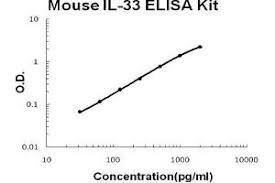
Mouse IL-33 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse IL-33. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-33
IL-33 (Interleukin-33) is released from physically damaged or necrotic cells. It triggers Th2-biased immune cell activation at sites of inflammation as well as regulatory T cell and M2 macrophage expansion. It additionally induces angiogenesis, promotes tumor cell migration and invasion, limits cardiac myocyte hypertrophy, and limits the development of atherosclerotic plaques. IL-33 signals through a receptor complex composed of ST2 and IL-1 RAcP. IL-1 RAcP also associates with IL-1 RI, IL-1 RII, IL-1 R6, and SCF R/c-kit. Soluble isoforms of ST2 and IL-1 RAcP function as decoy receptors that regulate IL-33 bioactivity.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse IL-33 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
54
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Intermittent ozone inhalation during house dust mite-induced sensitization primes for adverse asthma phenotype
Authors: Hussain, S;Majumder, N;Mazumder, MHH;Lewis, SE;Olapeju, O;Velayutham, M;Amin, MS;Brundage, K;Kelley, EE;Vanoirbeek, J;
Redox biology
Species: Mouse
Sample Types: Tissue Homogenates, BALF
-
Insulin and leptin oscillations license food-entrained browning and metabolic flexibility
Authors: Mattar, P;Reginato, A;Lavados, C;Das, D;Kalyani, M;Martinez-Lopez, N;Sharma, M;Skovbjerg, G;Skytte, JL;Roostalu, U;Subbarayan, R;Picarda, E;Zang, X;Zhang, J;Guha, C;Schwartz, G;Rajbhandari, P;Singh, R;
Cell reports
Species: Mouse, Transgenic Mouse
Sample Types: Serum
-
Active secretion of IL-33 from astrocytes is dependent on TMED10 and promotes central nervous system homeostasis
Authors: Jiao, M;Wang, C;Tang, X;Dai, C;Zhang, N;Fan, A;Qian, Z;Liu, S;Zhang, F;Li, B;Xu, Y;Tan, Z;Gong, F;Lu, Y;Zheng, F;
Brain, behavior, and immunity
Species: Mouse, Transgenic Mouse
Sample Types: Cell Culture Supernates
-
The IL-17A-neutrophil axis promotes epithelial cell IL-33 production during nematode lung migration
Authors: Ajendra, J;Papotto, PH;Parkinson, JE;Dodd, RJ;Bombeiro, AL;Pearson, S;Chan, BHK;Ribot, JC;McSorley, HJ;Sutherland, TE;Allen, JE;
Mucosal immunology
Species: Mouse, Transgenic Mouse
Sample Types: BALF
-
Heat-Labile Enterotoxin Decreases Macrophage Phagocytosis of Enterotoxigenic Escherichia coli
Authors: Hollifield, IE;Motyka, NI;Fernando, KA;Bitoun, JP;
Microorganisms
Species: Mouse
Sample Types: Cell Culture Supernates, Cell Lysates
-
Apoptosis inhibitor of macrophage (AIM)/CD5L is involved in the pathogenesis of COPD
Authors: Takimoto-Sato, M;Suzuki, M;Kimura, H;Ge, H;Matsumoto, M;Makita, H;Arai, S;Miyazaki, T;Nishimura, M;Konno, S;
Respiratory research
Species: Mouse
Sample Types: BALF
-
Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice
Authors: Maggi, L;Camelo, GMA;Rocha, IC;Pereira Alves, W;Moreira, JMP;Almeida Pereira, T;Tafuri, WL;Rabelo, ÉML;Correa, A;Ecco, R;Negrão-Corrêa, DA;
International journal of molecular sciences
Species: Mouse
Sample Types: Serum, Tissue Homogenates
-
Symbiotic microbiome Staphylococcus epidermidis restricts IL-33 production in allergic nasal epithelium via limiting the cellular necroptosis
Authors: Jeon, YJ;Gil, CH;Won, J;Jo, A;Kim, HJ;
BMC microbiology
Species: Mouse
Sample Types: Nasal Lavage Fluid
-
Crosstalk between ILC2s and Th2 cells varies among mouse models
Authors: RK Gurram, D Wei, Q Yu, MJ Butcher, X Chen, K Cui, G Hu, M Zheng, X Zhu, J Oh, B Sun, JF Urban, K Zhao, WJ Leonard, J Zhu
Cell Reports, 2023-02-02;42(2):112073.
