Mouse VEGFR3/Flt-4 Antibody Summary
Tyr25-Asp770
Accession # P35917
Customers also Viewed
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
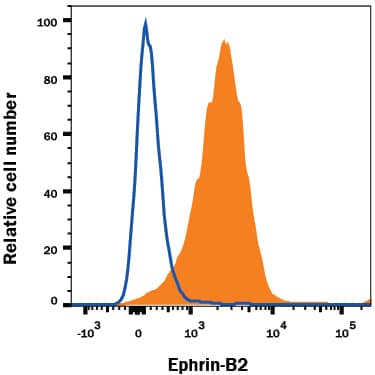
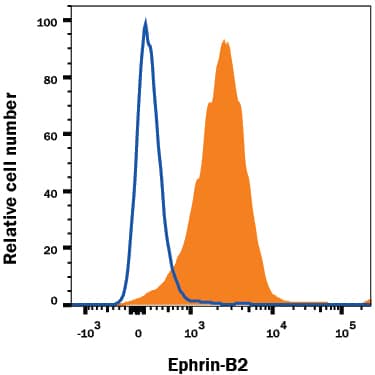
Detection of VEGFR3/Flt‑4 in bEnd.3 Mouse Cell Line by Flow Cytometry. bEnd.3 cells, a mouse endothelioma cell line, was stained with Goat Anti-Mouse VEGFR3/Flt-4 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF743, filled histogram) or isotype control antibody (Catalog # AB-108-C, open histogram), followed by PE-conjugated Anti-Goat IgG Secondary Antibody (Catalog # F0107).
 View Larger
View Larger
Detection of Mouse VEGFR3/Flt-4 by Immunocytochemistry/Immunofluorescence Cells with BLEC molecular markers are present within the mouse leptomeninges. a Coronal brain section of adult zebrafish brain indicating the imaging area in the dorsal optic tectum (TeO). b A 14 month old Tg(kdr-l:mCherry); Tg(flt4:mCitrine) double transgenic zebrafish has cells in the meninges (white bracket) that express flt4/vegfr3 ( alpha -GFP, green) near kdr-l positive ( alpha -RFP, red) blood vessels. DAPI (blue) labels the nuclei. Scale = 50 µm. c Coronal mouse brain section showing the imaging areas of the meninges. d As revealed by IHC, 17-week-old mouse brains express VEGFR3 (green) in the meninges (white bracket). Tie2-GFP;NG2-DsRed double reporter mice were used to distinguish arteries and veins. NG2 (red) labels pericytes and smooth muscle cells, Tie2 (magenta) labels vascular endothelial cells, and Hoechst (blue) stains nuclei. The image is rotated with the parenchyma at the bottom for ease of comparison with panel b. Scale = 50 µm. e-e′′′ As revealed by IHC, cells of the meninges co-express MRC1 (e, yellow), LYVE1 (e′, white), and VEGFR3 (e′′, green). Red arrows highlight cells expressing these three markers. The images are rotated with the parenchyma at the bottom. scale = 30 µm. f, g Quantification of the relative numbers of single and double-labelled cells in 2-month old mouse meninges. VEGFR3 and LYVE1 cell counts were from n = 2 brains, 3 coronal sections (10 area images)/brain. MRC1 and LYVE1 cell counts were from n = 3 brains, 3 coronal sections (4 area images)/brain. The mean values for each set are depicted Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VEGFR3/Flt-4 by Immunocytochemistry/Immunofluorescence Mouse LLECs take up A beta 1-40. a Schematic showing the site of dye and A beta 1-40 perfusion into the CSF via the cisterna magna (arrow) of a 2-month old mouse. The dotted line indicates the plane of section. A anterior, P posterior, D dorsal, V ventral. b Coronal brain section indicating the areas imaged. SF4 refers to area captured in Figure S4. c The percentage of each labelled cell type that internalized perfused A beta. Cells co-expressing VEGFR3 and LYVE1 take up A beta at a higher rate than MRC1, LYVE1 double-positive cells as well as MRC1-positive, LYVE1-negative cells (p ≤ 0.05, bootstrap). VEGFR3, LYVE1 counts, n = 2 brains (3 sections/brain). MRC1, LYVE1 counts, n = 3 brains (3 sections/brain). d–d′′′ Cells of the adult mouse meninges that co-express VEGFR3 (d, green) and LYVE1 (d′, white) internalize A beta 1-40 (d′′, cyan). Scale = 20 µm. e-e′′′) Cells of the adult mouse meninges that co-express VEGFR3 (e, green) and MRC1 (e′, white) internalize A beta 1-40 (e′′, cyan). Scale = 40 µm. f–f′′′) Cells of the adult mouse meninges that co-express MRC1 (f, magenta) and LYVE1 (f′, white) internalize A beta 1-40 (f′′, cyan). The walls of a blood vessel (white arrowhead, f′′) also accumulate A beta 1-40. Scale = 60 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human VEGFR3/Flt-4 by Immunocytochemistry/Immunofluorescence Cells of human meninges co-express LLEC markers. a–c DAB-IHC with single antibodies detects VEGFR3 (a), LYVE1 (b), and MRC1 (c) in the meninges of human post mortem brain showing no signs of neuropathology. These images are taken from a 38 year old male (sample P17/07, Table 1), and confirmed in n = 2 additional samples. P parenchyma. Scale = 150 µm (a); 40 µm (b); and 20 µm (c). d–f DAB-IHC with single antibodies detects VEGFR3 (b), LYVE1 (c), and MRC1 (d) in elderly human meninges (age: 89–92) with evidence of neuropathology and confirmed in n = 3 brains (Table 1). P, parenchyma. Scale = 20 µm. g–p IHC with fluorescent antibodies detects human meningeal cells that co-express MRC1 (h, m, yellow), LYVE1 (i, n, white), and VEGFR3 (j, o, green). Nuclei/RNA are labelled with DAPI (g, l, blue) and images are merged in (k, p). Scale = 10 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VEGFR3/Flt-4 by Immunocytochemistry/Immunofluorescence Endothelial changes after pericyte depletion. a–f Maximum intensity projection of confocal images from control and DTRiPC P6 retinas stained for IB4 (red) in combination with VEGF-A a, VEGFR2 b, VEGFR3 c, Tie2 d, Esm1 e, and Dll4 f (all in white), as indicated. Note local increase of VEGFR2, VEGFR3, and Esm1 (arrowheads in b, c, e) but not Tie2 or VEGF-A at the edge of the vessel plexus. Dll4 expression in DTRiPC sprouts is increased in some regions (arrowheads) but absent in others (arrows). Scale bar, 100 µm. g–j Quantitation of VEGF-A immunosignals area and intensity g, signal intensity for VEGFR2 h and VEGFR3 i and proportion of Esm1+ area with respect to vascular area j in the P6 control and DTRiPC angiogenic front. Error bars, s.e.m. p-values, Student’s t-test Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29146905), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VEGFR3/Flt-4 by Immunocytochemistry/Immunofluorescence Cells with BLEC molecular markers are present within the mouse leptomeninges. a Coronal brain section of adult zebrafish brain indicating the imaging area in the dorsal optic tectum (TeO). b A 14 month old Tg(kdr-l:mCherry); Tg(flt4:mCitrine) double transgenic zebrafish has cells in the meninges (white bracket) that express flt4/vegfr3 ( alpha -GFP, green) near kdr-l positive ( alpha -RFP, red) blood vessels. DAPI (blue) labels the nuclei. Scale = 50 µm. c Coronal mouse brain section showing the imaging areas of the meninges. d As revealed by IHC, 17-week-old mouse brains express VEGFR3 (green) in the meninges (white bracket). Tie2-GFP;NG2-DsRed double reporter mice were used to distinguish arteries and veins. NG2 (red) labels pericytes and smooth muscle cells, Tie2 (magenta) labels vascular endothelial cells, and Hoechst (blue) stains nuclei. The image is rotated with the parenchyma at the bottom for ease of comparison with panel b. Scale = 50 µm. e-e′′′ As revealed by IHC, cells of the meninges co-express MRC1 (e, yellow), LYVE1 (e′, white), and VEGFR3 (e′′, green). Red arrows highlight cells expressing these three markers. The images are rotated with the parenchyma at the bottom. scale = 30 µm. f, g Quantification of the relative numbers of single and double-labelled cells in 2-month old mouse meninges. VEGFR3 and LYVE1 cell counts were from n = 2 brains, 3 coronal sections (10 area images)/brain. MRC1 and LYVE1 cell counts were from n = 3 brains, 3 coronal sections (4 area images)/brain. The mean values for each set are depicted Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human VEGFR3/Flt-4 by Immunocytochemistry/Immunofluorescence Cells of human meninges co-express LLEC markers. a–c DAB-IHC with single antibodies detects VEGFR3 (a), LYVE1 (b), and MRC1 (c) in the meninges