Human VEGFR2/KDR/Flk-1 PE-conjugated Antibody Summary
Ala20-Glu764
Accession # P35968
Customers also Viewed
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of VEGF R2/KDR/Flk‑1 in HUVEC Human Cells by Flow Cytometry. HUVEC human umbilical vein endothelial cells were stained with Mouse Anti-Human VEGF R2/KDR/Flk-1 PE-conjugated Mono-clonal Antibody (Catalog # FAB357P, filled histogram) or isotype control antibody (Catalog # IC002P, open histogram). View our protocol for Staining Membrane-associated Proteins.
 View Larger
View Larger
Detection of Human VEGFR2/KDR/Flk-1 by Flow Cytometry Circulating EPC number in healthy controls and diabetes. a and b: Circulating EPC numbers were determined by flow cytometry for the co-expression of CD34 and VEGFR2 (b). Peripheral blood MNCs incubated with IgG isotype control (a) serve as a negative control to determine the intrinsic fluorescent intensity of the peripheral blood MNCs and to define positive area R2. c: Absolute number of circulating EPCs in healthy controls and diabetes as determined by the co-expression of CD34 and VEGFR2. d: Absolute number of circulating EPCs in healthy controls, diabetes with good and poor glycemic control as determined by the co-expression of CD34 and VEGFR2. e: Correlation between the circulating EPC numbers and FBS, f: Correlation between the circulating EPC numbers and HbA1C, g: Absolute number of circulating CD34+/VEGFR2- cells in healthy controls and diabetes. Data are presented as means ± SEM. Image collected and cropped by CiteAb from the following publication (https://bmcendocrdisord.biomedcentral.com/articles/10.1186/1472-6823-10-5), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human VEGFR2/KDR/Flk-1 by Flow Cytometry Phenotypic characterization of cultured PACs. A) PACs were characterized for surface marker expression by flow cytometry. In the side scatted (SSC) versus forward scatter (FSC) morphologic plot, lymphocytic cells (LYMPHs) and monocyte-macrophages (MONOs) were identified and gated separately. Histograms reporting the expression of relevant leukocyte (CD45, CD14, CD68) and endothelial markers (CD31, KDR, CD34) are shown, together with mean percent expression from 3 replicates. The red line indicates negative control, while the blue line indicates the stained condition. B) Cells in the PACs culture were stained with the endothelial markers acLDL and Ulex Lectin. The fraction of cells that were positive for both markers were compared in cultures obtained from T2D or healthy control cells. *p < 0.05 T2D vs Ctrl. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24886621), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human VEGFR2/KDR/Flk-1 by Flow Cytometry Flow cytometry-derived EPC definitions.(A) Cells isolated from whole blood by density gradient separation were gated by forward and side scatter to select mononuclear cells by excluding erythrocytes, granulocytes and cell debris. (B) The mononuclear-gated cells that stained positive for CD34-FITC were analyzed for the presence or absence of CD45-PC5. (C) Mononuclear cells were also analyzed for co-staining of CD34-FITC and KDR-PE. MNC, mononuclear cell; CD34-FITC, fluorescein isothiocyanate-conjugated CD34; CD45-PC5, phycoerythrin-Cy5-conjugated CD45; KDR-PE, phycoerythrin-conjugated KDR. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24736282), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human VEGFR2/KDR/Flk-1 by Flow Cytometry Circulating EPC number in healthy controls and diabetes. a and b: Circulating EPC numbers were determined by flow cytometry for the co-expression of CD34 and VEGFR2 (b). Peripheral blood MNCs incubated with IgG isotype control (a) serve as a negative control to determine the intrinsic fluorescent intensity of the peripheral blood MNCs and to define positive area R2. c: Absolute number of circulating EPCs in healthy controls and diabetes as determined by the co-expression of CD34 and VEGFR2. d: Absolute number of circulating EPCs in healthy controls, diabetes with good and poor glycemic control as determined by the co-expression of CD34 and VEGFR2. e: Correlation between the circulating EPC numbers and FBS, f: Correlation between the circulating EPC numbers and HbA1C, g: Absolute number of circulating CD34+/VEGFR2- cells in healthy controls and diabetes. Data are presented as means ± SEM. Image collected and cropped by CiteAb from the following publication (https://bmcendocrdisord.biomedcentral.com/articles/10.1186/1472-6823-10-5), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, 2 to 8 °C as supplied.
Background: VEGFR2/KDR/Flk-1
VEGF R2 (KDR/Flk-1), VEGF R1 (Flt-1) and VEGF R3 (Flt-4) belong to the class III subfamily of receptor tyrosine kinases (RTKs). All three receptors contain seven immunoglobulin-like repeats in their extracellular domains and kinase insert domains in their intracellular regions. The expression of VEGF R1, 2, and 3 is almost exclusively restricted to the endothelial cells. These receptors are likely to play essential roles in vasculogenesis and angiogenesis. Mature VEGF R2 is composed of a 745 aa extracellular domain, a 25 aa transmembrane domain and a 567 aa cytoplasmic domain. In contrast to VEGF R1 which binds both PlGF and VEGF with high affinity, VEGF R2 binds VEGF but not PlGF with high affinity. The recombinant soluble VEGF R2/Fc chimera binds VEGF with high affinity and is a potent VEGF antagonist.
Product Datasheets
Citations for Human VEGFR2/KDR/Flk-1 PE-conjugated Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
122
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Mapping molecular landmarks of human skeletal ontogeny and pluripotent stem cell-derived articular chondrocytes
Authors: GB Ferguson, B Van Handel, M Bay, P Fiziev, T Org, S Lee, R Shkhyan, NW Banks, M Scheinberg, L Wu, B Saitta, J Elphingsto, AN Larson, SM Riester, AD Pyle, NM Bernthal, HK Mikkola, J Ernst, AJ van Wijnen, M Bonaguidi, D Evseenko
Nat Commun, 2018-09-07;9(1):3634.
-
Endothelial damage in major depression patients is modulated by SSRI treatment, as demonstrated by circulating biomarkers and an in vitro cell model
Authors: I Lopez-Vilchez, M Diaz-Ricart, V Navarro, S Torramade, J Zamorano-Leon, A Lopez-Farre et al.
Translational Psychiatry
-
Surface PD-L1,�E-cadherin, CD24, and VEGFR2 as markers of epithelial cancer stem cells associated with rapid tumorigenesis
Authors: GG Jinesh, GC Manyam, CO Mmeje, KA Baggerly, AM Kamat
Sci Rep, 2017-08-29;7(1):9602.