Species: Mouse
Sample Types: BALF
-
Allergen protease-activated stress granule assembly and gasdermin D fragmentation control interleukin-33 secretion
Authors: W Chen, S Chen, C Yan, Y Zhang, R Zhang, M Chen, S Zhong, W Fan, S Zhu, D Zhang, X Lu, J Zhang, Y Huang, L Zhu, X Li, D Lv, Y Fu, H Iv, Z Ling, L Ma, H Jiang, G Long, J Zhu, D Wu, B Wu, B Sun
Nature Immunology, 2022-07-06;23(7):1021-1030.
Species: Mouse
Sample Types: BALF
-
Macrophage-intrinsic DUOX1 contributes to type 2 inflammation and mucus metaplasia during allergic airway disease
Authors: CR Morris, A Habibovic, CM Dustin, C Schiffers, MC Lin, JL Ather, YMW Janssen-He, ME Poynter, O Utermohlen, M Krönke, A van der Vl
Mucosal Immunology, 2022-06-02;0(0):.
Species: Mouse
Sample Types: BALF
-
NLRP3-Inflammasome Inhibition during Respiratory Virus Infection Abrogates Lung Immunopathology and Long-Term Airway Disease Development
Authors: CA Malinczak, CF Schuler, AJ Duran, AJ Rasky, MM Mire, G Núñez, NW Lukacs, W Fonseca
Viruses, 2021-04-16;13(4):.
Species: Mouse
Sample Types: BALF
-
Epithelial IL-33 appropriates exosome trafficking for secretion in chronic airway disease
Authors: E Katz-Kiria, DF Steinberg, CE Kluender, OA Osorio, C Newsom-Ste, A Baronia, DE Byers, MJ Holtzman, D Katafiasz, KL Bailey, SL Brody, MJ Miller, J Alexander-
JCI Insight, 2021-02-22;0(0):.
Species: Mouse
Sample Types: BALF
-
Interleukin (IL)-33 is dispensable for Schistosoma mansoni worm maturation and the maintenance of egg-induced pathology in intestines of infected mice
Authors: JPK Mukendi, R Nakamura, S Uematsu, S Hamano
Parasites & vectors, 2021-01-22;14(1):70.
Species: Mouse
Sample Types: Tissue Homogenates
-
Gut microbiota modulation induced by Zika virus infection in immunocompetent mice
Authors: R Corrêa, I de Oliveir, HA Braz-de-Me, LP de Sant'An, R das Neves, G Pasquarell, PS Prado, GP Kobinger, CF Maurice, KG Magalhães
Scientific Reports, 2021-01-14;11(1):1421.
Species: Mouse
Sample Types: Tissue Homogenates
-
Severity of Experimental Autoimmune Uveitis Is Reduced by Pretreatment with Live Probiotic Escherichia coli Nissle 1917
Authors: O Dusek, A Fajstova, A Klimova, P Svozilkova, T Hrncir, M Kverka, S Coufal, J Slemin, H Tlaskalova, JV Forrester, J Heissigero
Cells, 2020-12-25;10(1):.
Species: Mouse
Sample Types: Tissue Culture Supernates
-
Oleanolic acid ameliorates intestinal alterations associated with EAE
Authors: B Gutierrez, I Gallardo, L Ruiz, Y Alvarez, V Cachofeiro, A Margolles, M Hernandez, ML Nieto
J Neuroinflammation, 2020-11-27;17(1):363.
Species: Mouse
Sample Types: Serum
-
Symbiotic microbiome Staphylococcus aureus from human nasal mucus modulates IL-33-mediated type 2 immune responses in allergic nasal mucosa
Authors: YJ Jeon, CH Gil, J Won, A Jo, HJ Kim
BMC Microbiol, 2020-10-07;20(1):301.