of human post mortem brain showing no signs of neuropathology. These images are taken from a 38 year old male (sample P17/07, Table 1), and confirmed in n = 2 additional samples. P parenchyma. Scale = 150 µm (a); 40 µm (b); and 20 µm (c). d–f DAB-IHC with single antibodies detects VEGFR3 (b), LYVE1 (c), and MRC1 (d) in elderly human meninges (age: 89–92) with evidence of neuropathology and confirmed in n = 3 brains (Table 1). P, parenchyma. Scale = 20 µm. g–p IHC with fluorescent antibodies detects human meningeal cells that co-express MRC1 (h, m, yellow), LYVE1 (i, n, white), and VEGFR3 (j, o, green). Nuclei/RNA are labelled with DAPI (g, l, blue) and images are merged in (k, p). Scale = 10 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VEGFR3/Flt-4 by Immunohistochemistry Cultured murine LECs express VDR.To check lymphatic origin of mouse LECs, we used three different well-established markers expressed by LECs. (a) Lymphatic origin of murine LECs was confirmed by IHC for Prox-1, VEGFR3 and Podoplanin (400x). Scale bar: 50 μm. (b) VDR expression of in vitro grown murine LECs was evaluated by western blot. Murine renal tubular epithelial cells (MTCs) served as positive control. (c) VDR expression of murine LECs was assessed by immunofluorescence (antibody D6) staining (200x). Scale bar: 50 μm. Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/srep44403), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VEGFR3/Flt-4 by Immunohistochemistry Vascular alterations after intraocular VEGF-A injection. a Morphology of IB4-stained P6 wild-type retinal vessels at 4 h after administration of human VEGF-A165 (0.5 µl at a concentration of 5 μg μl−1). Note blunt appearance of the vessel front after VEGF-A injection but not for vehicle (PBS) control. Scale bar, 200 µm. b Quantitation of sprouts and filopodia at the front of the P6 vessel plexus after injection of VEGF-A165 or vehicle control. Error bars, s.e.m. p-values, Student’s t-test. c PDGFR beta + (green) pericytes are unaffected by short-term VEGF-A administration, whereas VEGFR2 immunosignals (white) are increased in IB4+ (red) ECs (arrowheads). Images shown correspond to insets in a. Scale bar, 100 µm. d Quantitation of VEGFR2 immunosignals intensity in the peripheral plexus of P6 retinas after injection of VEGF-A165 or vehicle control. Error bars, s.e.m. p-values, Student’s t-test. e Confocal images showing increased Esm1 immunostaining (white) in IB4+ (red) ECs in the peripheral plexus (arrowheads) after VEGF-A injection in P6 pups. Scale bar, 200 µm. f VEGF-A165 injection-mediated increase of Esm1 immunosignals (normalized to IB4+ EC area) in the peripheral capillary plexus but not at the edge of the angiogenic front in comparison to PBS-injected controls at P6. Error bars, s.e.m. p-values, Student’s t-test. NS, not statistically significant. g Short-term VEGF-A165 administration leads to clustering of Erg1+ (green) and IB4+ (red) ECs, as indicated, in thick sprout-like structures of P6 retinas. Panels in the center and on the right (scale bar, 20 µm) show higher magnification of the insets on the left (scale bar, 100 µm). Dashed lines in panels on the right outline IB4+ vessels. h Quantitation of EC density in the leading front vessel and emerging sprouts of the P6 angiogenic front after injection of VEGF-A165 or vehicle control. Error bars, s.e.m. p-values, Student’s t-test Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29146905), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human VEGFR3/Flt-4 by Immunocytochemistry/Immunofluorescence Cells of human meninges co-express LLEC markers. a–c DAB-IHC with single antibodies detects VEGFR3 (a), LYVE1 (b), and MRC1 (c) in the meninges of human post mortem brain showing no signs of neuropathology. These images are taken from a 38 year old male (sample P17/07, Table 1), and confirmed in n = 2 additional samples. P parenchyma. Scale = 150 µm (a); 40 µm (b); and 20 µm (c). d–f DAB-IHC with single antibodies detects VEGFR3 (b), LYVE1 (c), and MRC1 (d) in elderly human meninges (age: 89–92) with evidence of neuropathology and confirmed in n = 3 brains (Table 1). P, parenchyma. Scale = 20 µm. g–p IHC with fluorescent antibodies detects human meningeal cells that co-express MRC1 (h, m, yellow), LYVE1 (i, n, white), and VEGFR3 (j, o, green). Nuclei/RNA are labelled with DAPI (g, l, blue) and images are merged in (k, p). Scale = 10 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human VEGFR3/Flt-4 by Immunocytochemistry/Immunofluorescence Cells of human meninges co-express LLEC markers. a–c DAB-IHC with single antibodies detects VEGFR3 (a), LYVE1 (b), and MRC1 (c) in the meninges of human post mortem brain showing no signs of neuropathology. These images are taken from a 38 year old male (sample P17/07, Table 1), and confirmed in n = 2 additional samples. P parenchyma. Scale = 150 µm (a); 40 µm (b); and 20 µm (c). d–f DAB-IHC with single antibodies detects VEGFR3 (b), LYVE1 (c), and MRC1 (d) in elderly human meninges (age: 89–92) with evidence of neuropathology and confirmed in n = 3 brains (Table 1). P, parenchyma. Scale = 20 µm. g–p IHC with fluorescent antibodies detects human meningeal cells that co-express MRC1 (h, m, yellow), LYVE1 (i, n, white), and VEGFR3 (j, o, green). Nuclei/RNA are labelled with DAPI (g, l, blue) and images are merged in (k, p). Scale = 10 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VEGFR3/Flt-4 by Immunohistochemistry Kinase activity of EphB4 is required for lymphatic valve development.(a) Visualization of mesenteric lymphatic vessels and valves (arrows) by staining for Prox-1 and VEGFR3 2 days following treatment of P3 neonatal mice with NVP-BHG712, a selective EphB4 inhibitor. Blood vessels are highlighted by strong alpha -smooth muscle actin ( alpha SMA) staining. Scale bar, 200 μm. (b) Quantification of mesenteric lymphatic valves, ***P< 0.001 (two-tailed, unpaired student's t-test), n=3 per treatment group (error bars, s.d.). (c) NVP-BHG712 inhibits EphB4 phosphorylation in P2 neonatal mice. Lung tissue lysates were subjected to anti-EphB4 immunoprecipitation followed by anti-pY or anti-EphB4 immunoblotting. Ratios of pEphB4 to total EphB4 (pEphB4: EphB4) are graphed. *P<0.05 (two-tailed, unpaired student's t-test), n=4 per treatment group (error bars, s.d.). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25865237), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VEGFR3/Flt-4 by Immunocytochemistry/Immunofluorescence Mouse LLECs take up A beta 1-40. a Schematic showing the site of dye and A beta 1-40 perfusion into the CSF via the cisterna magna (arrow) of a 2-month old mouse. The dotted line indicates the plane of section. A anterior, P posterior, D dorsal, V ventral. b Coronal brain section indicating the areas imaged. SF4 refers to area captured in Figure S4. c The percentage of each labelled cell type that internalized perfused A beta. Cells co-expressing VEGFR3 and LYVE1 take up A beta at a higher rate than MRC1, LYVE1 double-positive cells as well as MRC1-positive, LYVE1-negative cells (p ≤ 0.05, bootstrap). VEGFR3, LYVE1 counts, n = 2 brains (3 sections/brain). MRC1, LYVE1 counts, n = 3 brains (3 sections/brain). d–d′′′ Cells of the adult mouse meninges that co-express VEGFR3 (d, green) and LYVE1 (d′, white) internalize A beta 1-40 (d′′, cyan). Scale = 20 µm. e-e′′′) Cells of the adult mouse meninges that co-express VEGFR3 (e, green) and MRC1 (e′, white) internalize A beta 1-40 (e′′, cyan). Scale = 40 µm. f–f′′′) Cells of the adult mouse meninges that co-express MRC1 (f, magenta) and LYVE1 (f′, white) internalize A beta 1-40 (f′′, cyan). The walls of a blood vessel (white arrowhead, f′′) also accumulate A beta 1-40. Scale = 60 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31696318), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Mouse VEGFR3/Flt-4 Antibody by Immunohistochemistry YAP and TAZ are required for the maintenance of LVs. The lymphatic vessels in the dorsal skin of E16.5 and E18.5 control and Lyve1-Cre;Yapf/f;Tazf/f embryos were analyzed by whole-mount immunohistochemistry. (A,B) LVs were observed in the collecting lymphatic vessels of E16.5 control and Lyve1-Cre;Yapf/f;Tazf/f embryos (arrows). (C,D) The migrating front of E16.5 control (C) and Lyve1-Cre;Yapf/f;Tazf/f (D) embryos appeared comparable. (E-G) At E18.5, the lymphatic vessels from the left and right sides have merged to form a network in control embryos (E). In contrast, huge gaps were observed in between the migrating fronts of E18.5 Lyve1-Cre;Yapf/f;Tazf/f embryos (F, magenta lines). The lymphatic vessels of mutant embryos were also dilated. The distance between the migrating fronts and the diameter of vessels are quantified in G. (H,I) LVs were observed in the collecting lymphatic vessels of E18.5 control embryos (H, yellow arrows). In contrast, the dilated lymphatic vessels of E18.5 Lyve1-Cre;Yapf/f;Tazf/f embryos lacked LVs (I). The various parameters of lymphatic vascular patterning were quantified and are plotted in G. n=4 embryos per each genotype. ****P<0.0001. Data are mean±s.e.m. Scale bars: 200 µm in A-D; 500 µm in E,F; 200 µm in H,I. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33060128), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Mouse VEGFR3/Flt-4 Antibody by Immunohistochemistry Cultured murine LECs express VDR.To check lymphatic origin of mouse LECs, we used three different well-established markers expressed by LECs. (a) Lymphatic origin of murine LECs was confirmed by IHC for Prox-1, VEGFR3 and Podoplanin (400x). Scale bar: 50 μm. (b) VDR expression of in vitro grown murine LECs was evaluated by western blot. Murine renal tubular epithelial cells (MTCs) served as positive control. (c) VDR expression of murine LECs was assessed by immunofluorescence (antibody D6) staining (200x). Scale bar: 50 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28303937), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Mouse VEGFR3/Flt-4 Antibody by Immunohistochemistry YAP and TAZ are required for the maintenance of LVs. The lymphatic vessels in the dorsal skin of E16.5 and E18.5 control and Lyve1-Cre;Yapf/f;Tazf/f embryos were analyzed by whole-mount immunohistochemistry. (A,B) LVs were observed in the collecting lymphatic vessels of E16.5 control and Lyve1-Cre;Yapf/f;Tazf/f embryos (arrows). (C,D) The migrating front of E16.5 control (C) and Lyve1-Cre;Yapf/f;Tazf/f (D) embryos appeared comparable. (E-G) At E18.5, the lymphatic vessels from the left and right sides have merged to form a network in control embryos (E). In contrast, huge gaps were observed in between the migrating fronts of E18.5 Lyve1-Cre;Yapf/f;Tazf/f embryos (F, magenta lines). The lymphatic vessels of mutant embryos were also dilated. The distance between the migrating fronts and the diameter of vessels are quantified in G. (H,I) LVs were observed in the collecting lymphatic vessels of E18.5 control embryos (H, yellow arrows). In contrast, the dilated lymphatic vessels of E18.5 Lyve1-Cre;Yapf/f;Tazf/f embryos lacked LVs (I). The various parameters of lymphatic vascular patterning were quantified and are plotted in G. n=4 embryos per each genotype. ****P<0.0001. Data are mean±s.e.m. Scale bars: 200 µm in A-D; 500 µm in E,F; 200 µm in H,I. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/33060128), licensed under a CC-BY license. Not internally tested by R&D Systems.
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: VEGFR3/Flt-4
VEGFR3 (Flt-4), together with VEGFR1 (Flt-1) and VEGFR2 (KDR/Flk-1), belong to the class III subfamily of receptor tyrosine kinases (RTKs). All three receptors contain seven immunoglobulin-like repeats in their extracellular domains and kinase insert domains in their intracellular regions. The expression of these receptors is almost exclusively restricted to the endothelial cells. These receptors are likely to play essential roles in vasculogenesis and angiogenesis.
In adults, VEGFR3 expression is restricted to the endothelial cells of the lymphatic vessels. Mouse VEGFR3 cDNA encodes a 1363 amino acid (aa) residue precursor protein with a 24 aa residue signal peptide. Mature VEGFR3 has a 751 aa residue extracellular domain, a 22 aa residue hydrophobic transmembrane domain and a 565 aa residue cytoplasmic domain. The polypeptide sequences of murine Flt-4 is 88% identical to the human homologue. VEGFR3 has been reported to serve as the receptors for VEGF-C and VEGF-D.
- Finnerty, H. et al. (1993) Oncogene 8:2293.
- Joukov, V. et al. (1996) EMBO J. 15:290.
- Achen, M. et al. (1998) Proc. Natl. Acad. Sci. USA 95:548.
Product Datasheets
Citations for Mouse VEGFR3/Flt-4 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
142
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Imaging Blood Vessels and Lymphatics in Mouse Trachea Wholemounts
Authors: Peter Baluk, Donald M. McDonald
Methods in Molecular Biology
-
Polydom/SVEP1 binds to Tie1 and promotes migration of lymphatic endothelial cells
Authors: Ryoko Sato-Nishiuchi, Masamichi Doiguchi, Nanami Morooka, Kiyotoshi Sekiguchi
Journal of Cell Biology
-
Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation
Authors: Reto Huggenberger, Stefan Ullmann, Steven T. Proulx, Bronislaw Pytowski, Kari Alitalo, Michael Detmar
Journal of Experimental Medicine
-
An Unexpected Role of Semaphorin3A–Neuropilin-1 Signaling in Lymphatic Vessel Maturation and Valve Formation
Authors: Giorgia Jurisic, Hélène Maby-El Maby-El Hajjami, Sinem Karaman, Alexandra M. Ochsenbein, Annamari Alitalo, Shoib S. Siddiqui et al.
Circulation Research
-
Sarm1-mediated neurodegeneration within the enteric nervous system protects against local inflammation of the colon
Authors: Yue Sun, Qi Wang, Yi Wang, Wenran Ren, Ying Cao, Jiali Li et al.
Protein & Cell
-
Negative pressure wound therapy induces early wound healing by increased and accelerated expression of vascular endothelial growth factor receptors
Authors: Tsuruhito Tanaka, Nirmal Panthee, Yoshifumi Itoda, Naoko Yamauchi, Masashi Fukayama, Minoru Ono
European Journal of Plastic Surgery
-
Organogenesis and distribution of the ocular lymphatic vessels in the anterior eye
Authors: Yifan Wu, Young Jin Seong, Kin Li, Dongwon Choi, Eunkyung Park, George H. Daghlian et al.
JCI Insight
-
Netrin-4 induces lymphangiogenesis in vivo
Authors: Frederic Larrieu-Lahargue, Alana L. Welm, Kirk R. Thomas, Dean Y. Li
Blood
-
Shear stimulation of FOXC1 and FOXC2 differentially regulates cytoskeletal activity during lymphatic valve maturation
Authors: Pieter R Norden, Amélie Sabine, Ying Wang, Cansaran Saygili Demir, Ting Liu, Tatiana V Petrova et al.
eLife
-
Blocking Fibroblast Growth Factor Receptor Signaling Inhibits Tumor Growth, Lymphangiogenesis, and Metastasis
Authors: Frédéric Larrieu-Lahargue, Alana L. Welm, Marion Bouchecareilh, Kari Alitalo, Dean Y. Li, Andreas Bikfalvi et al.
