDR/Flk-1 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
61
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
A predictive microfluidic model of human glioblastoma to assess trafficking of blood–brain barrier-penetrant nanoparticles
Authors: Joelle P. Straehla, Cynthia Hajal, Hannah C. Safford, Giovanni S. Offeddu, Natalie Boehnke, Tamara G. Dacoba et al.
Proceedings of the National Academy of Sciences
-
KLK3/PSA and cathepsin D activate VEGF-C and VEGF-D
Authors: SK Jha, K Rauniyar, E Chronowska, K Mattonet, EW Maina, H Koistinen, UH Stenman, K Alitalo, M Jeltsch
Elife, 2019-05-17;8(0):.
-
VEGF-A, -C, -D, VEGFR1, -2, -3, PDGF-BB and FGF-2 join forces to induce vascular and lymphatic angiogenesis during bone healing of hip implants
Authors: Cassuto, J;Folestad, A;Göthlin, J;Malchau, H;Kärrholm, J;
Bone reports
Species: Human
Sample Types: Plasma
Applications: ELISA Capture -
Specialized post-arterial capillaries facilitate adult bone remodelling
Authors: Mohanakrishnan, V;Sivaraj, KK;Jeong, HW;Bovay, E;Dharmalingam, B;Bixel, MG;Dinh, VV;Petkova, M;Paredes Ugarte, I;Kuo, YT;Gurusamy, M;Raftrey, B;Chu, NTL;Das, S;Rios Coronado, PE;Stehling, M;Sävendahl, L;Chagin, AS;Mäkinen, T;Red-Horse, K;Adams, RH;
Nature cell biology
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
c-Src-induced vascular malformations require localised matrix degradation at focal adhesions
Authors: Essebier, P;Keyser, M;Yordanov, T;Hill, B;Yu, A;Noordstra, I;Yap, AS;Stehbens, SJ;Lagendijk, AK;Schimmel, L;Gordon, EJ;
Journal of cell science
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
E2 ubiquitin-conjugating enzymes, UBE2D1 and UBE2D2, regulate VEGFR2 dynamics and endothelial function
Authors: Critchley, WR;Smith, GA;Zachary, IC;Harrison, MA;Ponnambalam, S;
Journal of cell science
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Critical role of mitogen-inducible gene 6 in restraining endothelial cell permeability to maintain vascular homeostasis
Authors: Liying Xing, Guanqun Huang, Rongyuan Chen, Lijuan Huang, Juanxi Liu, Xiangrong Ren et al.
Journal of Cell Communication and Signaling
-
Galectin-3 Enhances Vascular Endothelial Growth Factor-A Receptor 2 Activity in the Presence of Vascular Endothelial Growth Factor
Authors: Issahy Cano, Zhengping Hu, Dina B. AbuSamra, Magali Saint-Geniez, Yin Shan Eric Ng, Pablo Argüeso et al.
Frontiers in Cell and Developmental Biology
-
The endosomal RIN2/Rab5C machinery prevents VEGFR2 degradation to control gene expression and tip cell identity during angiogenesis
Authors: Lanette Kempers, Yuki Wakayama, Ivo van der Bijl, Charita Furumaya, Iris M. De Cuyper, Aldo Jongejan et al.
Angiogenesis
-
The P-type ATPase transporter ATP7A promotes angiogenesis by limiting autophagic degradation of VEGFR2
Authors: D Ash, V Sudhahar, SW Youn, MN Okur, A Das, JP O'Bryan, M McMenamin, Y Hou, JH Kaplan, T Fukai, M Ushio-Fuka
Nature Communications, 2021-05-25;12(1):3091.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation, Western Blot -
The Role of Heparan Sulfate and Neuropilin 2 in VEGFA Signaling in Human Endothelial Tip Cells and Non-Tip Cells during Angiogenesis In Vitro
Authors: MG Dallinga, YI Habani, AWM Schimmel, GM Dallinga-T, CJF van Noorde, I Klaassen, RO Schlingema
Cells, 2021-04-16;10(4):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Paladin is a phosphoinositide phosphatase regulating endosomal VEGFR2 signalling and angiogenesis
Authors: Anja Nitzsche, Riikka Pietilä, Dominic T Love, Chiara Testini, Takeshi Ninchoji, Ross O Smith et al.