Species: Mouse
Sample Types: Nasal Lavage Fluid
-
Eosinophil function in adipose tissue is regulated by Kr�ppel-like factor 3 (KLF3)
Authors: AJ Knights, EJ Vohralik, PJ Houweling, ES Stout, LJ Norton, SJ Alexopoulo, JJ Yik, H Mat Jusoh, EM Olzomer, KS Bell-Ander, KN North, KL Hoehn, M Crossley, KGR Quinlan
Nat Commun, 2020-06-10;11(1):2922.
Species: Mouse
Sample Types: Tissue Homogenates
-
The Role of ST2 Receptor in the Regulation of Brucella abortus Oral Infection
Authors: R Santos, PC Campos, M Rungue, V Rocha, D Santos, V Mendes, FV Marinho, F Martins, MF Ricci, DCD Reis, GD Cassali, JC Alves-Filh, AT Vieira, SC Oliveira
Pathogens, 2020-04-28;9(5):.
Species: Mouse
Sample Types: Cell culture supernate
-
Uric acid pathway activation during respiratory virus infection promotes Th2 immune response via innate cytokine production and ILC2 accumulation
Authors: W Fonseca, CA Malinczak, CF Schuler, SKK Best, AJ Rasky, SB Morris, TX Cui, AP Popova, NW Lukacs
Mucosal Immunol, 2020-02-11;0(0):.
Species: Mouse
Sample Types: BALF
-
Interleukin 1 Receptor-Like 1 (IL1RL1) Promotes Airway Bacterial and Viral Infection and Inflammation
Authors: N Schaunaman, A Sanchez, KG Dimasuay, N Pavelka, M Numata, R Alam, RJ Martin, HW Chu
Infect. Immun., 2019-06-20;87(7):.
Species: Mouse
Sample Types: BALF
-
IL-33 drives group 2 innate lymphoid cell-mediated protection during Clostridium difficile infection
Authors: AL Frisbee, MM Saleh, MK Young, JL Leslie, ME Simpson, MM Abhyankar, CA Cowardin, JZ Ma, P Pramoonjag, SD Turner, AP Liou, EL Buonomo, WA Petri
Nat Commun, 2019-06-20;10(1):2712.
Species: Mouse
Sample Types: Tissue Homogenates
-
TET1 contributes to allergic airway inflammation and regulates interferon and aryl hydrocarbon receptor signaling pathways in bronchial epithelial cells
Authors: JD Burleson, D Siniard, VK Yadagiri, X Chen, MT Weirauch, BP Ruff, EB Brandt, GKK Hershey, H Ji
Sci Rep, 2019-05-14;9(1):7361.
Species: Mouse
Sample Types: Tissue Homogenates
-
Conjugated bile acids attenuate allergen-induced airway inflammation and hyperresposiveness by inhibiting UPR transducers
Authors: EM Nakada, NR Bhakta, BR Korwin-Mih, A Kumar, N Chamberlai, SR Bruno, DG Chapman, SM Hoffman, N Daphtary, M Aliyeva, CG Irvin, AE Dixon, PG Woodruff, S Amin, ME Poynter, DH Desai, V Anathy
JCI Insight, 2019-05-02;4(9):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Roles for T/B lymphocytes and ILC2s in experimental chronic obstructive pulmonary disease
Authors: C Donovan, MR Starkey, RY Kim, BMJ Rana, JL Barlow, B Jones, TJ Haw, P Mono Nair, K Budden, GJM Cameron, JC Horvat, PA Wark, PS Foster, ANJ McKenzie, PM Hansbro
J. Leukoc. Biol., 2018-09-27;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
T cell microvilli constitute immunological synaptosomes that carry messages to antigen-presenting cells
Authors: HR Kim, Y Mun, KS Lee, YJ Park, JS Park, JH Park, BN Jeon, CH Kim, Y Jun, YM Hyun, M Kim, SM Lee, CS Park, SH Im, CD Jun
Nat Commun, 2018-09-07;9(1):3630.
Species: Mouse
Sample Types: Cell Lysates
-
IL-33/ST2 axis mediates hyperplasia of intrarenal urothelium in obstructive renal injury
Authors: WY Chen, JL Yang, YH Wu, LC Li, RF Li, YT Chang, LH Dai, WC Wang, YJ Chang
Exp. Mol. Med., 2018-04-20;50(4):36.