-
Change in Circulating Levels of Endothelial Progenitor Cells and Sexual Function in Women With Type 1 Diabetes
Authors: Antonietta Maio, Maria Ida Maiorino, Miriam Longo, Lorenzo Scappaticcio, Vlenia Pernice, Paolo Cirillo et al.
The Journal of Clinical Endocrinology & Metabolism
-
Clinical significance of 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester-labeled microspheres for detecting endothelial progenitor cells in human peripheral blood
Authors: Chaolin Qiu, Denghai Zhang, Yongbin Chi, Qing Chen, Limin Xu, Qiuhua Xie
Experimental and Therapeutic Medicine
-
Vasculogenic Conditioning of Peripheral Blood Mononuclear Cells Promotes Endothelial Progenitor Cell Expansion and Phenotype Transition of Anti‐Inflammatory Macrophage and T Lymphocyte to Cells With Regenerative Potential
Authors: Haruchika Masuda, Rica Tanaka, Satoshi Fujimura, Masakazu Ishikawa, Hiroshi Akimaru, Tomoko Shizuno et al.
Journal of the American Heart Association
-
Isolation, differentiation and characterization of vascular cells derived from human embryonic stem cells
Authors: Shulamit Levenberg, Lino S Ferreira, Limor Chen-Konak, Thomas P Kraehenbuehl, Robert Langer
Nature Protocols
-
The Differentiation of Pluripotent Stem Cells towards Endothelial Progenitor Cells - Potential Application in Pulmonary Arterial Hypertension
Authors: Kezhou Qin, Jia Lei, Jun Yang
International Journal of Stem Cells
-
Circulating Mesenchymal Stem Cells Microparticles in Patients with Cerebrovascular Disease
Authors: Suk Jae Kim, Gyeong Joon Moon, Yeon Hee Cho, Ho Young Kang, Na Kyum Hyung, Donghee Kim et al.
PLoS ONE
-
Association of endothelial progenitor cells and peptic ulcer treatment in patients with type 2 diabetes mellitus
Authors: ZHIHONG NIE, LIMIN XU, CHUANYUAN LI, TAO TIAN, PINGPING XIE, XIA CHEN et al.
Experimental and Therapeutic Medicine
-
The Histone Demethylase Jumonji Coordinates Cellular Senescence Including Secretion of Neural Stem Cell–Attracting Cytokines
Authors: Patrick M. Perrigue, Michael E. Silva, Charles D. Warden, Nathan L. Feng, Michael A. Reid, Daniel J. Mota et al.
Molecular Cancer Research
-
Inhaled NO Contributes to Lung Repair in Piglets with Acute Respiratory Distress Syndrome via Increasing Circulating Endothelial Progenitor Cells
Authors: Yuanyuan Qi, Liling Qian, Bo Sun, Lijuan Liu, Panpan Wu, Libo Sun
PLoS ONE
-
Effects of shRNA-mediated silencing of PDE5A3 on intracellular cGMP and free Ca(2+) levels and human prostate smooth muscle cell proliferation from benign prostatic hyperplasia
Authors: Xu Z, Ge Y, Jiang K et al
Exp Ther Med
-
COMPARE CPM-RMI Trial: Intramyocardial Transplantation of Autologous Bone Marrow-Derived CD133+ Cells and MNCs during CABG in Patients with Recent MI: A Phase II/III, Multicenter, Placebo-Controlled, Randomized, Double-Blind Clinical Trial
Authors: Mohammad Hassan Naseri, Hoda Madani, Seyed Hossein Ahmadi Tafti, Maryam Moshkani Farahani, Davood Kazemi Saleh, Hossein Hosseinnejad et al.
Cell J
-
Endothelial Cells Promote Expansion of Long-Term Engrafting Marrow Hematopoietic Stem and Progenitor Cells in Primates
Authors: Jennifer L. Gori, Jason M. Butler, Balvir Kunar, Michael G. Poulos, Michael Ginsberg, Daniel J. Nolan et al.
Stem Cells Translational Medicine
-
Blebbishields, the emergency program for cancer stem cells: sphere formation and tumorigenesis after apoptosis
Authors: G G Jinesh, W Choi, J B Shah, E K Lee, D L Willis, A M Kamat
Cell Death & Differentiation
-
GATA6 regulates WNT and BMP programs to pattern precardiac mesoderm during the earliest stages of human cardiogenesis
Authors: Bisson, JA;Gordillo, M;Kumar, R;de Silva, N;Yang, E;Banks, KM;Shi, ZD;Lee, K;Yang, D;Chung, WK;Huangfu, D;Evans, T;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Human iPSC-derived Committed Cardiac Progenitors Generate Cardiac Tissue Grafts in a Swine Ischemic Cardiomyopathy Model without Triggering Ventricular Arrhythmias
Authors: Raval, AN;Schmuck, EG;Roy, S;Saito, Y;Zhou, T;Conklin, J;Hacker, TA;Koonce, C;Boyer, M;Stack, K;Hebron, E;Nagle, SK;Hsieh, PCH;Kamp, TJ;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Generation of a human Tropomyosin 1 knockout iPSC line
Authors: Wilken, MB;Maguire, JA;Dungan, LV;Gagne, A;Osorio-Quintero, C;Waxman, EA;Chou, ST;Gadue, P;French, DL;Thom, CS;
Stem cell research
Species: Human
Sample Types: Whole Tissue
Applications: Flow Cytometry -
Augmenting the Angiogenic Profile and Functionality of Cord Blood Endothelial Colony-Forming Cells by Indirect Priming with Bone-Marrow-Derived Mesenchymal Stromal Cells
Authors: Bansal, A;Singh, A;Nag, TC;Sharma, D;Garg, B;Bhatla, N;Choudhury, SD;Ramakrishnan, L;
Biomedicines
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Generation of a human Tropomyosin 1 knockout iPSC line
Authors: Wilken, MB;Maguire, JA;Dungan, LV;Gagne, A;Osorio-Quintero, C;Waxman, EA;Chou, ST;Gadue, P;French, DL;Thom, CS;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Impaired repair properties of endothelial colony-forming cells in patients with granulomatosis with polyangiitis
Authors: APT Del Rio, JO Frade-Guan, S Ospina-Pri, BKL Duarte, MB Bertolo, MC Ozelo, Z Sachetto
Journal of Cellular and Molecular Medicine, 2022-09-02;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Functional Impairment of Endothelial Colony Forming Cells (ECFC) in Patients with Severe Atherosclerotic Cardiovascular Disease (ASCVD)
Authors: S Simoncini, S Toupance, C Labat, S Gautier, C Dumoulin, L Arnaud, MG Stathopoul, S Visvikis-S, PM Rossi, A Benetos, F Dignat-Geo, F Sabatier
International Journal of Molecular Sciences, 2022-08-11;23(16):.