EMBO reports
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Immunoprecipitation -
Pigment Epithelium-Derived Factor (PEDF) Receptors Are Involved in Survival of Retinal Neurons
Authors: S Bürger, J Meng, A Zwanzig, M Beck, M Pankonin, P Wiedemann, W Eichler, JD Unterlauft
International Journal of Molecular Sciences, 2020-12-31;22(1):.
Species: Mouse, Rat
Sample Types: Whole Cells
Applications: Neutralization -
Elements of the Endomucin Extracellular Domain Essential for VEGF-Induced VEGFR2 Activity
Authors: Z Hu, I Cano, KL Saez-Torre, ME LeBlanc, M Saint-Geni, YS Ng, P Argüeso, PA D'Amore
Cells, 2020-06-05;9(6):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Shear stress activates ADAM10 sheddase to regulate Notch1 via the Piezo1 force sensor in endothelial cells
Authors: Vincenza Caolo, Marjolaine Debant, Naima Endesh, T Simon Futers, Laeticia Lichtenstein, Fiona Bartoli et al.
eLife
-
Atypical chemokine receptor ACKR3/CXCR7 controls postnatal vasculogenesis and arterial specification by mesenchymal stem cells via Notch signaling
Authors: ST Wei, YC Huang, ML Hsieh, YJ Lin, WC Shyu, HC Chen, CH Hsieh
Cell Death Dis, 2020-05-04;11(5):307.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Western Blot -
ATF-2 and Tpl2 regulation of endothelial cell cycle progression and apoptosis
Authors: GW Fearnley, AM Latham, M Hollstein, AF Odell, S Ponnambala
Cell. Signal., 2019-11-21;66(0):109481.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Targeting chemoresistant colorectal cancer via systemic administration of a BMP7 variant
Authors: V Veschi, LR Mangiapane, A Nicotra, S Di Franco, E Scavo, T Apuzzo, DS Sardina, M Fiori, A Benfante, ML Colorito, G Cocorullo, F Giuliante, C Cipolla, G Pistone, MR Bongiorno, A Rizzo, CM Tate, X Wu, S Rowlinson, LF Stancato, M Todaro, R De Maria, G Stassi
Oncogene, 2019-10-07;0(0):.
Species: Xenograft
Sample Types: Whole Tissue
Applications: IHC-P -
Co‐existent pilocytic astrocytoma with acute B‐cell leukemia within the cerebellum
Authors: Richard Hickman, Rebecca Leeman‐Neill, Marc Rosenblum, Richard Anderson, James Goldman
Neuropathology
-
Weibel-Palade Bodies Orchestrate Pericytes During Angiogenesis
Authors: Mélissande Cossutta, Marie Darche, Gilles Carpentier, Claire Houppe, Matteo Ponzo, Fabio Raineri et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Endophilin-A2 dependent VEGFR2 endocytosis promotes sprouting angiogenesis
Authors: G Genet, K Boyé, T Mathivet, R Ola, F Zhang, A Dubrac, J Li, N Genet, L Henrique G, L Benedetti, S Künzel, L Pibouin-Fr, JL Thomas, A Eichmann
Nat Commun, 2019-05-28;10(1):2350.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Tpl2 is required for VEGF-A-stimulated signal transduction and endothelial cell function
Authors: Gareth W. Fearnley, Izma Abdul-Zani, Antony M. Latham, Monica C. Hollstein, John E. Ladbury, Stephen B. Wheatcroft et al.
Biology Open
-
Identification of Cross Talk between FoxM1 and RASSF1A as a Therapeutic Target of Colon Cancer
Authors: Thomas G. Blanchard, Steven J. Czinn, Vivekjyoti Banerjee, Neha Sharda, Andrea C. Bafford, Fahad Mubariz et al.
Cancers (Basel)
-
RCAN1.4 regulates VEGFR-2 internalisation, cell polarity and migration in human microvascular endothelial cells
Authors: Ahmad F. Alghanem, Emma L. Wilkinson, Maxine S. Emmett, Mohammad A. Aljasir, Katherine Holmes, Beverley A. Rothermel et al.