PLoS ONE
-
Incomplete Restoration of Angiotensin II - Induced Renal Extracellular Matrix Deposition and Inflammation Despite Complete Functional Recovery in Rats
Authors: Anne-Roos S. Frenay, Saleh Yazdani, Miriam Boersema, Anne Marijn van der Graaf, Femke Waanders, Jacob van den Born et al.
PLOS ONE
-
Cdk5 controls lymphatic vessel development and function by phosphorylation of Foxc2
Authors: Johanna Liebl, Siwei Zhang, Markus Moser, Yan Agalarov, Cansaran Saygili Demir, Bianca Hager et al.
Nature Communications
-
Syndecan 4 controls lymphatic vasculature remodeling during mouse embryonic development
Authors: Yingdi Wang, Nicolas Baeyens, Federico Corti, Keiichiro Tanaka, Jennifer S. Fang, Jiasheng Zhang et al.
Development
-
Cardiac lymphatics are heterogeneous in origin and respond to injury.
Authors: Klotz L, Norman S, Vieira JM et al.
Nature.
-
Loss of the Sympathetic Signal Produces Sterile Inflammation of the Prostate
Authors: Hao Hu, Yiwen Cui, Jing Yang, Ying Cao
Frontiers in Molecular Neuroscience
-
ADAM10 controls the differentiation of the coronary arterial endothelium
Authors: Gregory Farber, Matthew M. Parks, Nicole Lustgarten Guahmich, Yi Zhang, Sébastien Monette, Scott C. Blanchard et al.
Angiogenesis
-
Lymphatic Endothelial Cells Produce M‐CSF, Causing Massive Bone Loss in Mice
Authors: Wensheng Wang, Hua Wang, Xichao Zhou, Xing Li, Wen Sun, Michael Dellinger et al.
Journal of Bone and Mineral Research
-
Structural and Functional Changes in Aged Skin Lymphatic Vessels
Authors: Raghu P. Kataru, Hyeung Ju Park, Jinyeon Shin, Jung Eun Baik, Ananta Sarker, Stav Brown et al.
Frontiers in Aging
-
Mitochondrial respiration controls the Prox1-Vegfr3 feedback loop during lymphatic endothelial cell fate specification and maintenance
Authors: Wanshu Ma, Hyea Jin Gil, Xiaolei Liu, Lauren P. Diebold, Marc A. Morgan, Michael J. Oxendine-Burns et al.
Science Advances
-
NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization
Authors: Fusanori Yotsumoto, Weon-Kyoo You, Pilar Cejudo-Martin, Karolina Kucharova, Kenji Sakimura, William B Stallcup
OncoImmunology
-
Imaging Lymphatics in Mouse Lungs
Authors: Peter Baluk, Donald M. McDonald
Methods in Molecular Biology
-
VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts
Authors: Ingrid Nilsson, Fuad Bahram, Xiujuan Li, Laura Gualandi, Sina Koch, Malin Jarvius et al.
The EMBO Journal
-
Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice
Authors: Xianghu Qu, Kevin Tompkins, Lorene E Batts, Mira Puri, H Scott Baldwin, Scott Baldwin
Development
-
Interplay of vascular endothelial growth factor receptors in organ-specific vessel maintenance
Authors: Sinem Karaman, Satu Paavonsalo, Krista Heinolainen, Madeleine H. Lackman, Amanda Ranta, Karthik A. Hemanthakumar et al.
Journal of Experimental Medicine
-
Reduced Prenatal Pulmonary Lymphatic Function Is Observed in Clp1 K/K Embryos With Impaired Motor Functions Including Fetal Breathing Movements in Preparation of the Developing Lung for Inflation at Birth
Authors: Kitti Szoták-Ajtay, Dániel Szõke, Gábor Kovács, Judit Andréka, Gábor B. Brenner, Zoltán Giricz et al.
Frontiers in Bioengineering and Biotechnology
-
Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex
Authors: Brian G. Coon, Nicolas Baeyens, Jinah Han, Madhusudhan Budatha, Tyler D. Ross, Jennifer S. Fang et al.
Journal of Cell Biology
-
An ocular glymphatic clearance system removes beta -amyloid from the rodent eye
Authors: Xiaowei Wang, Nanhong Lou, Allison Eberhardt, Yujia Yang, Peter Kusk, Qiwu Xu et al.
Science Translational Medicine
-
Hyperoxia Disrupts Lung Lymphatic Homeostasis in Neonatal Mice
Authors: Nithyapriya Shankar, Shyam Thapa, Amrit Kumar Shrestha, Poonam Sarkar, M. Waleed Gaber, Roberto Barrios et al.
Antioxidants (Basel)
-
Multi-species meta-analysis identifies transcriptional signatures associated with cardiac endothelial responses in the ischaemic heart
Authors: Ziwen Li, Emmanouil G Solomonidis, Bronwyn Berkeley, Michelle Nga Huen Tang, Katherine Ross Stewart, Daniel Perez-Vicencio et al.
Cardiovascular Research
-
Donor-host Lymphatic Anastomosis After Murine Lung Transplantation
Authors: Hasina Outtz Outtz Reed, Liqing Wang, Mark L. Kahn, Wayne W. Hancock
Transplantation
-
MT1-MMP sheds LYVE-1 on lymphatic endothelial cells and suppresses VEGF-C production to inhibit lymphangiogenesis
Authors: Hoi Leong Xavier Wong, Guoxiang Jin, Renhai Cao, Shuo Zhang, Yihai Cao, Zhongjun Zhou
Nature Communications
-
Tie1 is required for lymphatic valve and collecting vessel development
Authors: Xianghu Qu, Bin Zhou, H. Scott Scott Baldwin
Developmental Biology
-
Influenza induces lung lymphangiogenesis independent of YAP/TAZ activity in lymphatic endothelial cells..