Species: Human
Sample Types: Whole Cells
-
CD1d expression demarcates CDX4+ hemogenic mesoderm with definitive hematopoietic potential
Authors: J Philip Cre, SA Luff, H Yu, CM Sturgeon
Stem Cell Research, 2022-05-08;62(0):102808.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Specific mesoderm subset derived from human pluripotent stem cells ameliorates microvascular pathology in type 2 diabetic mice
Authors: CH Gil, D Chakrabort, CP Vieira, N Prasain, SL Calzi, SD Fortmann, P Hu, K Banno, M Jamal, C Huang, MS Sielski, Y Lin, X Huang, MD Dupont, JL Floyd, R Prasad, ALF Longhini, TJ McGill, HM Chung, MP Murphy, DN Kotton, ME Boulton, MC Yoder, MB Grant
Science Advances, 2022-03-04;8(9):eabm5559.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Role of endothelial colony forming cells (ECFCs) Tetrahydrobiopterin (BH4) in determining ECFCs functionality in coronary artery disease (CAD) patients
Authors: A Sen, A Singh, A Roy, S Mohanty, N Naik, M Kalaivani, L Ramakrishn
Scientific Reports, 2022-02-23;12(1):3076.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Stage-specific regulation of DNA methylation by TET enzymes during human cardiac differentiation
Authors: Y Lan, KM Banks, H Pan, N Verma, GR Dixon, T Zhou, B Ding, O Elemento, S Chen, D Huangfu, T Evans
Cell Reports, 2021-12-07;37(10):110095.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, ICC, Western Blot -
Reduced angiovasculogenic and increased inflammatory profiles of cord blood cells in severe but not mild preeclampsia
Authors: S Cho, YD Sohn, S Kim, A Rajakumar, ML Badell, N Sidell, YS Yoon
Scientific Reports, 2021-02-11;11(1):3630.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Tropomyosin 1 genetically constrains in vitro hematopoiesis
Authors: CS Thom, CD Jobaliya, K Lorenz, JA Maguire, A Gagne, P Gadue, DL French, BF Voight
BMC Biol., 2020-05-14;18(1):52.
Species: Human
Sample Types:
Applications: FLOW -
Rationale and design of the granulocyte-macrophage colony stimulating factor in peripheral arterial disease (GPAD-3) study
Authors: A Mehta, K Mavromatis, YA Ko, SC Rogers, DS Dhindsa, C Goodwin, R Patel, MA Martini, M Prasad, A Mokhtari, IG Hesaroieh, SC Frohwein, MH Kutner, A Harzand, BJ Wells, Y Duwayri, O Alabi, RR Rajani, LP Brewster, EK Waller, AA Quyyumi
Contemp Clin Trials, 2020-03-04;91(0):105975.
Species: Human
Sample Types: Whole Blood
Applications: Neutralization -
Effects of exergaming in postmenopausal women with high cardiovascular risk: A randomized controlled trial
Authors: EA Jo, SS Wu, HR Han, JJ Park, SJ Park, KI Cho
Clin Cardiol, 2019-12-28;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Systemic microvascular rarefaction is correlated with dysfunction of late endothelial progenitor cells in mild hypertension: a substudy of EXCAVATION-CHN1
Authors: J Liang, Y Li, L Chen, W Xia, G Wu, X Tong, C Su, J He, X Lin, J Tao
J Transl Med, 2019-11-12;17(1):368.
Species: Human
Sample Types: Whole Cell
Applications: Flow Cytometry -
Predicting Angiogenesis by Endothelial Progenitor Cells Relying on In-Vitro Function Assays and VEGFR-2 Expression Levels
Authors: N Sabbah, T Tamari, R Elimelech, O Doppelt, U Rudich, H Zigdon-Gil
Biomolecules, 2019-11-08;9(11):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Essential Gene Profiles for Human Pluripotent Stem Cells Identify Uncharacterized Genes and Substrate Dependencies
Authors: B Mair, J Tomic, SN Masud, P Tonge, A Weiss, M Usaj, AHY Tong, JJ Kwan, KR Brown, E Titus, M Atkins, KSK Chan, L Munsie, A Habsid, H Han, M Kennedy, B Cohen, G Keller, J Moffat
Cell Rep, 2019-04-09;27(2):599-615.e12.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Anti-Angiogenic miR-222, miR-195, and miR-21a Plasma Levels in T1DM Are Improved by Metformin Therapy, Thus Elucidating Its Cardioprotective Effect: The MERIT Study
Authors: FW Ahmed, S Bakhashab, IT Bastaman, RE Crossland, M Glanville, JU Weaver
Int J Mol Sci, 2018-10-19;19(10):.
Species: Human
Sample Types: Whole Blood
Applications: Flow Cytometry -
Linking cell function with perfusion: insights from the transcatheter delivery of bone marrow-derived CD133+ cells in ischemic refractory cardiomyopathy trial (RECARDIO)
Authors: B Bassetti, C Carbucicch, V Catto, E Gambini, E Rurali, A Bestetti, G Gaipa, D Belotti, F Celeste, M Parma, S Righetti, L Biava, M Arosio, A Bonomi, P Agostoni, P Scacciatel, F Achilli, G Pompilio
Stem Cell Res Ther, 2018-09-14;9(1):235.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Preparation of iPS cell-derived CD31+ endothelial cells using three-dimensional suspension culture
Authors: S Masuda, K Matsuura, T Shimizu
Regen Ther, 2018-07-07;9(0):1-9.