Angiogenesis
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Affimer proteins are versatile and renewable affinity reagents
Authors: C Tiede, R Bedford, SJ Heseltine, G Smith, I Wijetunga, R Ross, D AlQallaf, AP Roberts, A Balls, A Curd, RE Hughes, H Martin, SR Needham, LC Zanetti-Do, Y Sadigh, TP Peacock, AA Tang, N Gibson, H Kyle, GW Platt, N Ingram, T Taylor, LP Coletta, I Manfield, M Knowles, S Bell, F Esteves, A Maqbool, RK Prasad, M Drinkhill, RS Bon, V Patel, SA Goodchild, M Martin-Fer, RJ Owens, JE Nettleship, ME Webb, M Harrison, JD Lippiat, S Ponnambala, M Peckham, A Smith, PK Ferrigno, M Johnson, MJ McPherson, DC Tomlinson
Elife, 2017-06-27;6(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Lymphatic endothelial progenitors originate from plastic myeloid cells activated by toll-like receptor-4
Authors: LD Volk-Drape, KL Hall, AC Wilber, S Ran
PLoS ONE, 2017-06-09;12(6):e0179257.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Endoglin prevents vascular malformation by regulating flow-induced cell migration and specification through VEGFR2 signalling
Authors: Y Jin, L Muhl, M Burmakin, Y Wang, AC Duchez, C Betsholtz, HM Arthur, L Jakobsson
Nat. Cell Biol., 2017-05-22;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Blood vessel endothelium-directed tumor cell streaming in breast tumors requires the HGF/C-Met signaling pathway
Authors: E Leung, A Xue, Y Wang, P Rougerie, V P Sharma, R Eddy et al.
Oncogene
-
VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling
Authors: K Heinolaine, S Karaman, G D'Amico, T Tammela, R Sormunen, L Eklund, K Alitalo, G Zarkada
Circ. Res, 2017-03-15;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Luteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cells
Authors: Matthew T Cook, Yayun Liang, Cynthia Besch-Williford, Salman M Hyder
Breast Cancer: Targets and Therapy
-
VEGF induces signalling and angiogenesis by directing VEGFR2 internalisation via macropinocytosis
J Cell Sci, 2016-09-21;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
VEGF-A isoforms program differential VEGFR2 signal transduction, trafficking and proteolysis
Authors: Gareth W. Fearnley, Gina A. Smith, Izma Abdul-Zani, Nadira Yuldasheva, Nadeem A. Mughal, Shervanthi Homer-Vanniasinkam et al.
Biology Open
-
Placenta growth factor and neuropilin-1 collaborate in promoting melanoma aggressiveness
Authors: ELENA PAGANI, FEDERICA RUFFINI, GIAN CARLO ANTONINI Antonini Cappellini, ALESSANDRO SCOPPOLA, CRISTINA FORTES, PAOLO MARCHETTI et al.
International Journal of Oncology
-
Differential sensitivity of prostate tumor derived endothelial cells to sorafenib and sunitinib.
Authors: Fiorio Pla, Alessand, Brossa, Alessia, Bernardini, Michela, Genova, Tullio, Grolez, Guillaum, Villers, Arnaud, Leroy, Xavier, Prevarskaya, Natalia, Gkika, Dimitra, Bussolati, Benedett
BMC Cancer, 2014-12-12;14(0):939.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
VEGF-A isoforms differentially regulate ATF-2-dependent VCAM-1 gene expression and endothelial-leukocyte interactions.
Authors: Fearnley G, Odell A, Latham A, Mughal N, Bruns A, Burgoyne N, Homer-Vanniasinkam S, Zachary I, Hollstein M, Wheatcroft S, Ponnambalam S
Mol Biol Cell, 2014-06-25;25(16):2509-21.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Control of angiogenesis by galectins involves the release of platelet-derived proangiogenic factors.
Authors: Etulain J, Negrotto S, Tribulatti M, Croci D, Carabelli J, Campetella O, Rabinovich G, Schattner M
PLoS ONE, 2014-04-30;9(4):e96402.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling.
Authors: Shvartsman, Dmitry, Storrie-White, Hannah, Lee, Kangwon, Kearney, Cathal, Brudno, Yevgeny, Ho, Nhi, Cezar, Christin, McCann, Corey, Anderson, Erin, Koullias, John, Tapia, Juan Car, Vandenburgh, Herman, Lichtman, Jeff W, Mooney, David J
Mol Ther, 2014-04-28;22(7):1243-53.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Endothelial cell adhesion to soluble vascular endothelial growth factor receptor-1 triggers a cell dynamic and angiogenic phenotype.
Authors: Orecchia A, Mettouchi A, Uva P, Simon G, Arcelli D, Avitabile S, Ragone G, Meneguzzi G, Pfenninger K, Zambruno G, Failla C
FASEB J, 2013-10-30;28(2):692-704.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
MicroRNA-24 suppression of N-deacetylase/N-sulfotransferase-1 (NDST1) reduces endothelial cell responsiveness to vascular endothelial growth factor A (VEGFA).
Authors: Kasza Z, Fredlund Fuchs P, Tamm C, Eriksson A, O'Callaghan P, Heindryckx F, Spillmann D, Larsson E, Le Jan S, Eriksson I, Gerwins P, Kjellen L, Kreuger J
J Biol Chem, 2013-07-24;288(36):25956-63.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Neuropilin-1 expression promotes invasiveness of melanoma cells through vascular endothelial growth factor receptor-2-dependent and -independent mechanisms
Authors: FEDERICA RUFFINI, STEFANIA D’ATRI, PEDRO M. LACAL
International Journal of Oncology
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
KDR identifies a conserved human and murine hepatic progenitor and instructs early liver development.
Authors: Goldman O, Han S, Sourisseau M, Dziedzic N, Hamou W, Corneo B, D'Souza S, Sato T, Kotton D, Bissig K, Kalir T, Jacobs A, Evans T, Evans M, Gouon-Evans V
Cell Stem Cell, 2013-06-06;12(6):748-60.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Vascular endothelial-cadherin stimulates syndecan-1-coupled insulin-like growth factor-1 receptor and cross-talk between alpha V beta 3 integrin and vascular endothelial growth factor receptor 2 at the onset of endothelial cell dissemination during angiogenesis
Authors: Alan C. Rapraeger, Brian J. Ell, Madhuchhanda Roy, Xuehui Li, Orrianne R. Morrison, Grant M. Thomas et al.
FEBS Journal
-
Ex Vivo Reconstitution of Arterial Endothelium by Embryonic Stem Cell-Derived Endothelial Progenitor Cells in Baboons
Authors: Qiang Shi, Vida Hodara, Calvin R. Simerly, Gerald P. Schatten, John L. VandeBerg
Stem Cells and Development
-
Essential role for endocytosis in the growth factor-stimulated activation of ERK1/2 in endothelial cells.
Authors: Gourlaouen, Morgane, Welti, Jonathan, Vasudev, Naveen S, Reynolds, Andrew R
J Biol Chem, 2013-01-22;288(11):7467-80.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Vascular Endothelial Growth Factor Receptor-3 Directly Interacts with Phosphatidylinositol 3-Kinase to Regulate Lymphangiogenesis
Authors: Sanja Coso, Yiping Zeng, Kenneth Opeskin, Elizabeth D. Williams
PLoS ONE
-
Differential expression of Vegfr-2 and its soluble form in preeclampsia.
Authors: Munaut C, Lorquet S, Pequeux C, Coulon C, Le Goarant J, Chantraine F, Noel A, Goffin F, Tsatsaris V, Subtil D, Foidart JM
PLoS ONE, 2012-03-12;7(3):e33475.
Species: Human
Sample Types: Plasma, Whole Tissue
Applications: IHC-P, Western Blot -
VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling
Authors: Tuomas Tammela, Georgia Zarkada, Harri Nurmi, Lars Jakobsson, Krista Heinolainen, Denis Tvorogov et al.
Nature Cell Biology
-
Expression of the soluble vascular endothelial growth factor receptor-1 in cutaneous melanoma: role in tumour progression
Authors: F. Ruffini, C.M. Failla, A. Orecchia, M.R. Bani, A.S. Dorio, C. Fortes et al.