Authors: Crossey, E;Carty, S;Shao, F;Henao-Vasquez, J;Ysasi, AB;Zeng, M;Hinds, A;Lo, M;Tilston-Lunel, A;Varelas, X;Jones, MR;Fine, A;
Scientific reports
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
FLT1 activation in cancer cells promotes PARP-inhibitor resistance in breast cancer
Authors: Tai, Y;Chow, A;Han, S;Coker, C;Ma, W;Gu, Y;Estrada Navarro, V;Kandpal, M;Hibshoosh, H;Kalinsky, K;Manova-Todorova, K;Safonov, A;Walsh, EM;Robson, M;Norton, L;Baer, R;Merghoub, T;Biswas, AK;Acharyya, S;
EMBO molecular medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Vascular architecture regulates mesenchymal stromal cell heterogeneity via P53-PDGF signaling in the mouse incisor
Authors: Guo, T;Pei, F;Zhang, M;Yamada, T;Feng, J;Jing, J;Ho, TV;Chai, Y;
Cell stem cell
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Influenza Induces Lung Lymphangiogenesis Independent of YAP/TAZ Activity in Lymphatic Endothelial Cells
Authors: Crossey, E;Carty, S;Shao, F;Henao-Vasquez, J;Ysasi, AB;Zeng, M;Hinds, A;Lo, M;Tilston-Lunel, A;Varelas, X;Jones, MR;Fine, A;
Research square
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Spatial heterogeneity of bone marrow endothelial cells unveils a distinct subtype in the epiphysis
Authors: Iga, T;Kobayashi, H;Kusumoto, D;Sanosaka, T;Fujita, N;Tai-Nagara, I;Ando, T;Takahashi, T;Matsuo, K;Hozumi, K;Ito, K;Ema, M;Miyamoto, T;Matsumoto, M;Nakamura, M;Okano, H;Shibata, S;Kohyama, J;Kim, KK;Takubo, K;Kubota, Y;
Nature cell biology
Species: Mouse
Sample Types: Whole Cells
Applications: IHC -
Self-organized and directed branching results in optimal coverage in developing dermal lymphatic networks
Authors: Uçar, MC;Hannezo, E;Tiilikainen, E;Liaqat, I;Jakobsson, E;Nurmi, H;Vaahtomeri, K;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VEGFR3 is required for button junction formation in lymphatic vessels
Authors: Jannaway, M;Iyer, D;Mastrogiacomo, DM;Li, K;Sung, DC;Yang, Y;Kahn, ML;Scallan, JP;
Cell reports
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Three-Dimensional Histological Characterization of the Placental Vasculature Using Light Sheet Microscopy
Authors: Freise, L;Behncke, RY;Allerkamp, HH;Sandermann, TH;Chu, NH;Funk, EM;Hondrich, LJ;Riedel, A;Witzel, C;Hansmeier, NR;Danyel, M;Gellhaus, A;Dechend, R;Hägerling, R;
Biomolecules
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VEGFR-3 signaling restrains the neuron-macrophage crosstalk during neurotropic viral infection
Authors: Qi, L;Li, X;Zhang, F;Zhu, X;Zhao, Q;Yang, D;Hao, S;Li, T;Li, X;Tian, T;Feng, J;Sun, X;Wang, X;Gao, S;Wang, H;Ye, J;Cao, S;He, Y;Wang, H;Wei, B;
Cell reports
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
A Prox1 enhancer represses haematopoiesis in the lymphatic vasculature
Authors: J Kazenwadel, P Venugopal, A Oszmiana, J Toubia, L Arriola-Ma, V Panara, SG Piltz, C Brown, W Ma, AW Schreiber, K Koltowska, S Taoudi, PQ Thomas, HS Scott, NL Harvey
Nature, 2023-01-25;614(7947):343-348.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
An analysis modality for vascular structures combining tissue-clearing technology and topological data analysis
Authors: K Takahashi, K Abe, SI Kubota, N Fukatsu, Y Morishita, Y Yoshimatsu, S Hirakawa, Y Kubota, T Watabe, S Ehata, HR Ueda, T Shimamura, K Miyazono
Nature Communications, 2022-09-12;13(1):5239.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system
Authors: X Li, L Qi, D Yang, S Hao, F Zhang, X Zhu, Y Sun, C Chen, J Ye, J Yang, L Zhao, DM Altmann, S Cao, H Wang, B Wei
Nature Neuroscience, 2022-05-06;25(5):577-587.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Live imaging of neolymphangiogenesis identifies acute antimetastatic roles of dsRNA mimics
Authors: D Olmeda, D Cerezo-Wal, C Mucientes, TG Calvo, E Cañón, D Alonso-Cur, N Ibarz, J Muñoz, JL Rodriguez-, P Ortiz-Rome, S Ortega, MS Soengas
Embo Molecular Medicine, 2021-11-11;0(0):e12924.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Vegfr3-tdTomato, a reporter mouse for microscopic visualization of lymphatic vessel by multiple modalities
Authors: E Redder, N Kirschnick, S Bobe, R Hägerling, NR Hansmeier, F Kiefer
PLoS ONE, 2021-09-20;16(9):e0249256.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Chronic VEGFR-3 signaling preserves dendritic arborization and sensitization under stress
Authors: A Chakrabort, R Upadhya, TA Usman, AK Shetty, JM Rutkowski
Brain, Behavior, and Immunity, 2021-08-11;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Dynamic cell contacts between periportal mesenchyme and ductal epithelium act as a rheostat for liver cell proliferation
Authors: L Cordero-Es, AM Dowbaj, TN Kohler, B Strauss, O Sarlidou, G Belenguer, C Pacini, NP Martins, R Dobie, JR Wilson-Kan, R Butler, N Prior, P Serup, F Jug, NC Henderson, F Hollfelder, M Huch
Cell Stem Cell, 2021-08-02;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Targeting local lymphatics to ameliorate heterotopic ossification via FGFR3-BMPR1a pathway
Authors: D Zhang, J Huang, X Sun, H Chen, S Huang, J Yang, X Du, Q Tan, F Luo, R Zhang, S Zhou, W Jiang, Z Ni, Z Wang, M Jin, M Xu, F Li, L Chen, M Liu, N Su, X Luo, L Yin, Y Zhu, JQ Feng, D Chen, H Qi, L Chen, Y Xie
Nature Communications, 2021-07-19;12(1):4391.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Efficient aortic lymphatic drainage is necessary for atherosclerosis regression induced by ezetimibe
Authors: KP Yeo, HY Lim, CH Thiam, SH Azhar, C Tan, Y Tang, WQ See, XH Koh, MH Zhao, ML Phua, A Balachande, Y Tan, SY Lim, HS Chew, LG Ng, V Angeli
Science Advances, 2020-12-11;6(50):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Blood and lymphatic systems are segregated by the FLCN tumor suppressor
Authors: I Tai-Nagara, Y Hasumi, D Kusumoto, H Hasumi, K Okabe, T Ando, F Matsuzaki, F Itoh, H Saya, C Liu, W Li, YS Mukouyama, W Marston Li, X Liu, M Hirashima, Y Suzuki, S Funasaki, Y Satou, M Furuya, M Baba, Y Kubota
Nature Communications, 2020-12-09;11(1):6314.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Distinct fibroblast subsets regulate lacteal integrity through YAP/TAZ-induced VEGF-C in intestinal villi
Authors: SP Hong, MJ Yang, H Cho, I Park, H Bae, K Choe, SH Suh, RH Adams, K Alitalo, D Lim, GY Koh
Nat Commun, 2020-08-14;11(1):4102.
Species: Mouse
Sample Types: Serum, Whole Tissue
Applications: IHC, Western Blot -
S1PR1 regulates the quiescence of lymphatic vessels by inhibiting laminar shear stress-dependent VEGF-C signaling
Authors: X Geng, K Yanagida, RG Akwii, D Choi, L Chen, Y Ho, B Cha, MR Mahamud, K Berman de, H Ichise, H Chen, J Wythe, CM Mikelis, T Hla, RS Srinivasan
JCI Insight, 2020-07-23;0(0):.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Blockade of VEGF-C signaling inhibits lymphatic malformations driven by oncogenic PIK3CA mutation
Authors: I Martinez-C, Y Zhang, M Petkova, H Ortsäter, S Sjöberg, SD Castillo, P Brouillard, L Libbrecht, D Saur, M Graupera, K Alitalo, L Boon, M Vikkula, T Mäkinen
Nat Commun, 2020-06-08;11(1):2869.
Species: Mouse
Sample Types: Serum, Whole Tissue
Applications: IHC, Western Blot -
Atypical cadherin Fat4 orchestrates lymphatic endothelial cell polarity in response to flow
Authors: KL Betterman, DL Sutton, GA Secker, J Kazenwadel, A Oszmiana, L Lim, N Miura, L Sorokin, BM Hogan, ML Kahn, H McNeill, NL Harvey
J. Clin. Invest., 2020-06-01;0(0):.
Species: Mouse
Sample Types: Cell Culture Lysates, Whole Tissue
Applications: IHC, Western Blot -
Transcriptional landscape of pulmonary lymphatic endothelial cells during fetal gestation
Authors: TA Norman, AC Gower, F Chen, A Fine
PLoS ONE, 2019-05-13;14(5):e0216795.