Species: Human
Sample Types: Whole Cells
Applications: Cell Selection -
Putative mechanisms Underlying Myocardial infarction onset and Emotions (PUME): a randomised controlled study protocol
Authors: I Ensari, MM Burg, KM Diaz, J Fu, AT Duran, JM Suls, JA Sumner, R Monane, JE Julian, S Zhao, WF Chaplin, D Shimbo
BMJ Open, 2018-05-31;8(5):e020525.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Quality-Quantity Control Culture Enhances Vasculogenesis and Wound Healing Efficacy of Human Diabetic Peripheral Blood CD34+ Cells
Authors: R Tanaka, H Masuda, S Fujimura, R Ito-Hirano, K Arita, Y Kakinuma, H Hagiwara, M Kado, A Hayashi, T Mita, T Ogawa, H Watada, H Mizuno, N Sawada, T Asahara
Stem Cells Transl Med, 2018-03-24;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Endothelial cell functions impaired by interferon in vitro: Insights into the molecular mechanism of thrombotic microangiopathy associated with interferon therapy
Authors: H Jia, C Thelwell, P Dilger, C Bird, S Daniels, M Wadhwa
Thromb. Res., 2018-02-06;163(0):105-116.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Effect of intensive multifactorial treatment on vascular progenitor cells in hypertensive patients
Authors: C Maroun-Eid, A Ortega-Her, J Modrego, M Abad-Cardi, JA García-Don, L Reinares, N Martell-Cl, D Gómez-Garr
PLoS ONE, 2018-01-05;13(1):e0190494.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Sex Differences in Circulating Progenitor Cells
Authors: ML Topel, SS Hayek, YA Ko, PB Sandesara, A Samman Tah, I Hesaroieh, E Mahar, GS Martin, EK Waller, AA Quyyumi
J Am Heart Assoc, 2017-10-03;6(10):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Hydrogen Sulfide-Preconditioning of Human Endothelial Progenitor Cells Transplantation Improves Re-Endothelialization in Nude Mice with Carotid Artery Injury
Authors: X Ke, J Zou, Q Hu, X Wang, C Hu, R Yang, J Liang, X Shu, R Nie, C Peng
Cell. Physiol. Biochem., 2017-08-31;43(1):308-319.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
The blebbishield emergency program overrides chromosomal instability and phagocytosis checkpoints in cancer stem cells
Authors: GG Jinesh, AM Kamat
Cancer Res., 2017-08-30;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Chromatin and Transcriptional Analysis of Mesoderm Progenitor Cells Identifies HOPX as a Regulator of Primitive Hematopoiesis
Authors: NJ Palpant, Y Wang, B Hadland, RJ Zaunbreche, M Redd, D Jones, L Pabon, R Jain, J Epstein, WL Ruzzo, Y Zheng, I Bernstein, A Margolin, CE Murry
Cell Rep, 2017-08-15;20(7):1597-1608.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
VEGF-induced intracellular Ca2+ oscillations are down-regulated and do not stimulate angiogenesis in breast cancer-derived endothelial colony forming cells
Authors: F Lodola, U Laforenza, F Cattaneo, FA Ruffinatti, V Poletto, M Massa, R Tancredi, E Zuccolo, DA Khdar, A Riccardi, M Biggiogera, V Rosti, G Guerra, F Moccia
Oncotarget, 2017-08-14;8(56):95223-95246.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Targeted Disruption of TCF12 Reveals HEB as Essential in Human Mesodermal Specification and Hematopoiesis
Authors: Y Li, PM Brauer, J Singh, S Xhiku, K Yoganathan, JC Zúñiga-Pfl, MK Anderson
Stem Cell Reports, 2017-08-10;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Effects of a New Nutraceutical Formulation (Berberine, Red Yeast Rice and Chitosan) on Non-HDL Cholesterol Levels in Individuals with Dyslipidemia: Results from a Randomized, Double Blind, Placebo-Controlled Study
Authors: V Spigoni, R Aldigeri, M Antonini, MM Micheli, F Fantuzzi, A Fratter, M Pellizzato, E Derlindati, I Zavaroni, RC Bonadonna, A Dei Cas
Int J Mol Sci, 2017-07-12;18(7):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
A quantitative method for screening and identifying molecular targets for nanomedicine
Authors: P Guo, J Yang, DR Bielenberg, D Dillon, D Zurakowski, MA Moses, DT Auguste
J Control Release, 2017-03-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Increased serum levels of fractalkine and mobilisation of CD34(+)CD45(-) endothelial progenitor cells in systemic sclerosis
Authors: A Benyamine, J Magalon, S Cointe, R Lacroix, L Arnaud, N Bardin, P Rossi, Y Francès, F Bernard-Gu, G Kaplanski, JR Harlé, PJ Weiller, P Berbis, D Braunstein, E Jouve, N Lesavre, F Couranjou, F Dignat-Geo, F Sabatier, P Paul, B Granel
Arthritis Res. Ther, 2017-03-20;19(1):60.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Circulating microparticle subpopulations in systemic lupus erythematosus are affected by disease activity
Authors: P López, J Rodríguez-, A Martínez-Z, L Caminal-Mo, A Suárez
Int. J. Cardiol, 2017-03-01;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Effects of Chronic Exercise on Endothelial Progenitor Cells and Microparticles in Professional Runners
Authors: CRO Bittencour, MCO Izar, CN França, VL Schwerz, RMDS Póvoa, FAH Fonseca
Arq. Bras. Cardiol., 2017-03-01;108(3):212-216.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Vildagliptin, but not glibenclamide, increases circulating endothelial progenitor cell number: a 12-month randomized controlled trial in patients with type 2 diabetes
Authors: A Dei Cas, V Spigoni, M Cito, R Aldigeri, V Ridolfi, E Marchesi, M Marina, E Derlindati, R Aloe, RC Bonadonna, I Zavaroni
Cardiovasc Diabetol, 2017-02-23;16(1):27.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Cell therapy in critical limb ischemia: A comprehensive analysis of two cell therapy products
Authors: Claire Tournois
Cytotherapy, 2016-11-30;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Endothelial cell colony forming units derived from malignant breast diseases are resistant to tumor necrosis factor-?-induced apoptosis
Sci Rep, 2016-11-24;6(0):37450.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
BMP4/Id2 signaling pathway is a novel therapeutic target for late outgrowth endothelial progenitor cell-mediated endothelial injury repair
Authors: Jun Tao
Int. J. Cardiol., 2016-11-09;228(0):796-804.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Somatic GNAQ Mutation is Enriched in Brain Endothelial Cells in�Sturge-Weber Syndrome
Authors: Lan Huang
Pediatr. Neurol, 2016-10-21;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Mycobacterium tuberculosis exploits the formation of new blood vessels for its dissemination
Sci Rep, 2016-09-12;6(0):33162.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Cloche is a bHLH-PAS transcription factor that drives haemato-vascular specification
Nature, 2016-07-14;535(7611):294-8.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Dysregulation of ossification related microRNAs in circulating osteogenic progenitor cells obtained from patients with aortic stenosis
Authors: K Takahashi, M Satoh, Y Takahashi, T Osaki, T Nasu, M Tamada, H Okabayashi, M Nakamura, Y Morino
Clin Sci, 2016-04-14;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
MicroRNA-134 Contributes to Glucose-Induced Endothelial Cell Dysfunction and This Effect Can Be Reversed by Far-Infrared Irradiation.