Species: Mouse
Sample Types: Urine
-
Toll-Interacting Protein, Tollip, Inhibits IL-13-Mediated Pulmonary Eosinophilic Inflammation in Mice
Authors: Y Ito, N Schaefer, A Sanchez, D Francisco, R Alam, RJ Martin, JG Ledford, C Stevenson, D Jiang, L Li, M Kraft, HW Chu
J Innate Immun, 2018-01-27;0(0):.
Species: Mouse
Sample Types: BALF
-
Behavioral Changes in Mice Lacking Interleukin-33
Authors: E Dohi, EY Choi, IVL Rose, AS Murata, S Chow, M Niwa, SI Kano
eNeuro, 2017-12-26;4(6):.
Species: Mouse
Sample Types: Serum
-
STAT1 Represses Cytokine-Producing Group 2 and Group 3 Innate Lymphoid Cells during Viral Infection
Authors: MT Stier, K Goleniewsk, JY Cephus, DC Newcomb, TP Sherrill, KL Boyd, MH Bloodworth, ML Moore, K Chen, JK Kolls, RS Peebles
J. Immunol., 2017-06-02;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Experimental atopic dermatitis depends on IL-33R signaling via MyD88 in dendritic cells
Authors: C Li, I Maillet, C Mackowiak, C Viala, F Di Padova, M Li, D Togbe, V Quesniaux, Y Lai, B Ryffel
Cell Death Dis, 2017-04-06;8(4):e2735.
Species: Mouse
Sample Types: Tissue Homogenates
-
Interleukin-33 and RANK-L Interplay in the Alveolar Bone Loss Associated to Periodontitis
PLoS ONE, 2016-12-19;11(12):e0168080.
Species: Mouse
Sample Types: Serum
-
Determinants of Divergent Adaptive Immune Responses after Airway Sensitization with Ligands of Toll-Like Receptor 5 or Toll-Like Receptor 9
PLoS ONE, 2016-12-15;11(12):e0167693.
Species: Mouse
Sample Types: Tissue Homogenates
-
Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung
Authors: Ismé M de Kleer
Immunity, 2016-12-06;45(6):1285-1298.
Species: Mouse
Sample Types: Tissue Homogenates
-
DUOX1 mediates persistent epithelial EGFR activation, mucous cell metaplasia, and airway remodeling during allergic asthma
JCI Insight, 2016-11-03;1(18):e88811.
Species: Mouse
Sample Types: BALF
-
Pulmonary ORMDL3 is critical for induction of Alternaria-induced allergic airways disease
Authors: S Löser, LG Gregory, Y Zhang, K Schaefer, SA Walker, J Buckley, L Denney, CH Dean, WOC Cookson, MF Moffatt, CM Lloyd
J. Allergy Clin. Immunol., 2016-09-10;139(5):1496-1507.e3.
Species: Mouse
Sample Types: Tissue Homogenates
-
Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation
Nat Commun, 2016-09-01;7(0):12651.
Species: Rat
Sample Types: Pleural Lavage Fluid
-
Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding.
Authors: Jovicic N, Jeftic I, Jovanovic I, Radosavljevic G, Arsenijevic N, Lukic M, Pejnovic N
PLoS ONE, 2015-07-28;10(7):e0134089.
Species: Mouse
Sample Types: Tissue Homogenates
-
The Inflammatory Caspases-1 and -11 Mediate the Pathogenesis of Dermatitis in Sharpin-Deficient Mice.
Authors: Douglas T, Champagne C, Morizot A, Lapointe J, Saleh M
J Immunol, 2015-07-27;195(5):2365-73.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Critical role of IL-33 receptor ST2 in experimental cerebral malaria development.
Authors: Palomo J, Reverchon F, Piotet J, Besnard A, Couturier-Maillard A, Maillet I, Tefit M, Erard F, Mazier D, Ryffel B, Quesniaux V
Eur J Immunol, 2015-03-20;45(5):1354-65.
Species: Mouse
Sample Types: Tissue Homogenates
-
Dietary galacto-oligosaccharides prevent airway eosinophilia and hyperresponsiveness in a murine house dust mite-induced asthma model.
Authors: Verheijden K, Willemsen L, Braber S, Leusink-Muis T, Delsing D, Garssen J, Kraneveld A, Folkerts G
Respir Res, 2015-02-07;16(0):17.
Species: Mouse
Sample Types: Tissue Homogenates
-
Type II cytokines impair host defense against an intracellular fungal pathogen by amplifying macrophage generation of IL-33.