Species: Mouse
Sample Types: fetal lung tissue
Applications: IHC-P -
Dynamic signature of lymphangiogenesis during acute kidney injury and chronic kidney disease
Authors: A Zarjou, LM Black, S Bolisetty, AM Traylor, SA Bowhay, MZ Zhang, RC Harris, A Agarwal
Lab. Invest., 2019-04-24;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC-P, Western Blot -
Lymphatic impairment leads to pulmonary tertiary lymphoid organ formation and alveolar damage
Authors: HO Reed, L Wang, J Sonett, M Chen, J Yang, L Li, P Aradi, Z Jakus, J D'Armiento, WW Hancock, ML Kahn
J. Clin. Invest., 2019-04-04;129(6):2514-2526.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Complementary Wnt Sources Regulate Lymphatic Vascular Development via PROX1-Dependent Wnt/?-Catenin Signaling
Authors: B Cha, X Geng, MR Mahamud, JY Zhang, L Chen, W Kim, EH Jho, Y Kim, D Choi, JB Dixon, H Chen, YK Hong, L Olson, TH Kim, BJ Merrill, MJ Davis, RS Srinivasan
Cell Rep, 2018-10-16;25(3):571-584.e5.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Unexpected contribution of lymphatic vessels to promotion of distant metastatic tumor spread
Authors: Q Ma, LC Dieterich, K Ikenberg, SB Bachmann, J Mangana, ST Proulx, VC Amann, MP Levesque, R Dummer, P Baluk, DM McDonald, M Detmar
Sci Adv, 2018-08-08;4(8):eaat4758.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
HHEX is a transcriptional regulator of the VEGFC/FLT4/PROX1 signaling axis during vascular development
Authors: S Gauvrit, A Villasenor, B Strilic, P Kitchen, MM Collins, R Marín-Juez, S Guenther, HM Maischein, N Fukuda, MA Canham, JM Brickman, CW Bogue, PS Jayaraman, DYR Stainier
Nat Commun, 2018-07-13;9(1):2704.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Expression of the Atypical Chemokine Receptor ACKR4 Identifies a Novel Population of Intestinal Submucosal Fibroblasts That Preferentially Expresses Endothelial Cell Regulators
Authors: CA Thomson, SA van de Pav, M Stakenborg, E Labeeuw, G Matteoli, AM Mowat, RJB Nibbs
J. Immunol., 2018-05-14;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program
Authors: M Frye, A Taddei, C Dierkes, I Martinez-C, M Fielden, H Ortsäter, J Kazenwadel, DP Calado, P Ostergaard, M Salminen, L He, NL Harvey, F Kiefer, T Mäkinen
Nat Commun, 2018-04-17;9(1):1511.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Heterogeneity in VEGFR3 levels drives lymphatic vessel hyperplasia through cell-autonomous and non-cell-autonomous mechanisms
Authors: Y Zhang, MH Ulvmar, L Stanczuk, I Martinez-C, M Frye, K Alitalo, T Mäkinen
Nat Commun, 2018-04-03;9(1):1296.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Impaired angiopoietin/Tie2 signaling compromises Schlemm's canal integrity and induces glaucoma
Authors: J Kim, DY Park, H Bae, DY Park, D Kim, CK Lee, S Song, TY Chung, DH Lim, Y Kubota, YK Hong, Y He, HG Augustin, G Oliver, GY Koh
J. Clin. Invest., 2017-09-18;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Efficient activation of the lymphangiogenic growth factor VEGF-C requires the C-terminal domain of VEGF-C and the N-terminal domain of CCBE1
Authors: SK Jha, K Rauniyar, T Karpanen, VM Leppänen, P Brouillard, M Vikkula, K Alitalo, M Jeltsch
Sci Rep, 2017-07-07;7(1):4916.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine
Authors: D Olmeda, D Cerezo-Wal, E Riveiro-Fa, PC Pennacchi, M Contreras-, N Ibarz, M Cifdaloz, X Catena, TG Calvo, E Cañón, D Alonso-Cur, J Suarez, L Osterloh, O Graña, F Mulero, D Megías, M Cañamero, JL Martínez-T, C Mondal, J Di Martino, D Lora, I Martinez-C, JJ Bravo-Cord, J Muñoz, S Puig, P Ortiz-Rome, JL Rodriguez-, S Ortega, MS Soengas
Nature, 2017-06-28;546(7660):676-680.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Apelin modulates pathological remodeling of lymphatic endothelium after myocardial infarction
Authors: F Tatin, E Renaud-Gab, AC Godet, F Hantelys, F Pujol, F Morfoisse, D Calise, F Viars, P Valet, B Masri, AC Prats, B Garmy-Susi
JCI Insight, 2017-06-15;2(12):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VEGFR2 but not VEGFR3 governs integrity and remodeling of thyroid angiofollicular unit in normal state and during goitrogenesis
Authors: JY Jang, SY Choi, I Park, DY Park, K Choe, P Kim, YK Kim, BJ Lee, M Hirashima, Y Kubota, JW Park, SY Cheng, A Nagy, YJ Park, K Alitalo, M Shong, GY Koh
EMBO Mol Med, 2017-06-01;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
FGF-dependent metabolic control of vascular development
Authors: P Yu, K Wilhelm, A Dubrac, JK Tung, TC Alves, JS Fang, Y Xie, J Zhu, Z Chen, F De Smet, J Zhang, SW Jin, L Sun, H Sun, RG Kibbey, KK Hirschi, N Hay, P Carmeliet, TW Chittenden, A Eichmann, M Potente, M Simons
Nature, 2017-05-03;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Vitamin D inhibits lymphangiogenesis through VDR-dependent mechanisms
Authors: S Yazdani, F Poosti, L Toro, J Wedel, R Mencke, K Mirkovi?, MH de Borst, JS Alexander, G Navis, H van Goor, J van den Bo, JL Hillebrand
Sci Rep, 2017-03-17;7(0):44403.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling
Authors: K Heinolaine, S Karaman, G D'Amico, T Tammela, R Sormunen, L Eklund, K Alitalo, G Zarkada
Circ. Res, 2017-03-15;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Cells
Applications: ICC, Western Blot -
Cell-matrix signals specify bone endothelial cells during developmental osteogenesis
Authors: UH Langen, ME Pitulescu, JM Kim, R Enriquez-G, KK Sivaraj, AP Kusumbe, A Singh, J Di Russo, MG Bixel, B Zhou, L Sorokin, JM Vaquerizas, RH Adams
Nat. Cell Biol, 2017-02-20;19(3):189-201.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Basophil-derived IL-6 regulates TH17 cell differentiation and CD4 T cell immunity
Authors: CM Yuk, HJ Park, BI Kwon, SJ Lah, J Chang, JY Kim, KM Lee, SH Park, S Hong, SH Lee
Sci Rep, 2017-01-30;7(0):41744.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
G-Protein-Coupled Receptor-2-Interacting Protein-1 Controls Stalk Cell Fate by Inhibiting Delta-like 4-Notch1 Signaling
Cell Rep, 2016-12-06;17(10):2532-2541.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Defective lymphatic valve development and chylothorax in mice with a lymphatic-specific deletion of Connexin43
Authors: Alexander M Simon
Dev. Biol., 2016-11-27;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Semaphorin 3G Provides a Repulsive Guidance Cue to Lymphatic Endothelial Cells via Neuropilin-2/PlexinD1
Cell Rep, 2016-11-22;17(9):2299-2311.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mechanotransduction activates canonical Wnt/?-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves
Authors: Boksik Cha
Genes Dev, 2016-06-16;30(12):1454-69.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina.
Authors: Zhou Y, Williams J, Smallwood P, Nathans J
PLoS ONE, 2015-12-02;10(12):e0143650.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Delayed Healing of Sickle Cell Ulcers Is due to Impaired Angiogenesis and CXCL12 Secretion in Skin Wounds.
Authors: Nguyen V, Nassar D, Batteux F, Raymond K, Tharaux P, Aractingi S
J Invest Dermatol, 2015-11-18;136(2):497-506.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules.
Authors: Aspelund A, Antila S, Proulx S, Karlsen T, Karaman S, Detmar M, Wiig H, Alitalo K
J Exp Med, 2015-06-15;212(7):991-9.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Cdk5 controls lymphatic vessel development and function by phosphorylation of Foxc2
Authors: Johanna Liebl, Siwei Zhang, Markus Moser, Yan Agalarov, Cansaran Saygili Demir, Bianca Hager et al.
Nature Communications
Applications: Immunohistochemistry, Western Blot -
Structural and functional features of central nervous system lymphatic vessels.
Authors: Louveau A, Smirnov I, Keyes T, Eccles J, Rouhani S, Peske J, Derecki N, Castle D, Mandell J, Lee K, Harris T, Kipnis J
Nature, 2015-06-01;523(7560):337-41.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
EphB4 forward signalling regulates lymphatic valve development.