British Journal of Dermatology
-
Expression of growth factor receptors and targeting of EGFR in cholangiocarcinoma cell lines
Authors: Ling Xu, Martin Hausmann, Wolfgang Dietmaier, Silvia Kellermeier, Theresa Pesch, Manuela Stieber-Gunckel et al.
BMC Cancer
-
Vascular endothelial growth factor drives autocrine epithelial cell proliferation and survival in chronic rhinosinusitis with nasal polyposis.
Authors: Lee HS, Myers A, Kim J
Am. J. Respir. Crit. Care Med., 2009-09-17;180(11):1056-67.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
The Down syndrome critical region gene 1 short variant promoters direct vascular bed-specific gene expression during inflammation in mice.
Authors: Minami T, Yano K, Miura M, Kobayashi M, Suehiro J, Reid PC, Hamakubo T, Ryeom S, Aird WC, Kodama T
J. Clin. Invest., 2009-07-13;119(8):2257-70.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Placenta growth factor in diabetic wound healing: altered expression and therapeutic potential.
Authors: Cianfarani F, Zambruno G, Brogelli L, Sera F, Lacal PM, Pesce M, Capogrossi MC, Failla CM, Napolitano M, Odorisio T
Am. J. Pathol., 2006-10-01;169(4):1167-82.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Neutralization, Western Blot -
Enhancement of angiogenic effectors through hypoxia-inducible factor in preterm primate lung in vivo.
Authors: Asikainen TM, Waleh NS, Schneider BK, Clyman RI, White CW
Am. J. Physiol. Lung Cell Mol. Physiol., 2006-05-05;291(4):L588-95.
Species: Primate - Papio anubis (Olive Baboon)
Sample Types: Tissue Homogenates
Applications: Western Blot -
Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung.
Authors: Asikainen TM, Schneider BK, Waleh NS, Clyman RI, Ho WB, Flippin LA, Gunzler V, White CW
Proc. Natl. Acad. Sci. U.S.A., 2005-07-11;102(29):10212-7.
Species: Human
Sample Types: Whole Cells
Applications: ICC, Neutralization -
PEDF derived from glial Muller cells: a possible regulator of retinal angiogenesis.
Authors: Eichler W, Yafai Y, Keller T, Wiedemann P, Reichenbach A
Exp. Cell Res., 2004-09-10;299(1):68-78.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2.
Authors: Usui T, Ishida S, Yamashiro K, Kaji Y, Poulaki V, Moore J, Moore T, Amano S, Horikawa Y, Dartt D, Golding M, Shima DT, Adamis AP
Invest. Ophthalmol. Vis. Sci., 2004-02-01;45(2):368-74.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Malignant mesothelioma growth inhibition by agents that target the VEGF and VEGF-C autocrine loops.
Authors: Masood R, Kundra A, Zhu S, Xia G, Scalia P, Smith DL, Gill PS
Int. J. Cancer, 2003-05-01;104(5):603-10.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Proangiogenic properties of human myeloma cells: production of angiopoietin-1 and its potential relationship to myeloma-induced angiogenesis.
Authors: Giuliani N, Colla S, Lazzaretti M, Sala R, Roti G, Mancini C, Bonomini S, Lunghi P, Hojden M, Genestreti G, Svaldi M, Coser P, Fattori PP, Sammarelli G, Gazzola GC, Almici C, Caramatti C, Mangoni L, Rizzoli V
Blood, 2003-03-20;102(2):638-45.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects.
Authors: Okruzhnov Y, Nicholas J
J. Virol., 2001-11-01;75(22):10933-40.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Induced hepatic stem cells are suitable for human hepatocyte production
Authors: Yoshiki Nakashima, Chika Miyagi-Shiohira, Issei Saitoh, Masami Watanabe, Masayuki Matsushita, Masayoshi Tsukahara et al.
iScience
-
Perivascular Neuropilin-1 expression is an independent marker of improved survival in renal cell carcinoma
Authors: Abdallah MG, Buchanan-Vega JA, Lee KJ, et al.
J Pathol.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human VEGFR2/KDR/Flk-1 Antibody
Average Rating: 3.5 (Based on 2 Reviews)
Have you used Human VEGFR2/KDR/Flk-1 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
First lane- nontargeting, second lane- AP1 knockdown. Top ladder band 220kDa.