Human VEGFR2/KDR Quantikine ELISA Kit Summary
Customers also Viewed
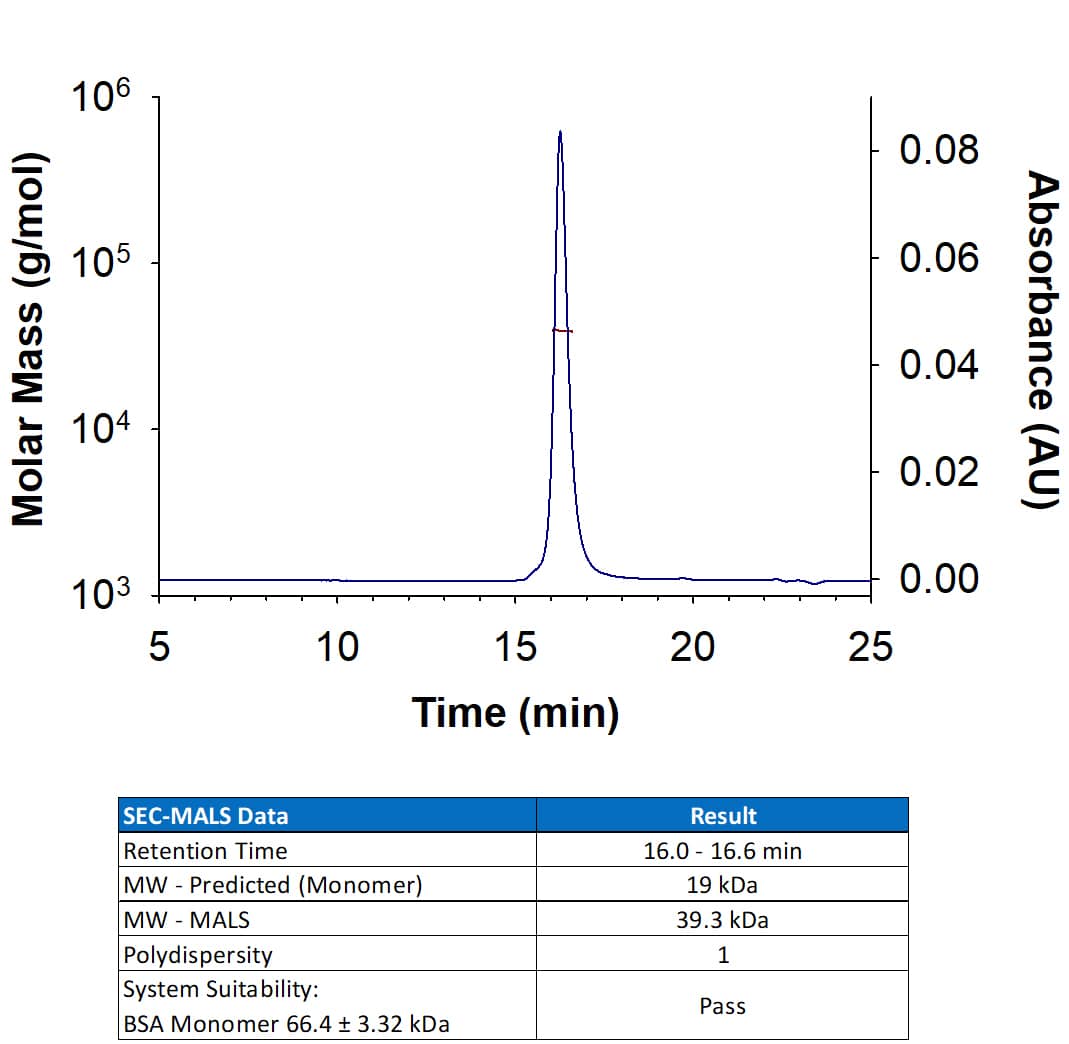
Product Summary
Precision
Cell Culture Supernates, Cell Lysates, Serum, EDTA Plasma, Heparin Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 465 | 1269 | 2995 | 455 | 1233 | 2962 |
| Standard Deviation | 19.4 | 45.5 | 87.9 | 32.1 | 84.8 | 169 |
| CV% | 4.2 | 3.6 | 2.9 | 7 | 6.9 | 5.7 |
Recovery
The recovery of VEGF R2 spiked to levels throughout the range of the assay was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=4) | 99 | 92-104 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: VEGFR2/KDR/Flk-1
VEGF R1 (Flt-1), VEGF R2 (KDR/Flk-1), and VEGF R3 (Flt-4) belong to the class III subfamily of receptor tyrosine kinases (RTKs). All three receptors contain seven immunoglobulin-like repeats in their extracellular domain and kinase insert domains in their intracellular region. They are best known for regulating VEGF family-mediated vasculogenesis, angiogenesis, and lymphangiogenesis. They are also mediators of neurotrophic activity and regulators of hematopoietic development. VEGF R2 is thought to be the primary inducer of VEGF-mediated blood vessel growth, while VEGF R3 plays a significant role in VEGF-C and VEGF-D-mediated lymphangiogenesis.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 µL of Assay Diluent to each well.