Authors: Wang H, Su S, Wang Y, Chang S, Liao K, Lo H, Chiu Y, Hsieh T, Huang T, Lin C, Cheng S, Cheng C
PLoS ONE, 2016-01-22;11(1):e0147067.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
The inflammation, vascular repair and injury responses to exercise in fit males with and without Type 1 diabetes: an observational study.
Authors: West D, Campbell M, Gonzalez J, Walker M, Stevenson E, Ahmed F, Wijaya S, Shaw J, Weaver J
Cardiovasc Diabetol, 2015-06-05;14(0):71.
Species: Human
Sample Types: Complex Sample Type
Applications: Flow Cytometry -
Generation of articular chondrocytes from human pluripotent stem cells.
Authors: Craft A, Rockel J, Nartiss Y, Kandel R, Alman B, Keller G
Nat Biotechnol, 2015-05-11;33(6):638-45.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells.
Authors: Gori J, Butler J, Chan Y, Chandrasekaran D, Poulos M, Ginsberg M, Nolan D, Elemento O, Wood B, Adair J, Rafii S, Kiem H
J Clin Invest, 2015-02-09;125(3):1243-54.
Species: Primate - Macaca nemestrina (Southern Pig-tailed Macaque)
Sample Types: Whole Cells
Applications: Flow Cytometry -
BRAF Inhibition Stimulates Melanoma-Associated Macrophages to Drive Tumor Growth.
Authors: Wang T, Xiao M, Ge Y, Krepler C, Belser E, Lopez-Coral A, Xu X, Zhang G, Azuma R, Liu Q, Liu R, Li L, Amaravadi R, Xu W, Karakousis G, Gangadhar T, Schuchter L, Lieu M, Khare S, Halloran M, Herlyn M, Kaufman R
Clin Cancer Res, 2015-01-23;21(7):1652-64.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Glucose transporter 1-positive endothelial cells in infantile hemangioma exhibit features of facultative stem cells.
Authors: Huang L, Nakayama H, Klagsbrun M, Mulliken J, Bischoff J
Stem Cells, 2015-01-01;33(1):133-45.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Hypoxia-controlled EphA3 marks a human endometrium-derived multipotent mesenchymal stromal cell that supports vascular growth.
Authors: To C, Farnsworth R, Vail M, Chheang C, Gargett C, Murone C, Llerena C, Major A, Scott A, Janes P, Lackmann M
PLoS ONE, 2014-11-24;9(11):e112106.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Bone marrow-derived cells are implicated as a source of lymphatic endothelial progenitors in human breast cancer.
Authors: Van't Hull E, Bron S, Henry L, Ifticene-Treboux A, Turrini R, Coukos G, Delaloye J, Doucey M
Oncoimmunology, 2014-06-25;3(0):e29080.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Laminin promotes vascular network formation in 3D in vitro collagen scaffolds by regulating VEGF uptake.
Authors: Stamati K, Priestley J, Mudera V, Cheema U
Exp Cell Res, 2014-06-05;327(1):68-77.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Endothelial CD276 (B7-H3) expression is increased in human malignancies and distinguishes between normal and tumour-derived circulating endothelial cells.
Authors: Kraan, J, van den Broek, P, Verhoef, C, Grunhagen, D J, Taal, W, Gratama, J W, Sleijfer, S
Br J Cancer, 2014-06-03;111(1):149-56.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells.
Authors: Orlova, Valeria, van den Hil, Francijn, Petrus-Reurer, Sandra, Drabsch, Yvette, Ten Dijke, Peter, Mummery, Christin
Nat Protoc, 2014-05-29;9(6):1514-31.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
The dipeptidyl peptidase-4 inhibitor saxagliptin improves function of circulating pro-angiogenic cells from type 2 diabetic patients.
Authors: Poncina N, Albiero M, Menegazzo L, Cappellari R, Avogaro A, Fadini G
Cardiovasc Diabetol, 2014-05-14;13(0):92.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Characterization of circulating and endothelial progenitor cells in patients with extreme-duration type 1 diabetes.
Authors: Hernandez S, Gong J, Chen L, Wu I, Sun J, Keenan H, King G
Diabetes Care, 2014-04-29;37(8):2193-201.
Species: Human
Sample Types: Whole Blood
Applications: Flow Cytometry -
The relationship between endothelial progenitor cell populations and epicardial and microvascular coronary disease-a cellular, angiographic and physiologic study.
Authors: Chan, Kim H, Simpson, Philippa, Yong, Andy S, Dunn, Louise L, Chawantanpipat, Chirapan, Hsu, Chijen, Yu, Young, Keech, Anthony, Celermajer, David S, Ng, Martin K
PLoS ONE, 2014-04-15;9(4):e93980.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Endothelial progenitor cells in morbid obesity.
Authors: Graziani F, Leone A, Basile E, Cialdella P, Tritarelli A, Bona R, Liuzzo G, Nanni G, Iaconelli A, Iaconelli A, Mingrone G, Biasucci L, Crea F
Circ J, 2014-02-27;78(4):977-85.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Migratory activity of circulating mononuclear cells is associated with cardiovascular mortality in type 2 diabetic patients with critical limb ischemia.
Authors: Spinetti G, Specchia C, Fortunato O, Sangalli E, Clerici G, Caminiti M, Airoldi F, Losa S, Emanueli C, Faglia E, Madeddu P
Diabetes Care, 2014-02-26;37(5):1410-7.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Circulating angiogenic cell dysfunction in patients with hereditary hemorrhagic telangiectasia.
Authors: Zucco L, Zhang Q, Kuliszewski M, Kandic I, Faughnan M, Stewart D, Kutryk M
PLoS ONE, 2014-02-25;9(2):e89927.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Phenotyping of human melanoma cells reveals a unique composition of receptor targets and a subpopulation co-expressing ErbB4, EPO-R and NGF-R.
Authors: Mirkina I, Hadzijusufovic E, Krepler C, Mikula M, Mechtcheriakova D, Strommer S, Stella A, Jensen-Jarolim E, Holler C, Wacheck V, Pehamberger H, Valent P
PLoS ONE, 2014-01-29;9(1):e84417.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
IFNalpha serum levels are associated with endothelial progenitor cells imbalance and disease features in rheumatoid arthritis patients.
Authors: Rodriguez-Carrio J, de Paz B, Lopez P, Prado C, Alperi-Lopez M, Ballina-Garcia F, Suarez A
PLoS ONE, 2014-01-21;9(1):e86069.
Species: Human
Sample Types: Whole Blood
Applications: Flow Cytometry -
Circulating endothelial progenitor cells decrease in infants with bronchopulmonary dysplasia and increase after inhaled nitric oxide.
Authors: Qi, Yuanyuan, Jiang, Qian, Chen, Chao, Cao, Yun, Qian, Liling
PLoS ONE, 2013-11-11;8(11):e79060.
Species: Human
Sample Types: Plasma
Applications: Flow Cytometry -
The synergistic effect of everolimus and chloroquine on endothelial cell number reduction is paralleled by increased apoptosis and reduced autophagy occurrence.
Authors: Grimaldi A, Balestrieri M, D'Onofrio N, Di Domenico G, Nocera C, Lamberti M, Tonini G, Zoccoli A, Santini D, Caraglia M, Pantano F
PLoS ONE, 2013-11-11;8(11):e79658.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts.
Authors: Orlova, Valeria, Drabsch, Yvette, Freund, Christia, Petrus-Reurer, Sandra, van den Hil, Francijn, Muenthaisong, Suchitra, Dijke, Peter Te, Mummery, Christin
Arterioscler Thromb Vasc Biol, 2013-10-24;34(1):177-86.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Functional and molecular characterization of ex vivo cultured epiretinal membrane cells from human proliferative diabetic retinopathy.
Authors: Vereb Z, Lumi X, Andjelic S, Globocnik-Petrovic M, Urbancic M, Hawlina M, Facsko A, Petrovski G
Biomed Res Int, 2013-10-01;2013(0):492376.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
An affordable method to obtain cultured endothelial cells from peripheral blood.
Authors: Bueno-Beti C, Novella S, Lazaro-Franco M, Perez-Cremades D, Heras M, Sanchis J, Hermenegildo C
J Cell Mol Med, 2013-10-01;17(11):1475-83.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Efficiency of statin treatment on EPC recruitment depends on baseline EPC titer and does not improve angiographic outcome in coronary artery disease patients treated with the Genous stent.
Authors: den Dekker W, Houtgraaf J, Rowland S, Ligtenberg E, de Boer S, de Jong R, de Winter R, den Heijer P, Zijlstra F, Serruys P, Cheng C, Duckers H
Cell Transplant, 2013-04-03;23(12):1525-35.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
CXCR4 positive and angiogenic monocytes in myocardial infarction.
Thromb Haemost, 2012-12-06;109(2):255-62.
Species: Human
Sample Types: Whole Blood
Applications: Flow Cytometry -
Human haemato-endothelial precursors: cord blood CD34+ cells produce haemogenic endothelium.
Authors: Pelosi E, Castelli G, Martin-Padura I, Bordoni V, Santoro S, Conigliaro A, Cerio A, De Santis Puzzonia M, Marighetti P, Biffoni M, Alonzi T, Amicone L, Alcalay M, Bertolini F, Testa U, Tripodi M
PLoS ONE, 2012-12-04;7(12):e51109.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Diabetes impairs stem cell and proangiogenic cell mobilization in humans.
Authors: Fadini G, Albiero M, Vigili de Kreutzenberg S, Boscaro E, Cappellari R, Marescotti M, Poncina N, Agostini C, Avogaro A
Diabetes Care, 2012-10-30;36(4):943-9.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Cultivation and characterization of cornea limbal epithelial stem cells on lens capsule in animal material-free medium.
Authors: Albert R, Vereb Z, Csomos K, Moe M, Johnsen E, Olstad O, Nicolaissen B, Rajnavolgyi E, Fesus L, Berta A, Petrovski G
PLoS ONE, 2012-10-09;7(10):e47187.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC.
Authors: Fekete N, Rojewski M, Furst D, Kreja L, Ignatius A, Dausend J, Schrezenmeier H
PLoS ONE, 2012-08-14;7(8):e43255.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Successful In Vitro Expansion and Differentiation of Cord Blood Derived CD34+ Cells into Early Endothelial Progenitor Cells Reveals Highly Differential Gene Expression.
Authors: Ahrens I, Domeij H, Topcic D
PLoS ONE, 2011-08-12;6(8):e23210.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Oligo-guanosine nucleotide induces neuropilin-1 internalization in endothelial cells and inhibits angiogenesis.
Authors: Narazaki M, Segarra M, Hou X, Tanaka T, Li X, Tosato G
Blood, 2010-07-06;116(16):3099-107.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Comparison of endothelial progenitor cell function in type 2 diabetes with good and poor glycemic control.
Authors: Churdchomjan, Worachat, Kheolamai, Pakpoom, Manochantr, Sirikul, Tapanadechopone, Pirath, Tantrawatpan, Chairat, U-Pratya, Yaowalak, Issaragrisil, Surapol
BMC Endocr Disord, 2010-04-07;10(0):5.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Neuropilin-1 regulates platelet-derived growth factor receptor signalling in mesenchymal stem cells.
Authors: Ball SG, Bayley C, Shuttleworth CA, Kielty CM
Biochem. J., 2010-03-15;427(1):29-40.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Multiple receptor tyrosine kinases regulate HIF-1alpha and HIF-2alpha in normoxia and hypoxia in neuroblastoma: implications for antiangiogenic mechanisms of multikinase inhibitors.
Authors: Nilsson MB, Zage PE, Zeng L, Xu L, Cascone T, Wu HK, Saigal B, Zweidler-McKay PA, Heymach JV
Oncogene, 2010-03-08;29(20):2938-49.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
VEGFR2 is selectively expressed by FOXP3high CD4+ Treg.
Authors: Suzuki H, Onishi H, Wada J, Yamasaki A, Tanaka H, Nakano K, Morisaki T, Katano M
Eur. J. Immunol., 2010-01-01;40(1):197-203.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Distraction osteogenesis induces endothelial progenitor cell mobilization without inflammatory response in man.
Authors: Lee DY, Cho TJ, Lee HR, Park MS, Yoo WJ, Chung CY, Choi IH
Bone, 2009-10-22;46(3):673-9.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Colocalization of the VEGF-R2 and the common IL-3/GM-CSF receptor beta chain to lipid rafts leads to enhanced p38 activation.
Authors: Saulle E, Riccioni R, Coppola S, Parolini I, Diverio D, Riti V, Mariani G, Laufer S, Sargiacomo M, Testa U
Br. J. Haematol., 2009-02-24;145(3):399-411.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Highly efficient and feeder-free production of subculturable vascular endothelial cells from primate embryonic stem cells.
Authors: Saeki K, Yogiashi Y, Nakahara M, Nakamura N, Matsuyama S, Koyanagi A, Yagita H, Koyanagi M, Kondo Y, Yuo A
J. Cell. Physiol., 2008-10-01;217(1):261-80.
Species: Primate - Macaca fascicularis (Crab-eating Monkey or Cynomolgus Macaque)
Sample Types: Whole Cells
Applications: Flow Cytometry -
Endothelial progenitor cell levels in obese men with the metabolic syndrome and the effect of simvastatin monotherapy vs. simvastatin/ezetimibe combination therapy.
Authors: Westerweel PE, Visseren FL, Hajer GR, Olijhoek JK, Hoefer IE, de Bree P, Rafii S, Doevendans PA, Verhaar MC
Eur. Heart J., 2008-09-28;29(22):2808-17.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Late outgrowth endothelial progenitor cells in patients with age-related macular degeneration.
Authors: Thill M, Strunnikova NV, Berna MJ, Gordiyenko N, Schmid K, Cousins SW, Thompson DJ, Csaky KG
Invest. Ophthalmol. Vis. Sci., 2008-06-01;49(6):2696-708.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Tumor necrosis factor-alpha and endothelial cells modulate Notch signaling in the bone marrow microenvironment during inflammation.
Authors: Fernandez L, Rodriguez S, Huang H, Chora A, Fernandes J, Mumaw C, Cruz E, Pollok K, Cristina F, Price JE, Ferkowicz MJ, Scadden DT, Clauss M, Cardoso AA, Carlesso N
Exp. Hematol., 2008-05-01;36(5):545-558.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population.
Authors: Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM
Nature, 2008-04-23;453(7194):524-8.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Microtubule-targeted drugs inhibit VEGF receptor-2 expression by both transcriptional and post-transcriptional mechanisms.
Authors: Meissner M, Pinter A, Michailidou D, Hrgovic I, Kaprolat N, Stein M, Holtmeier W, Kaufmann R, Gille J
J. Invest. Dermatol., 2008-03-06;128(8):2084-91.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Sulfated polysaccharides identified as inducers of neuropilin-1 internalization and functional inhibition of VEGF165 and semaphorin3A.
Authors: Narazaki M, Segarra M, Tosato G
Blood, 2008-02-13;111(8):4126-36.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Alternative splicing of vasohibin-1 generates an inhibitor of endothelial cell proliferation, migration, and capillary tube formation.
Authors: Kern J, Bauer M, Rychli K, Wojta J, Ritsch A, Gastl G, Gunsilius E, Untergasser G
Arterioscler. Thromb. Vasc. Biol., 2008-01-10;28(3):478-84.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells.
Authors: Zhang P, Li J, Tan Z, Wang C, Liu T, Chen L, Yong J, Jiang W, Sun X, DU L, Ding M, Deng H
Blood, 2007-11-27;111(4):1933-41.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus.
Authors: Lee PY, Li Y, Richards HB, Chan FS, Zhuang H, Narain S, Butfiloski EJ, Sobel ES, Reeves WH, Segal MS
Arthritis Rheum., 2007-11-01;56(11):3759-69.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Notch alters VEGF responsiveness in human and murine endothelial cells by direct regulation of VEGFR-3 expression.
Authors: Shawber CJ, Funahashi Y, Francisco E, Vorontchikhina M, Kitamura Y, Stowell SA, Borisenko V, Feirt N, Podgrabinska S, Shiraishi K, Chawengsaksophak K, Rossant J, Accili D, Skobe M, Kitajewski J
J. Clin. Invest., 2007-11-01;117(11):3369-82.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
VEGFR2 expressing circulating (progenitor) cell populations in volunteers and cancer patients.
Authors: Vroling L, Yuana Y, Schuurhuis GJ, van Hinsbergh VW, Gundy C, de Haas R, van Cruijsen H, Boven E, Hoekman K, Broxterman HJ
Thromb. Haemost., 2007-08-01;98(2):440-50.
Species: Human
Sample Types: Whole Blood
Applications: Flow Cytometry -
Increased circulating endothelial progenitor cells in septic patients: correlation with survival.
Authors: Rafat N, Hanusch C, Brinkkoetter PT, Schulte J, Brade J, Zijlstra JG, van der Woude FJ, van Ackern K, Yard BA, Beck GCh
Crit. Care Med., 2007-07-01;35(7):1677-84.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor.
Authors: Norden-Zfoni A, Desai J, Manola J, Beaudry P, Force J, Maki R, Folkman J, Bello C, Baum C, DePrimo SE, Shalinsky DR, Demetri GD, Heymach JV
Clin. Cancer Res., 2007-05-01;13(9):2643-50.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Vascular endothelial growth factor can signal through platelet-derived growth factor receptors.
Authors: Ball SG, Shuttleworth CA, Kielty CM
J. Cell Biol., 2007-04-30;177(3):489-500.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Isolation of an adult blood-derived progenitor cell population capable of differentiation into angiogenic, myocardial and neural lineages.
Authors: Porat Y, Porozov S, Belkin D, Shimoni D, Fisher Y, Belleli A, Czeiger D, Silverman WF, Belkin M, Battler A, Fulga V, Savion N
Br. J. Haematol., 2006-12-01;135(5):703-14.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Enalapril increases ischemia-induced endothelial progenitor cell mobilization through manipulation of the CD26 system.
Authors: Wang CH, Verma S, Hsieh IC, Chen YJ, Kuo LT, Yang NI, Wang SY, Wu MY, Hsu CM, Cheng CW, Cherng WJ
J. Mol. Cell. Cardiol., 2006-05-05;41(1):34-43.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Ligand-induced internalization selects use of common receptor neuropilin-1 by VEGF165 and semaphorin3A.
