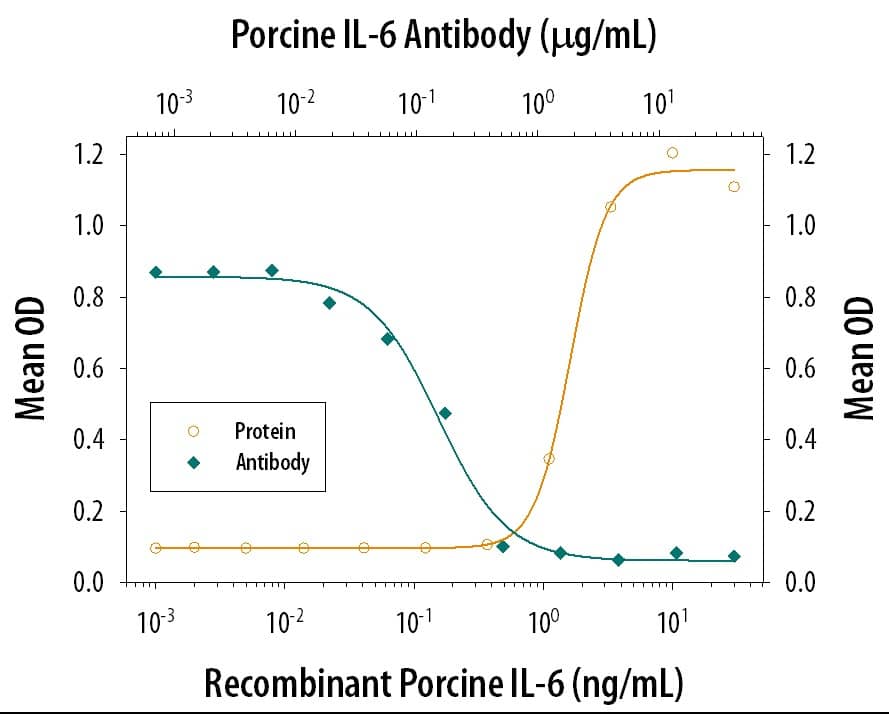
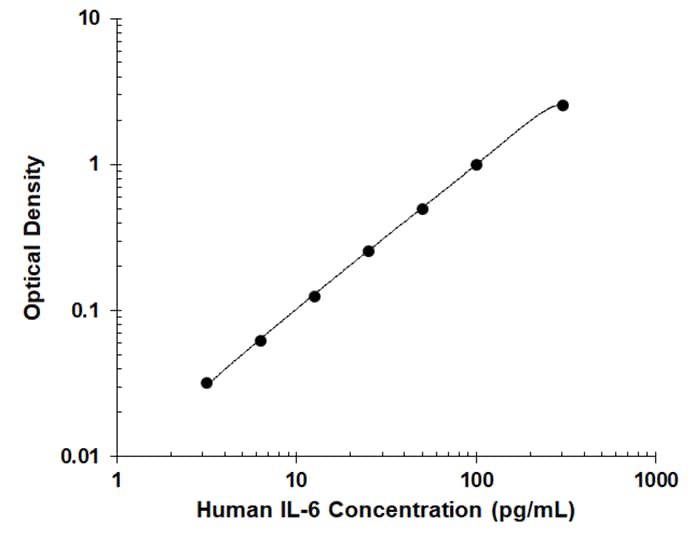
Porcine IL-6 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant porcine IL-6. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Customers also Viewed
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-6
Interleukin 6 (IL-6) is a pleiotropic, alpha -helical, 22 - 28 kDa phosphorylated and variably glycosylated cytokine that plays important roles in the acute phase reaction, inflammation, hematopoiesis, bone metabolism, and cancer progression (1-5). Mature porcine IL-6 is 183 amino acids (aa) in length and shares 58%, 39% and 39% aa sequence identity with human, mouse, and rat IL-6, respectively (6). Cells known to express IL-6 include CD8+ T cells, fibroblasts, synoviocytes, adipocytes, osteoblasts, megakaryocytes, endothelial cells (under the influence of endothelins), sympathetic neurons, cerebral cortex neurons, adrenal medulla chromaffin cells, retinal pigment cells, mast cells, keratinocytes, Langerhans cells, fetal and adult astrocytes, neutrophils, monocytes, eosinophils, colonic epithelial cells, B1 B cells and pancreatic islet beta cells (2, 7-29). IL-6 production is generally correlated with cell activation and is normally kept in control by glucocorticoids, catecholamines, and secondary sex steroids (2). Normal human circulating IL-6 is in the 1 pg/mL range, with slight elevations during the menstrual cycle, modest elevations in certain cancers, and large elevations after surgery (30-34).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Porcine IL-6 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
37
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Efficacy of Saccharomyces yeast postbiotics on cell turnover, immune responses, and oxidative stress in the jejunal mucosa of young pigs
Authors: Duarte, ME;Kim, SW;
Scientific reports
Species: Porcine
Sample Types: Whole Tissue
-
The lipopolysaccharide structure affects the detoxifying ability of intestinal alkaline phosphatases
Authors: Vermeire, B;Walsh, M;Cox, E;Devriendt, B;
BMC veterinary research
Species: Porcine
Sample Types: Cell Culture Supernates
-
Predicting cytokine kinetics during sepsis; a modelling framework from a porcine sepsis model with live Escherichia coli
Authors: Bahnasawy, SM;Skorup, P;Hanslin, K;Lipcsey, M;Friberg, LE;Nielsen, EI;
Cytokine
Species: Porcine
Sample Types: Plasma
-
Efficacy of Feed Additive Containing Bentonite and Enzymatically Hydrolyzed Yeast on Intestinal Health and Growth of Newly Weaned Pigs under Chronic Dietary Challenges of Fumonisin and Aflatoxin
Authors: Deng, Z;Jang, KB;Jalukar, S;Du, X;Kim, SW;
Toxins
Species: Porcine
Sample Types: Tissue Homogenate
-
Anti-inflammatory properties of antiangiogenic fucoidan in retinal pigment epithelium cells
Authors: P Dörschmann, C Seeba, T Thalenhors, J Roider, A Klettner
Heliyon, 2023-04-10;9(4):e15202.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Anti-inflammatory properties of antiangiogenic fucoidan in retinal pigment epithelium cells
Authors: P Dörschmann, C Seeba, T Thalenhors, J Roider, A Klettner
Heliyon, 2023;9(4):e15202.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Pulmonary inflammatory response and immunomodulation to multiple trauma and hemorrhagic shock in pigs
Authors: MA Oestreich, K Seidel, W Bertrams, HH Müller, M Sassen, T Steinfeldt, H Wulf, B Schmeck
PLoS ONE, 2022-12-07;17(12):e0278766.
Species: Porcine
Sample Types: BALF
-
Use of a novel "Split" ventilation system in bench and porcine modeling of acute respiratory distress syndrome
Authors: P Geoghegan, J Clarke, G Hogan, A Keogh, H Marsh, K Donnelly, N McEvoy, A Doolan, SF Madden, I Martin-Loe, M Power, JG Laffey, GF Curley
Physiological Reports, 2022-09-01;10(17):e15452.
Species: Porcine
Sample Types: BALF
-
Glucose supply and glycolysis inhibition shape the clinical fate of Staphylococcus epidermidis-infected preterm newborns
Authors: T Muk, A Brunse, NL Henriksen, K Aasmul-Ols, DN Nguyen
JCI Insight, 2022-06-08;0(0):.
Species: Porcine
Sample Types: Plasma
-
Human-relevant near-organ neuromodulation of the immune system via the splenic nerve
Authors: M Donegà, CT Fjordbakk, J Kirk, DM Sokal, I Gupta, GE Hunsberger, A Crawford, S Cook, J Viscasilla, TR Stathopoul, JA Miranda, WJ Dopson, D Goodwin, A Rowles, P McGill, A McSloy, D Werling, J Witheringt, DJ Chew, JD Perkins
Proceedings of the National Academy of Sciences of the United States of America, 2021-05-18;118(20):.
Species: Porcine
Sample Types: Plasma
-
Lung-protective ventilation increases cerebral metabolism and non-inflammatory brain injury in porcine experimental sepsis
Authors: A Nyberg, E Gremo, J Blixt, J Sperber, A Larsson, M Lipcsey, A Pikwer, M Castegren
Bmc Neuroscience, 2021-04-29;22(1):31.
Species: Porcine
Sample Types: Plasma
-
A comprehensive assessment of multi-system responses to a renal inoculation of uropathogenic E. coli in swine
Authors: MH Tiba, BM McCracken, RP Dickson, JA Nemzek, CI Colmenero, DC Leander, TL Flott, RC Daniels, KE Konopka, JS VanEpps, KA Stringer, KR Ward
PLoS ONE, 2020-12-11;15(12):e0243577.