- Add 100 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes.
- Add 200 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours.
- Aspirate and wash 4 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Human VEGFR2/KDR Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
37
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Unraveling the Angiogenic Puzzle: Pre-Treatment sVEGFR1 and sVEGFR2 Levels as Promising Prognostic Indicators in Early-Stage Breast Cancer Patients
Authors: Zarychta, E;Bielawski, K;Wrzeszcz, K;Rhone, P;Ruszkowska-Ciastek, B;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Association between Biomarkers (VEGF-R2, VEGF-R3, VCAM-1) and Treatment Duration in Patients with Neuroendocrine Tumors Receiving Therapy with First-Generation Somatostatin Analogues
Authors: V Rosiek, K Janas, B Kos-Kud?a
Biomedicines, 2023-03-10;11(3):.
Species: Human
Sample Types: Serum
-
Early increase of plasma soluble VEGFR-2 is associated with clinical benefit from second-line treatment of paclitaxel and ramucirumab in advanced gastric cancer
Authors: L Fornaro, G Musettini, P Orlandi, I Pecora, C Vivaldi, M Banchi, F Salani, E Fini, V Massa, S Catanese, F Cucchiara, M Lencioni, G Masi, E Vasile, G Bocci
American journal of cancer research, 2022-07-15;12(7):3347-3356.
Species: Human
Sample Types: Plasma
-
Plasmatic MMP9 released from tumor-infiltrating neutrophils is predictive for bevacizumab efficacy in glioblastoma patients: an AVAglio ancillary study
Authors: C Jiguet-Jig, S Boissonnea, E Denicolai, V Hein, R Lasseur, J Garcia, S Romain, R Appay, T Graillon, W Mason, AF Carpentier, AA Brandes, L' Ouafik, W Wick, A Baaziz, JP Gigan, RJ Argüello, D Figarella-, O Chinot, E Tabouret
Acta neuropathologica communications, 2022-01-03;10(1):1.
Species: Human
Sample Types: Plasma
-
Residual alterations of cardiac and endothelial function in patients who recovered from Takotsubo cardiomyopathy
Authors: L Zilberman, A Zalik, I Fugenfirov, S Shimoni, J George, S Goland
Clinical cardiology, 2021-05-06;0(0):.
Species: Human
Sample Types: Serum
-
Hyperoxia sensitizes hypoxic HeLa cells to ionizing radiation by downregulating HIF?1&alpha and VEGF expression
Authors: D Dong, Y Fu, F Chen, J Zhang, H Jia, J Li, H Wang, J Wen
Mol Med Rep, 2020-11-20;23(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Matrix Metalloproteinases MMP-2 and MMP-9, Their Inhibitors TIMP-1 and TIMP-2, Vascular Endothelial Growth Factor and sVEGFR-2 as Predictive Markers of Ischemic Retinopathy in Patients with Systemic Sclerosis-Case Series Report
Authors: A Waszczykow, M Podgórski, M Waszczykow, Z Gerlicz-Ko, P Jurowski
Int J Mol Sci, 2020-11-18;21(22):.