Authors: Narazaki M, Tosato G
Blood, 2006-01-19;107(10):3892-901.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Expansion of human SCID-repopulating cells under hypoxic conditions.
Authors: Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC
J. Clin. Invest., 2003-07-01;112(1):126-35.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Structural and functional studies of the endothelial activation antigen endothelial leucocyte adhesion molecule-1 using a panel of monoclonal antibodies.
Authors: Pigott R, Needham LA, Edwards RM
J. Immunol., 1991-07-01;147(1):130-5.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Role of Gd2O3‑doped carbon‑11‑choline‑lenvatinib nanoparticles contrast agent PET/CT in the diagnosis of patients with lung cancer
Authors: Tong Zhou, Dongfang Hang, Ying Li, Jin Zhang, Huayang Wu, Hongyan Wang et al.
Oncology Letters
-
Vascular Tumor Recapitulated in Endothelial Cells from hiPSCs Engineered to Express the SERPINE1-FOSB Translocation
Authors: David G.P. van IJzendoorn, Daniela C.F. Salvatori, Xu Cao, Francijna van den Hil, Inge H. Briaire-de Bruijn, Danielle de Jong et al.
Cell Reports Medicine
-
Decreased pre-surgical CD34+/CD144+ cell number in patients undergoing coronary artery bypass grafting compared to coronary artery disease-free valvular patients
Authors: Santiago Redondo, Álvaro González-Rocafort, Jorge Navarro-Dorado, Marta Ramajo, Mihail Hristov, Antonio Gordillo-Moscoso et al.
Journal of Cardiothoracic Surgery
-
Long-term repair of porcine articular cartilage using cryopreservable, clinically compatible human embryonic stem cell-derived chondrocytes
Authors: Frank A. Petrigliano, Nancy Q. Liu, Siyoung Lee, Jade Tassey, Arijita Sarkar, Yucheng Lin et al.
npj Regenerative Medicine
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsIsotype Controls
Staining Reagents
Reviews for Human VEGFR2/KDR/Flk-1 PE-conjugated Antibody
Average Rating: 2.5 (Based on 2 Reviews)
Have you used Human VEGFR2/KDR/Flk-1 PE-conjugated Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
This specific lot number turned out completely negative on our positive control of endothelial cells.
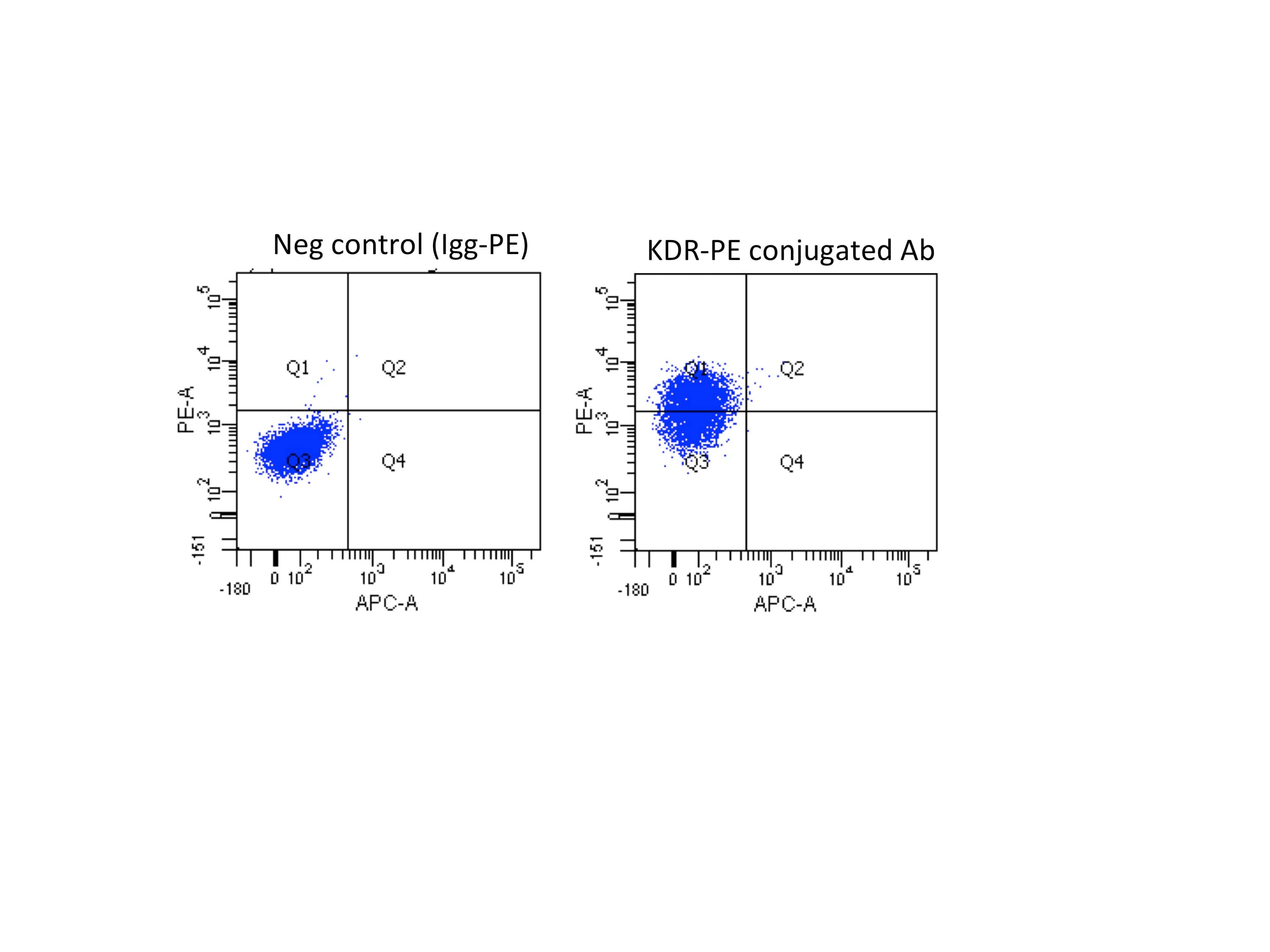
H1 cells were treated with ChIR 6uM for 48h. Then, 1x106 cells were incubated for 45min with 10ul of KDR-PE antibody or Igg-PE antibody and analyzed using LSRII cytometer.