Species: Porcine
Sample Types: Whole Blood
-
Pre-exposure to mechanical ventilation and endotoxemia increases Pseudomonas aeruginosa growth in lung tissue during experimental porcine pneumonia
Authors: J Sperber, A Nyberg, A Krifors, P Skorup, M Lipcsey, M Castegren
PLoS ONE, 2020-10-27;15(10):e0240753.
Species: Porcine
Sample Types: BALF
-
The antisecretory peptide AF-16 may modulate tissue edema but not inflammation in experimental peritonitis induced sepsis
Authors: A Barrueta T, J van der He, I Blokhin, F Massaro, HA Hansson, R Feinstein, A Larsson, A Larsson, J Tenhunen
PLoS ONE, 2020-08-21;15(8):e0232302.
Species: Porcine
Sample Types: Plasma
-
Activation of the NLRP3 Inflammasome by Particles from the Echinococcus granulosus Laminated Layer
Authors: C Casaravill, Á Pittini, D Rückerl, JE Allen, Á Díaz
Infect. Immun., 2020-08-19;88(9):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Lung-protective ventilation suppresses systemic and hepatic vein levels of cell-free DNA in porcine experimental post-operative sepsis
Authors: A Nyberg, A Larsson, J Jylhävä, M Hurme, J Sperber, M Lipcsey, M Castegren
BMC Pulm Med, 2020-07-31;20(1):206.
Species: Porcine
Sample Types: Plasma
-
The Effect of Zearalenone on the Cytokine Environment, Oxidoreductive Balance and Metabolism in Porcine Ileal Peyer's Patches
Authors: K Obremski, W Trybowski, P Wojtacha, M Gaj?cka, J Tyburski, ? Zielonka
Toxins (Basel), 2020-05-27;12(6):.
Species: Porcine
Sample Types: Whole Tissue
-
Analysis of Porcine Pro- and Anti-Inflammatory Cytokine Induction by S. suis In Vivo and In Vitro
Authors: FS Hohnstein, M Meurer, N de Buhr, M von Köckri, CG Baums, G Alber, N Schütze
Pathogens, 2020-01-03;9(1):.
Species: Porcine
Sample Types: Whole Cells
-
Efficacy of three innovative bacterin vaccines against experimental infection with Mycoplasma hyopneumoniae
Authors: AMF Matthijs, G Auray, F Boyen, A Schoos, A Michiels, O García-Nic, GT Barut, C Barnier-Qu, V Jakob, N Collin, B Devriendt, A Summerfiel, F Haesebrouc, D Maes
Vet. Res., 2019-11-08;50(1):91.
Species: Porcine
Sample Types: BALF
-
Classical swine fever virus non-structural proteins modulate Toll-like receptor signaling pathways in porcine monocyte-derived macrophages
Authors: Z Cao, Q Yang, M Zheng, H Lv, K Kang, Y Zhang
Vet. Microbiol., 2019-01-29;230(0):101-109.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Necrotizing enterocolitis is associated with acute brain responses in preterm pigs
Authors: J Sun, X Pan, LI Christians, XL Yuan, K Skovgaard, DEW Chatterton, SS Kaalund, F Gao, PT Sangild, S Pankratova
J Neuroinflammation, 2018-06-09;15(1):180.
Species: Porcine
Sample Types: Tissue Homogenates
-
Cardiac Depression in Pigs after Multiple Trauma - Characterization of Posttraumatic Structural and Functional Alterations
Authors: M Kalbitz, S Schwarz, B Weber, B Bosch, J Pressmar, FM Hoenes, CK Braun, K Horst, TP Simon, R Pfeifer, P Störmann, H Hummler, F Gebhard, HC Pape, M Huber-Lang, F Hildebrand, TREAT Rese
Sci Rep, 2017-12-19;7(1):17861.
Species: Porcine
Sample Types: Tissue Homogenates
-
Efficacy of one dose vaccination against experimental infection with two Mycoplasma hyopneumoniae strains
Authors: A Michiels, I Arsenakis, F Boyen, R Krejci, F Haesebrouc, D Maes
BMC Vet. Res., 2017-08-29;13(1):274.
Species: Porcine
Sample Types: BALF
-
Early Gut Microbiota Intervention Suppresses DSS-Induced Inflammatory Responses by Deactivating TLR/NLR Signalling in Pigs
Authors: Y Xiao, H Yan, H Diao, B Yu, J He, J Yu, P Zheng, X Mao, Y Luo, D Chen
Sci Rep, 2017-06-12;7(1):3224.
Species: Porcine
Sample Types: Tissue Culture Supernates
-
Host-pathogen interplay at primary infection sites in pigs challenged with Actinobacillus pleuropneumoniae
Authors: EL Sassu, J Frömbling, JC Duvigneau, I Miller, A Müllebner, AM Gutiérrez, T Grunert, M Patzl, A Saalmüller, A von Altroc, A Menzel, M Ganter, J Spergser, M Hewicker-T, J Verspohl, M Ehling-Sch, I Hennig-Pau
BMC Vet. Res, 2017-02-28;13(1):64.
Species: Porcine
Sample Types: BALF
-
The immune response against Chlamydia suis genital tract infection partially protects against re-infection.
Authors: De Clercq E, Devriendt B, Yin L, Chiers K, Cox E, Vanrompay D
Vet Res, 2014-09-25;45(0):95.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Lung protective ventilation induces immunotolerance and nitric oxide metabolites in porcine experimental postoperative sepsis.
Authors: Sperber J, Lipcsey M, Larsson A, Larsson A, Sjolin J, Castegren M
PLoS ONE, 2013-12-12;8(12):e83182.
Species: Porcine
Sample Types: Plasma
-
Immunopathogenesis of severe acute respiratory disease in Zaire ebolavirus-infected pigs.
Authors: Nfon, Charles, Leung, Anders, Smith, Greg, Embury-Hyatt, Carissa, Kobinger, Gary, Weingartl, Hana M
PLoS ONE, 2013-04-23;8(4):e61904.
Species: Porcine
Sample Types: Plasma
-
Characterization of homologous and heterologous adaptive immune responses in porcine reproductive and respiratory syndrome virus infection.
Authors: Diaz I, Gimeno M, Darwich L, Navarro N, Kuzemtseva L, Lopez S, Galindo I, Segales J, Martin M, Pujols J, Mateu E
Vet. Res., 2012-04-19;43(1):30.
Species: Porcine
Sample Types: Serum
-
N(pro) of Classical Swine Fever Virus Prevents Type I Interferon-Mediated Priming of Conventional Dendritic Cells for Enhanced Interferon-alpha Response.
Authors: Husser L, Ruggli N, Summerfield A
J. Interferon Cytokine Res., 2012-02-07;32(5):221-9.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Cytokine protein expression levels in tracheobronchial lymph node homogenates of pigs infected with pseudorabies virus.
Authors: Miller LC, Zanella EL, Waters WR
Clin. Vaccine Immunol., 2010-03-10;17(5):728-34.
Species: Porcine
Sample Types: Tissue Homogenates
-
Inflammatory and circulatory effects of the reduction of endotoxin concentration in established porcine endotoxemic shock--a model of endotoxin elimination.
Authors: Carlsson M, Lipcsey M, Larsson A, Tano E, Rubertsson S, Eriksson M, Sjolin J
Crit. Care Med., 2009-03-01;37(3):1031-e4.
Species: Porcine
Sample Types: Plasma
-
Early administration of probiotics alters bacterial colonization and limits diet-induced gut dysfunction and severity of necrotizing enterocolitis in preterm pigs.
Authors: Siggers RH, Siggers J, Boye M, Thymann T, Molbak L, Leser T, Jensen BB, Sangild PT
J. Nutr., 2008-08-01;138(8):1437-44.
Species: Porcine
Sample Types: Tissue Homogenates
-
Impact of topical cooling solution and prediction of pulmonary graft viability from non-heart-beating donors.
Authors: Inci I, Arni S, Inci D, Zhai W, Hillinger S, Leskosek B, Vogt P, Weder W
J. Heart Lung Transplant., 2008-07-21;27(9):1016-22.
Species: Porcine
Sample Types: BALF
-
In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid.
Authors: Saurer L, McCullough KC, Summerfield A
J. Immunol., 2007-09-15;179(6):3504-14.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Toll-like receptor 7 and MyD88 knockdown by lentivirus-mediated RNA interference to porcine dendritic cell subsets.
Authors: Alves MP, Neuhaus V, Guzylack-Piriou L, Ruggli N, McCullough KC, Summerfield A
Gene Ther., 2007-03-01;14(10):836-44.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Neutrophil and small intestinal lymphocyte migration after Salmonella typhimurium infection: impact of fermentable fiber.
Authors: Milo LA, Correa-Matos NJ, Donovan SM, Tappenden KA
J. Pediatr. Gastroenterol. Nutr., 2004-07-01;39(1):73-9.
Species: Porcine
Sample Types: Plasma
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Porcine IL-6 DuoSet ELISA
Average Rating: 4.5 (Based on 2 Reviews)
Have you used Porcine IL-6 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Works ok. As a reagent diluent RPMI medium can be used to dilute standard curve and samples.