Species: Human
Sample Types: Serum
-
Divergent Impact of Breast Cancer Laterality on Clinicopathological, Angiogenic, and Hemostatic Profiles: A Potential Role of Tumor Localization in Future Outcomes
Authors: RC Barbara, R Piotr, B Kornel, Z El?bieta, R Danuta, N Eduardo
J Clin Med, 2020-06-02;9(6):.
Species: Human
Sample Types: Plasma
-
A multicentre phase 1b/2 study of tivozanib in patients with advanced inoperable hepatocellular carcinoma
Authors: C Fountzilas, M Gupta, S Lee, S Krishnamur, B Estfan, K Wang, K Attwood, J Wilton, R Bies, W Bshara, R Iyer
Br. J. Cancer, 2020-02-10;0(0):.
Species: Human
Sample Types: Plasma
-
VWF/ADAMTS13 ratio as a potential biomarker for early detection of hepatocellular carcinoma
Authors: H Takaya, T Namisaki, M Kitade, K Kaji, K Nakanishi, Y Tsuji, N Shimozato, K Moriya, K Seki, Y Sawada, S Saikawa, S Sato, H Kawaratani, T Akahane, R Noguchi, M Matsumoto, H Yoshiji
BMC Gastroenterol, 2019-10-21;19(1):167.
Species: Human
Sample Types: Serum
-
Anti-angiogenic efficacy in invasive breast carcinoma patients depends on clinicopathological determinants
Authors: E Zarychta, P Rhone, K Bielawski, M Michalska, D Ro??, B Ruszkowska
Adv Med Sci, 2019-02-26;64(2):216-223.
Species: Human
Sample Types: Plasma
-
Characteristics of the specific humoral response in patients with advanced solid tumors after active immunotherapy with a VEGF vaccine, at different antigen doses and using two distinct adjuvants
Authors: J Sánchez Ra, Y Morera Día, M Bequet-Rom, F Hernández-, KH Selman-Hou, A de la Torr, ER Santiesteb, Y Martín Bau, CH Bermúdez B, J de la Torr, JV Gavilondo, M Ayala Avil
BMC Immunol., 2017-07-26;18(1):39.
Species: Human
Sample Types: Serum
-
Low grade inflammation inhibits VEGF induced HUVECs migration in p53 dependent manner
Authors: Sushil Panta
Biochem. Biophys. Res. Commun, 2016-12-18;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Kinetics of circulating endothelial progenitor cells in patients undergoing carotid artery surgery
Authors: Günay Kalender
Ther Clin Risk Manag, 2016-12-14;12(0):1841-1847.
Species: Human
Sample Types: Plasma
-
A randomized phase II study of capecitabine-based chemoradiation with or without bevacizumab in resectable locally advanced rectal cancer: clinical and biological features.
Authors: Salazar R, Capdevila J, Laquente B, Manzano J, Pericay C, Villacampa M, Lopez C, Losa F, Safont M, Gomez A, Alonso V, Escudero P, Gallego J, Sastre J, Gravalos C, Biondo S, Palacios A, Aranda E
BMC Cancer, 2015-02-26;15(0):60.
Species: Human
Sample Types: Plasma
-
Serum angiopoietin-2 and soluble VEGFR-2 levels predict malignancy of ovarian neoplasm and poor prognosis in epithelial ovarian cancer.
Authors: Sallinen H, Heikura T, Koponen J, Kosma V, Heinonen S, Yla-Herttuala S, Anttila M
BMC Cancer, 2014-09-23;14(0):696.
Species: Human
Sample Types: Serum
-
Analysis of plasma cytokines and angiogenic factors in patients with pretreated urothelial cancer receiving Pazopanib: the role of circulating interleukin-8 to enhance the prognostic accuracy.
Authors: Necchi A, Pennati M, Zaffaroni N, Landoni E, Giannatempo P, Raggi D, Schwartz L, Morosi C, Crippa F, Fare E, Nicolai N, Lanocita R, Sava T, Sacco C, Messina C, Ortega C, De Braud F, Salvioni R, Daidone M, Gianni A, Mariani L
Br J Cancer, 2013-11-14;110(1):26-33.