Authors: Verma, A, Kroetz, D N, Tweedle, J L, Deepe, G S Jr
Mucosal Immunol, 2014-08-13;8(2):380-9.
Species: Mouse
Sample Types: Tissue Homogenates
-
IRF-3, IRF-7, and IPS-1 promote host defense against acute human metapneumovirus infection in neonatal mice.
Authors: Spann K, Loh Z, Lynch J, Ullah A, Zhang V, Baturcam E, Werder R, Khajornjiraphan N, Rudd P, Loo Y, Suhrbier A, Gale M, Upham J, Phipps S
Am J Pathol, 2014-04-13;184(6):1795-806.
Species: Mouse
Sample Types: Tissue Homogenates
-
IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis.
Authors: Hams, Emily, Armstrong, Michelle, Barlow, Jillian, Saunders, Sean P, Schwartz, Christia, Cooke, Gordon, Fahy, Ruairi J, Crotty, Thomas B, Hirani, Nikhil, Flynn, Robin J, Voehringer, David, McKenzie, Andrew N, Donnelly, Seamas C, Fallon, Padraic
Proc Natl Acad Sci U S A, 2013-12-16;111(1):367-72.
Species: Mouse
Sample Types: Tissue Homogenates
-
Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation.
Authors: Roy A, Ganesh G, Sippola H, Bolin S, Sawesi O, Dagalv A, Schlenner S, Feyerabend T, Rodewald H, Kjellen L, Hellman L, Abrink M
J Biol Chem, 2013-11-20;289(1):237-50.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis.
Authors: McHedlidze T, Waldner M, Zopf S, Walker J, Rankin A, Schuchmann M, Voehringer D, McKenzie A, Neurath M, Pflanz S, Wirtz S
Immunity, 2013-08-15;39(2):357-71.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mice deficient in the St3gal3 gene product alpha2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation.
Authors: Kiwamoto T, Brummet M, Wu F, Motari M, Smith D, Schnaar R, Zhu Z, Bochner B
J Allergy Clin Immunol, 2013-07-02;133(1):240-7.e1-3.
Species: Mouse
Sample Types: BALF
-
The novel cytokine interleukin-33 activates acinar cell proinflammatory pathways and induces acute pancreatic inflammation in mice.
Authors: Kempuraj D, Twait E, Williard D, Yuan Z, Meyerholz D, Samuel I
PLoS ONE, 2013-02-13;8(2):e56866.
Species: Mouse, Rat
Sample Types: Tissue Homogenates
-
The Toll-like receptor 5 ligand flagellin promotes asthma by priming allergic responses to indoor allergens.
Authors: Wilson, Rhonda H, Maruoka, Shuichir, Whitehead, Gregory, Foley, Julie F, Flake, Gordon P, Sever, Michelle, Zeldin, Darryl C, Kraft, Monica, Garantziotis, Stavros, Nakano, Hideki, Cook, Donald N
Nat Med, 2012-10-14;18(11):1705-10.
Species: Mouse
Sample Types: BALF
-
Control of allergen-induced inflammation and hyperresponsiveness by the metalloproteinase ADAMTS-12.
Authors: Paulissen G, El Hour M, Rocks N, Gueders M, Bureau F, Foidart J, Lopez-Otin C, Noel A, Cataldo D
J Immunol, 2012-09-07;189(8):4135-43.
Species: Mouse
Sample Types: Tissue Homogenates
-
Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33.
J. Exp. Med., 2012-07-16;209(8):1505-17.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mast cells as sensors of cell injury through IL-33 recognition.
Authors: Enoksson M, Lyberg K, Moller-Westerberg C, Fallon PG, Nilsson G, Lunderius-Andersson C
J. Immunol., 2011-01-14;186(4):2523-8.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway.
Authors: Kearley J, Buckland KF, Mathie SA, Lloyd CM
Am. J. Respir. Crit. Care Med., 2009-01-29;179(9):772-81.
Species: Mouse
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse IL-33 DuoSet ELISA
Average Rating: 4.6 (Based on 7 Reviews)
Have you used Mouse IL-33 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Great kit, very useful for cell culture work and with mouse BALF.
Useful and easy to use kit. Good results with cell culture and animal samples,