Authors: Zhang, Gu, Brady, John, Liang, Wei-Chin, Wu, Yan, Henkemeyer, Mark, Yan, Minhong
Nat Commun, 2015-04-13;6(0):6625.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Quantitative assessment of angiogenesis, perfused blood vessels and endothelial tip cells in the postnatal mouse brain.
Authors: Walchli T, Mateos J, Weinman O, Babic D, Regli L, Hoerstrup S, Gerhardt H, Schwab M, Vogel J
Nat Protoc, 2014-12-11;10(1):53-74.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The Schlemm's canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel.
Authors: Aspelund A, Tammela T, Antila S, Nurmi H, Leppanen V, Zarkada G, Stanczuk L, Francois M, Makinen T, Saharinen P, Immonen I, Alitalo K
J Clin Invest, 2014-07-25;124(9):3975-86.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Lymphatic regulator PROX1 determines Schlemm's canal integrity and identity.
Authors: Park D, Lee J, Park I, Choi D, Lee S, Song S, Hwang Y, Hong K, Nakaoka Y, Makinen T, Kim P, Alitalo K, Hong Y, Koh G
J Clin Invest, 2014-07-25;124(9):3960-74.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Schlemm's canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process.
Authors: Kizhatil, Krishnak, Ryan, Margaret, Marchant, Jeffrey, Henrich, Stephen, John, Simon W
PLoS Biol, 2014-07-22;12(7):e1001912.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions.
Authors: Zheng W, Nurmi H, Appak S, Sabine A, Bovay E, Korhonen E, Orsenigo F, Lohela M, D'Amico G, Holopainen T, Leow C, Dejana E, Petrova T, Augustin H, Alitalo K
Genes Dev, 2014-07-15;28(14):1592-603.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Identification of novel genes associated with renal tertiary lymphoid organ formation in aging mice.
Authors: Huang Y, Caputo C, Noordmans G, Yazdani S, Monteiro L, van den Born J, van Goor H, Heeringa P, Korstanje R, Hillebrands J
PLoS ONE, 2014-03-17;9(3):e91850.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Endothelial Notch activity promotes angiogenesis and osteogenesis in bone.
Authors: Ramasamy S, Kusumbe A, Wang L, Adams R
Nature, 2014-03-12;507(7492):376-80.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Preferential lymphatic growth in bronchus-associated lymphoid tissue in sustained lung inflammation.
Authors: Baluk P, Adams A, Phillips K, Feng J, Hong Y, Brown M, McDonald D
Am J Pathol, 2014-03-11;184(5):1577-92.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Molecular identification of venous progenitors in the dorsal aorta reveals an aortic origin for the cardinal vein in mammals.
Authors: Lindskog, Henrik, Kim, Yung Hae, Jelin, Eric B, Kong, Yupeng, Guevara-Gallardo, Salvador, Kim, Tyson N, Wang, Rong A
Development, 2014-03-01;141(5):1120-8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Fusing VE-cadherin to alpha-catenin impairs fetal liver hematopoiesis and lymph but not blood vessel formation.
Authors: Dartsch N, Schulte D, Hagerling R, Kiefer F, Vestweber D
Mol Cell Biol, 2014-02-24;34(9):1634-48.
Species: Mouse
Sample Types: Embryo, Whole Tissue
Applications: IHC, IHC-P -
Lenalidomide inhibits lymphangiogenesis in preclinical models of mantle cell lymphoma.
Authors: Song K, Herzog B, Sheng M, Fu J, McDaniel J, Chen H, Ruan J, Xia L
Cancer Res, 2013-10-24;73(24):7254-64.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Absence of venous valves in mice lacking Connexin37
Authors: Stephanie J. Munger, John D. Kanady, Alexander M. Simon
Developmental Biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy.
Authors: Hagerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams R, Alitalo K, Andresen V, Schulte-Merker S, Kiefer F
EMBO J, 2013-01-08;32(5):629-44.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Proteinuria triggers renal lymphangiogenesis prior to the development of interstitial fibrosis.
Authors: Yazdani S, Poosti F, Kramer A, Mirkovic K, Kwakernaak A, Hovingh M, Slagman M, Sjollema K, de Borst M, Navis G, van Goor H, van den Born J
PLoS ONE, 2012-11-26;7(11):e50209.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Remodeling of the lymphatic vasculature during mouse mammary gland morphogenesis is mediated via epithelial-derived lymphangiogenic stimuli.
Authors: Betterman K, Paquet-Fifield S, Asselin-Labat M, Visvader J, Butler L, Stacker S, Achen M, Harvey N
Am J Pathol, 2012-10-11;181(6):2225-38.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling.
Authors: Benedito R, Rocha S, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams R
Nature, 2012-03-18;484(7392):110-4.
Species: Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: ELISA Development, IHC -
Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis.
Authors: Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH
Nature, 2010-05-27;465(7297):483-6.
Species: Mouse
Sample Types: Cell Lysates, Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr, Immunoprecipitation -
Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development.
Authors: Lin FJ, Chen X, Qin J, Hong YK, Tsai MJ, Tsai SY
J. Clin. Invest., 2010-04-01;120(5):1694-707.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Vascular endothelial growth factor-C induces lymphangitic carcinomatosis, an extremely aggressive form of lung metastases.
Authors: Das S, Ladell DS, Podgrabinska S
Cancer Res., 2010-02-23;70(5):1814-24.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3.
Authors: Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, Larrivee B, Del Toro R, Suchting S, Medvinsky A, Silva J, Yang J, Thomas JL, Koch AW, Alitalo K, Eichmann A, Bagri A
J. Cell Biol., 2010-01-11;188(1):115-30.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues.
Authors: Lohela M, Helotera H, Haiko P, Dumont DJ, Alitalo K
Am. J. Pathol., 2008-11-06;173(6):1891-901.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC, Immunoprecipitation -
Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation.
Authors: Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, Freitas C, Duarte A, Isoniemi H, Laakkonen P, Christofori G, Yla-Herttuala S, Shibuya M, Pytowski B, Eichmann A, Betsholtz C, Alitalo K
Nature, 2008-06-25;454(7204):656-60.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting.
Authors: Wirzenius M, Tammela T, Uutela M, He Y, Odorisio T, Zambruno G, Nagy JA, Dvorak HF, Yla-Herttuala S, Shibuya M, Alitalo K
J. Exp. Med., 2007-05-29;204(6):1431-40.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth.
Authors: Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L, Alitalo K
Cancer Res., 2007-01-15;67(2):593-9.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Visualization of IL-12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promote Th1 differentiation.
Authors: Reinhardt RL, Hong S, Kang SJ, Wang ZE, Locksley RM
J. Immunol., 2006-08-01;177(3):1618-27.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain.
Authors: Le Bras B, Barallobre MJ, Homman-Ludiye J, Ny A, Wyns S, Tammela T, Haiko P, Karkkainen MJ, Yuan L, Muriel MP, Chatzopoulou E, Breant C, Zalc B, Carmeliet P, Alitalo K, Eichmann A, Thomas JL
Nat. Neurosci., 2006-02-05;9(3):340-8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Desmoplakin and Plakoglobin--specific markers of lymphatic vessels in the skin?
Authors: Fedele C, Berens D, Rautenfeld V, Pabst R
Anat Histol Embryol, 2004-06-01;33(3):168-71.
Species: Equine
Sample Types: Whole Tissue
Applications: IHC -
B lymphocyte-specific c-Myc expression stimulates early and functional expansion of the vasculature and lymphatics during lymphomagenesis.
Authors: Ruddell A, Mezquita P, Brandvold KA, Farr A, Iritani BM
Am. J. Pathol., 2003-12-01;163(6):2233-45.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Sphingosine 1-phosphate-regulated transcriptomes in heterogenous arterial and lymphatic endothelium of the aorta
Authors: Eric Engelbrecht, Michel V Levesque, Liqun He, Michael Vanlandewijck, Anja Nitzsche, Hira Niazi et al.
eLife
-
Mechanoinduction of lymph vessel expansion
Authors: Lara Planas-Paz, Boris Strilić, Axel Goedecke, Georg Breier, Reinhard Fässler, Eckhard Lammert
The EMBO Journal
-
Interferon gamma constrains type 2 lymphocyte niche boundaries during mixed inflammation
Authors: Kelly M. Cautivo, Peri R. Matatia, Carlos O. Lizama, Nicholas M. Mroz, Madelene W. Dahlgren, Xiaofei Yu et al.