Species: Human
Sample Types: Plasma
-
Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study.
Authors: Briasoulis, Evangelo, Aravantinos, Gerasimo, Kouvatseas, George, Pappas, Periklis, Biziota, Eirini, Sainis, Ioannis, Makatsoris, Thomas, Varthalitis, Ioannis, Xanthakis, Ioannis, Vassias, Antonios, Klouvas, George, Boukovinas, Ioannis, Fountzilas, George, Syrigos, Kostanti, Kalofonos, Haralamb, Samantas, Epaminon
BMC Cancer, 2013-05-29;13(1):263.
Species: Human
Sample Types: Plasma
-
Angiogenic and vasculogenic factors in the vitreous from patients with proliferative diabetic retinopathy.
Authors: Abu El-Asrar, Ahmed M, Nawaz, Mohd Imt, Kangave, Dustan, Mairaj Siddiquei, Mohammed, Geboes, Karel
J Diabetes Res, 2013-03-10;0(0):539658.
Species: Human
Sample Types: Cell Lysates, Vitreous Humor
-
Prognostic and predictive value of VEGF, sVEGFR-2 and CEA in mCRC studies comparing cediranib, bevacizumab and chemotherapy.
Authors: Jurgensmeier J, Schmoll H, Robertson J, Brooks L, Taboada M, Morgan S, Wilson D, Hoff P
Br J Cancer, 2013-02-28;108(6):1316-23.
Species: Human
Sample Types: Plasma
-
Dasatinib plus capecitabine for advanced breast cancer: safety and efficacy in phase I study CA180004.
Authors: Somlo G, Atzori F, Strauss L, Geese W, Specht J, Gradishar W, Rybicki A, Sy O, Vahdat L, Cortes J
Clin Cancer Res, 2013-02-12;19(7):1884-93.
Species: Human
Sample Types: Plasma
-
A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a Children's Oncology Group Phase I Consortium report.
Authors: Widemann B, Kim A, Fox E, Baruchel S, Adamson P, Ingle A, Glade Bender J, Burke M, Weigel B, Stempak D, Balis F, Blaney S
Clin Cancer Res, 2012-09-07;18(21):6011-22.
Species: Human
Sample Types: Plasma
-
Cytokine and angiogenic factors associated with efficacy and toxicity of pazopanib in advanced soft-tissue sarcoma: an EORTC-STBSG study.
Br. J. Cancer, 2012-07-17;107(4):639-45.
Species: Human
Sample Types: Serum
-
Prognostic significance of angiogenic factors in uterine cervical cancer.
Authors: Landt S, Wehling M, Heidecke H, Jeschke S, Korlach S, Stoblen F, Schmid P, Blohmer JU, Lichtenegger W, Sehouli J, Kummel S
Anticancer Res., 2011-08-01;31(8):2589-95.
Species: Human
Sample Types: Serum
-
Mechanism-related circulating proteins as biomarkers for clinical outcome in patients with unresectable hepatocellular carcinoma receiving sunitinib.
Authors: Harmon CS, DePrimo SE, Raymond E, Cheng AL, Boucher E, Douillard JY, Lim HY, Kim JS, Lechuga MJ, Lanzalone S, Lin X, Faivre S
J Transl Med, 2011-07-25;9(0):120.
Species: Human
Sample Types: Plasma
-
Phase I and pharmacokinetic study of sunitinib in pediatric patients with refractory solid tumors: a children's oncology group study.
Authors: Dubois SG, Shusterman S, Ingle AM, Ahern CH, Reid JM, Wu B, Baruchel S, Glade-Bender J, Ivy P, Grier HE, Adamson PC, Blaney SM
Clin. Cancer Res., 2011-06-20;17(15):5113-22.
Species: Human
Sample Types: Plasma
-
Myeloid Biomarkers Associated with Glioblastoma Response to Anti-VEGF Therapy with Aflibercept.