Immunity
-
Kidins220/ARMS mediates the integration of the neurotrophin and VEGF pathways in the vascular and nervous systems
Authors: F Cesca, A Yabe, B Spencer-Dene, J Scholz-Starke, L Medrihan, C H Maden et al.
Cell Death & Differentiation
-
Conditional hypoxia inducible factor-1 alpha induction in embryonic pulmonary epithelium impairs maturation and augments lymphangiogenesis
Authors: James P. Bridges, Sui Lin, Machiko Ikegami, John M. Shannon
Developmental Biology
-
Spatiotemporal dynamics and heterogeneity of renal lymphatics in mammalian development and cystic kidney disease
Authors: Daniyal J Jafree, Dale Moulding, Maria Kolatsi-Joannou, Nuria Perretta Tejedor, Karen L Price, Natalie J Milmoe et al.
eLife
-
Development and plasticity of meningeal lymphatic vessels
Authors: Salli Antila, Sinem Karaman, Harri Nurmi, Mikko Airavaara, Merja H. Voutilainen, Thomas Mathivet et al.
Journal of Experimental Medicine
-
Carbohydrate-binding protein CLEC14A regulates VEGFR-2- and VEGFR-3-dependent signals during angiogenesis and lymphangiogenesis
Authors: Sungwoon Lee
J. Clin. Invest, 2016-12-19;0(0):.
-
YAP and TAZ maintain PROX1 expression in the developing lymphatic and lymphovenous valves in response to VEGF-C signaling
Authors: Boksik Cha, Yen-Chun Ho, Xin Geng, Md. Riaj Mahamud, Lijuan Chen, Yeunhee Kim et al.
Development
-
Lymphatic endothelial cells efferent to inflamed joints produce iNOS and inhibit lymphatic vessel contraction and drainage in TNF-induced arthritis in mice
Authors: Qianqian Liang, Yawen Ju, Yan Chen, Wensheng Wang, Jinlong Li, Li Zhang et al.
Arthritis Research & Therapy
-
Radiation-Induced Impairment in Lung Lymphatic Vasculature
Authors: Ye Cui, Julie Wilder, Cecilia Rietz, Andrew Gigliotti, Xiaomeng Tang, Yuanyuan Shi et al.
Lymphatic Research and Biology
-
The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis
Authors: Heidi I. Chen, Bikram Sharma, Brynn N. Akerberg, Harri J. Numi, Riikka Kivelä, Pipsa Saharinen et al.
Development
-
Structural and functional conservation of non-lumenized lymphatic endothelial cells in the mammalian leptomeninges
Authors: Shibata-Germanos S, Goodman JR, Grieg A et al.
Acta Neuropathol.
-
VEGF-C and aortic cardiomyocytes guide coronary artery stem development
Authors: Heidi I. Chen, Aruna Poduri, Harri Numi, Riikka Kivela, Pipsa Saharinen, Andrew S. McKay et al.
Journal of Clinical Investigation
-
Distinct roles of VE ‐cadherin for development and maintenance of specific lymph vessel beds
Authors: René Hägerling, Esther Hoppe, Cathrin Dierkes, Martin Stehling, Taija Makinen, Stefan Butz et al.
The EMBO Journal
-
GATA2 controls lymphatic endothelial cell junctional integrity and lymphovenous valve morphogenesis through miR-126
Authors: Md. Riaj Mahamud, Xin Geng, Yen-Chun Ho, Boksik Cha, Yuenhee Kim, Jing Ma et al.
Development
-
Genetic variants of VEGFA and FLT4 are determinants of survival in renal cell carcinoma patients treated with sorafenib
Authors: DJ Crona, AD Skol, VM Leppänen, DM Glubb, AS Etheridge, E Hilliard, CE Peña, YK Peterson, N Klauber-De, KK Alitalo, F Innocenti
Cancer Res., 2018-11-01;0(0):.
-
Cyclic AMP Response Element Binding Protein Mediates Pathological Retinal Neovascularization via Modulating DLL4-NOTCH1 Signaling.
Authors: Singh NK, Kotla S, Kumar R, Rao GN
EBioMedicine
-
The cardiopharyngeal mesoderm contributes to lymphatic vessel development in mouse
Authors: Kazuaki Maruyama, Sachiko Miyagawa-Tomita, Yuka Haneda, Mayuko Kida, Fumio Matsuzaki, Kyoko Imanaka-Yoshida et al.
eLife
-
Vascular endothelial growth factor-C ameliorates renal interstitial fibrosis through lymphangiogenesis in mouse unilateral ureteral obstruction
Authors: S Hasegawa, T Nakano, K Torisu, A Tsuchimoto, M Eriguchi, N Haruyama, K Masutani, K Tsuruya, T Kitazono
Lab. Invest., 2017-10-30;0(12):1439-1452.
-
Fluid shear stress regulates vascular remodeling via VEGFR-3 activation, although independently of its ligand, VEGF-C, in the uterus during pregnancy
Authors: Yang-Gyu Park, Jawun Choi, Hye-Kang Jung, In Kyu Song, Yongwhan Shin, Sang-Youel Park et al.
International Journal of Molecular Medicine
-
Smooth muscle cell recruitment to lymphatic vessels requires PDGFB and impacts vessel size but not identity
Authors: Yixin Wang, Yi Jin, Maarja Andaloussi Mäe, Yang Zhang, Henrik Ortsäter, Christer Betsholtz et al.
Development
-
VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling
Authors: Tuomas Tammela, Georgia Zarkada, Harri Nurmi, Lars Jakobsson, Krista Heinolainen, Denis Tvorogov et al.
Nature Cell Biology
-
RASA1 maintains the lymphatic vasculature in a quiescent functional state in mice.
Authors: Lapinski PE, Kwon S, Lubeck BA
J. Clin. Invest., 2012-01-09;122(2):733-47.
-
YAP1 and TAZ negatively control bone angiogenesis by limiting hypoxia-inducible factor signaling in endothelial cells
Authors: Sivaraj KK, Dharmalingam B, Mohanakrishnan V et al.
Elife
-
Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells
Authors: M Hsu, A Rayasam, JA Kijak, YH Choi, JS Harding, SA Marcus, WJ Karpus, M Sandor, Z Fabry
Nat Commun, 2019-01-16;10(1):229.
-
Identification of ILK as a critical regulator of VEGFR 3 signalling and lymphatic vascular growth
Authors: Sofia Urner, Lara Planas‐Paz, Laura Sophie Hilger, Carina Henning, Anna Branopolski, Molly Kelly‐Goss et al.
The EMBO Journal
-
Absence of venous valves in mice lacking Connexin37
Authors: Stephanie J. Munger, John D. Kanady, Alexander M. Simon
Developmental Biology
-
ADAMTS3 activity is mandatory for embryonic lymphangiogenesis and regulates placental angiogenesis
Authors: Lauriane Janssen, Laura Dupont, Mourad Bekhouche, Agnès Noel, Cédric Leduc, Marianne Voz et al.
Angiogenesis
-
Vascular Endothelial Growth Factor C for Polycystic Kidney Diseases
Authors: Jennifer L. Huang, Adrian S. Woolf, Maria Kolatsi-Joannou, Peter Baluk, Richard N. Sandford, Dorien J.M. Peters et al.
Journal of the American Society of Nephrology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse VEGFR3/Flt-4 Antibody
Average Rating: 4.6 (Based on 5 Reviews)
Have you used Mouse VEGFR3/Flt-4 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Whole-mounted mouse femur
5 ug of Ab used stained for 3 days at room temperature
Whole mount immunofluorescence of mouse mesenteric lymphatics. Mesentery was dissected from a P5 neonate, fixed in 2% PFA and stained using AF743 at a 1:200 dilution.