Authors: de Groot JF, Piao Y, Tran H, Gilbert M, Wu HK, Liu J, Bekele BN, Cloughesy T, Mehta M, Robins HI, Lassman A, Deangelis L, Camphausen K, Chen A, Yung W, Prados M, Wen PY, Heymach JV
Clin. Cancer Res., 2011-06-01;17(14):4872-4881.
Species: Human
Sample Types: Plasma
-
Pharmacodynamic and pharmacogenetic angiogenesis-related markers of first-line FOLFOXIRI plus bevacizumab schedule in metastatic colorectal cancer.
Authors: Loupakis F, Cremolini C, Fioravanti A
Br. J. Cancer, 2011-03-15;104(8):1262-9.
Species: Human
Sample Types: Plasma
-
Levels of circulating CD45(dim)CD34(+)VEGFR2(+) progenitor cells correlate with outcome in metastatic renal cell carcinoma patients treated with tyrosine kinase inhibitors.
Authors: Farace F, Gross-Goupil M, Tournay E, Taylor M, Vimond N, Jacques N, Billiot F, Mauguen A, Hill C, Escudier B
Br. J. Cancer, 2011-03-08;104(7):1144-50.
Species: Human
Sample Types: Plasma
-
Phase I and pharmacological study of the broad-spectrum tyrosine kinase inhibitor JNJ-26483327 in patients with advanced solid tumours.
Authors: Konings IR, de Jonge MJ, Burger H
Br. J. Cancer, 2010-09-07;103(7):987-92.
Species: Human
Sample Types: Serum
-
Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study.
Authors: Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J, Bhargava P, Meyerhardt J, Clark JW, Kwak EL, Hezel AF, Miksad R, Abrams TA, Enzinger PC, Fuchs CS, Ryan DP, Jain RK
J. Clin. Oncol., 2009-05-26;27(18):3027-35.
Species: Human
Sample Types: Plasma
-
Signs of reduced angiogenic activity after surgical removal of deeply infiltrating endometriosis.
Authors: Bourlev V, Iljasova N, Adamyan L, Larsson A, Olovsson M
Fertil. Steril., 2009-03-25;94(1):52-7.
Species: Human
Sample Types: Serum
-
Antiangiogenic gene therapy with soluble VEGFR-1, -2, and -3 reduces the growth of solid human ovarian carcinoma in mice.
Authors: Sallinen H, Anttila M, Narvainen J, Koponen J, Hamalainen K, Kholova I, Heikura T, Toivanen P, Kosma VM, Heinonen S, Alitalo K, Yla-Herttuala S
Mol. Ther., 2008-12-02;17(2):278-84.
Species: Human
Sample Types: Plasma
-
Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane.
Authors: Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE, Baum CM, Miller KD
J. Clin. Oncol., 2008-03-17;26(11):1810-6.
Species: Human
Sample Types: Plasma
-
Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy.
Authors: Saltz LB, Rosen LS, Marshall JL, Belt RJ, Hurwitz HI, Eckhardt SG, Bergsland EK, Haller DG, Lockhart AC, Rocha Lima CM, Huang X, DePrimo SE, Chow-Maneval E, Chao RC, Lenz HJ
J. Clin. Oncol., 2007-10-20;25(30):4793-9.
Species: Human
Sample Types: Plasma
-
Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor.
Authors: Norden-Zfoni A, Desai J, Manola J, Beaudry P, Force J, Maki R, Folkman J, Bello C, Baum C, DePrimo SE, Shalinsky DR, Demetri GD, Heymach JV
Clin. Cancer Res., 2007-05-01;13(9):2643-50.
Species: Human
Sample Types: Plasma
-
Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction.
Authors: Wallner W, Sengenberger R, Strick R, Strissel PL, Meurer B, Beckmann MW, Schlembach D
Clin. Sci., 2007-01-01;112(1):51-7.
Species: Human
Sample Types: Serum
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human VEGFR2/KDR Quantikine ELISA Kit
Average Rating: 5 (Based on 2 Reviews)
Have you used Human VEGFR2/KDR Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: