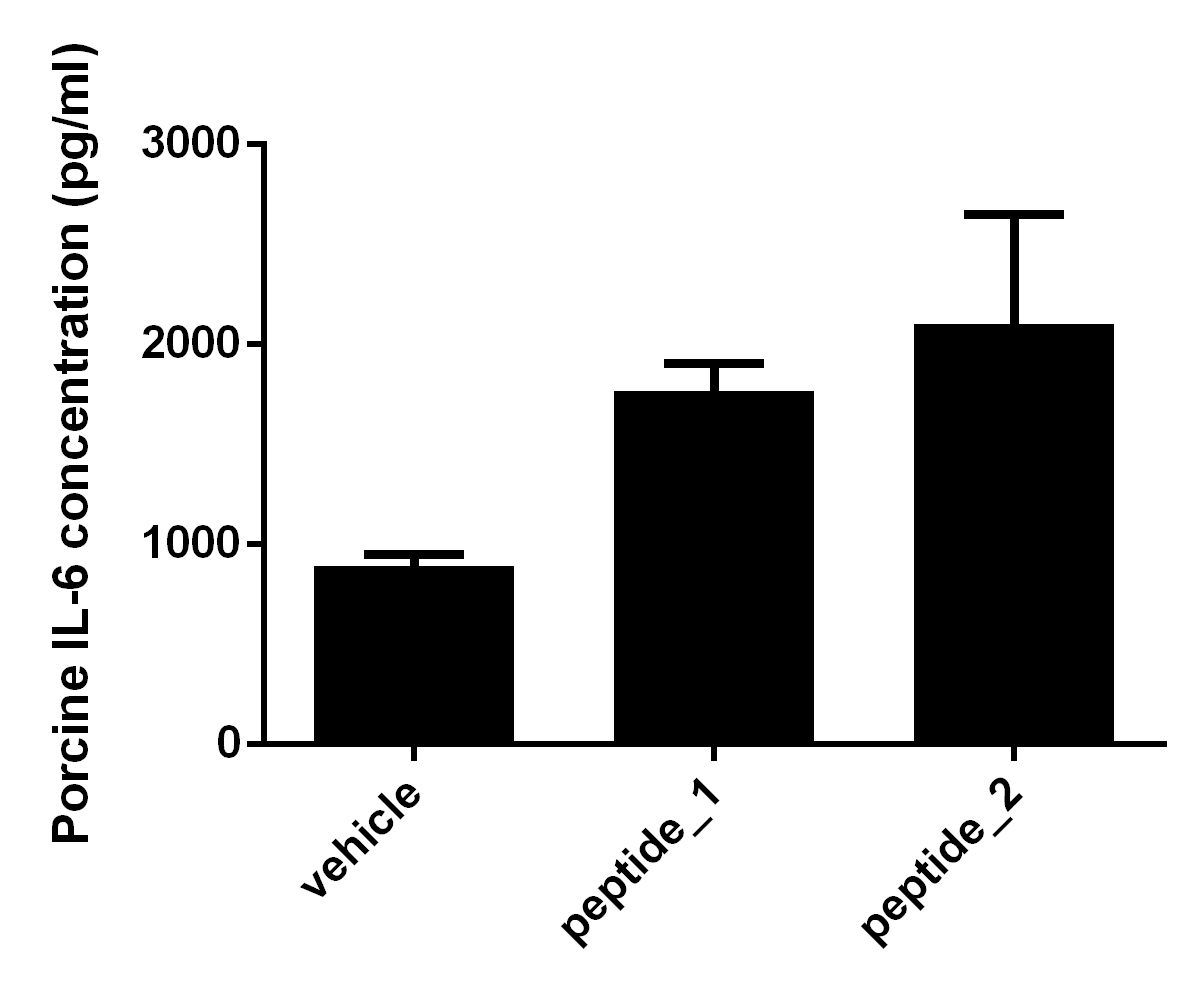
Porcine IL-6 Quantikine ELISA Kit Summary
Product Summary
Precision
Cell Culture Supernates, Serum, EDTA Plasma, Heparin Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 54.5 | 123 | 674 | 52.9 | 127 | 665 |
| Standard Deviation | 2.8 | 5.4 | 17.6 | 3.9 | 7.5 | 30.1 |
| CV% | 5.1 | 4.4 | 2.6 | 7.4 | 5.9 | 4.5 |
Recovery
The recovery of porcine IL-6 spiked to three levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Supernates (n=4) | 102 | 95-109 |
| EDTA Plasma (n=4) | 97 | 89-104 |
| Heparin Plasma (n=4) | 97 | 85-103 |
| Serum (n=4) | 96 | 87-110 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-6
Interleukin 6 (IL-6) is a pleiotropic, alpha -helical, 22 - 28 kDa phosphorylated and variably glycosylated cytokine that plays important roles in the acute phase reaction, inflammation, hematopoiesis, bone metabolism, and cancer progression (1-5). Mature porcine IL-6 is 183 amino acids (aa) in length and shares 58%, 39% and 39% aa sequence identity with human, mouse, and rat IL-6, respectively (6). Cells known to express IL-6 include CD8+ T cells, fibroblasts, synoviocytes, adipocytes, osteoblasts, megakaryocytes, endothelial cells (under the influence of endothelins), sympathetic neurons, cerebral cortex neurons, adrenal medulla chromaffin cells, retinal pigment cells, mast cells, keratinocytes, Langerhans cells, fetal and adult astrocytes, neutrophils, monocytes, eosinophils, colonic epithelial cells, B1 B cells and pancreatic islet beta cells (2, 7-29). IL-6 production is generally correlated with cell activation and is normally kept in control by glucocorticoids, catecholamines, and secondary sex steroids (2). Normal human circulating IL-6 is in the 1 pg/mL range, with slight elevations during the menstrual cycle, modest elevations in certain cancers, and large elevations after surgery (30-34).
Citations for Porcine IL-6 Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
66
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Analysis of the Model of Atherosclerosis Formation in Pig Hearts as a Result of Impaired Activity of DNA Repair Enzymes
Authors: Paslawski, R;Kowalczyk, P;Paslawska, U;Wi?niewski, J;Dzi?giel, P;Janiszewski, A;Kiczak, L;Zacharski, M;Gawdzik, B;Kramkowski, K;Szuba, A;
International journal of molecular sciences
Species: Porcine
Sample Types: Serum
-
Porcine circovirus type 2 infection inhibits macrophage M1 polarization induced by other pathogens via viral capsid protein and host gC1qR protein
Authors: Yang, X;Du, Q;Wang, X;Shi, J;Wang, T;Li, P;Zhong, J;Tong, D;Huang, Y;
Veterinary microbiology
Species: Porcine
Sample Types: Cell Culture Supernates
-
Aqueous cannabidiol ?-cyclodextrin complexed polymeric micelle nasal spray to attenuate in vitro and ex vivo SARS-CoV-2-induced cytokine storms
Authors: Changsan, N;Sawatdee, S;Suedee, R;Chunhachaichana, C;Srichana, T;
International journal of pharmaceutics
Species: Porcine
Sample Types: Tissue Homogenates
-
Mutating both relA and spoT of enteropathogenic Escherichia coli E2348/69 attenuates its virulence and induces interleukin 6 in vivo
Authors: JB Lee, SK Kim, D Han, JW Yoon
Frontiers in Microbiology, 2023-03-02;14(0):1121715.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Phytogenic feed additives alleviate pathogenic Escherichia coli-induced intestinal damage through improving barrier integrity and inhibiting inflammation in weaned pigs
Authors: SY Chang, MH Song, JH Lee, HJ Oh, YJ Kim, JW An, YB Go, DC Song, HA Cho, SY Cho, DJ Kim, MS Kim, HB Kim, JH Cho
Journal of animal science and biotechnology, 2022-09-02;13(1):107.
Species: Porcine
Sample Types: Serum
-
Levosimendan Ameliorates Cardiopulmonary Function but Not Inflammatory Response in a Dual Model of Experimental ARDS
Authors: R Rissel, M Gosling, J Kamuf, M Renz, R Ruemmler, A Ziebart, EK Hartmann
Biomedicines, 2022-04-29;10(5):.
Species: Porcine
Sample Types: Plasma
-
Effect of chronic and acute enterotoxigenic E. coli challenge on growth performance, intestinal inflammation, microbiome, and metabolome of weaned piglets
Authors: JX Boeckman, S Sprayberry, AM Korn, JS Suchodolsk, C Paulk, K Genovese, RR Rech, PR Giaretta, AK Blick, T Callaway, JJ Gill
Scientific Reports, 2022-03-23;12(1):5024.
Species: Porcine
Sample Types: Serum
-
Hemosiderin Accumulation in Liver Decreases Iron Availability in Tachycardia-Induced Porcine Congestive Heart Failure Model
Authors: M Kasztura, L Kiczak, U Pas?awska, J Bania, A Janiszewsk, A Tomaszek, M Zacharski, A Noszczyk-N, R Pas?awski, A Tabi?, P Kuropka, P Dzi?giel, P Ponikowski
International Journal of Molecular Sciences, 2022-01-18;23(3):.
Species: Porcine
Sample Types: Serum
-
Identification of Virulence Associated Region during Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus during Attenuation In Vitro: Complex Question with Different Strain Backgrounds
Authors: Y Jiang, W Tong, L Yu, L Li, F Gao, G Li, C Liu, P Chen, Q Shen, Y Zhang, Y Zhou, G Tong
Viruses, 2021-12-27;14(1):.
Species: Porcine
Sample Types: Serum
-
A novel pre-clinical strategy to deliver antimicrobial doses of inhaled nitric oxide
Authors: VS Michaelsen, RVP Ribeiro, E Brambate, A Ali, A Wang, L Pires, M Kawashima, Y Zhang, A Gazzalle, S Keshavjee, L Del Sorbo, M Cypel
PLoS ONE, 2021-10-13;16(10):e0258368.
Species: Porcine
Sample Types: Tissue Homogenates
-
Inhibition of MAVS Aggregation-Mediated Type-I Interferon Signaling by Foot-and-Mouth Disease Virus VP3
Authors: P Ekanayaka, BH Lee, A Weerawardh, K Chathurang, JH Park, JS Lee
Viruses, 2021-09-06;13(9):.
Species: Porcine
Sample Types: Cell Culture Supernates
-
A novel large animal model of smoke inhalation-induced acute respiratory distress syndrome
Authors: PD Leiphrakpa, HR Weber, A McCain, RR Matas, EM Duarte, KL Buesing
Respiratory Research, 2021-07-07;22(1):198.
Species: Porcine
Sample Types: BALF
-
SOFA Score, Hemodynamics and Body Temperature Allow Early Discrimination between Porcine Peritonitis-Induced Sepsis and Peritonitis-Induced Septic Shock
Authors: M Al-Obeidal, D Jarkovská, L Valešová, J Horák, J Jedli?ka, L Nalos, J Chvojka, J Švíglerová, J Kuncová, J Beneš, M Mat?jovi?, M Štengl
Journal of personalized medicine, 2021-02-28;11(3):.
Species: Porcine
Sample Types: Plasma
-
Hemodynamic effects of high frequency oscillatory ventilation with volume guarantee in a piglet model of respiratory distress syndrome
Authors: J Bhogal, AL Solevåg, M O'Reilly, TF Lee, C Joynt, LK Hornberger, GM Schmölzer, PY Cheung
PLoS ONE, 2021-02-16;16(2):e0246996.
Species: Porcine
Sample Types: Tissue Homogenates
-
Experimental lung injury induces cerebral cytokine mRNA production in pigs
Authors: J Kamuf, A Garcia Bar, A Ziebart, K Frauenknec, K Folkert, J Schwab, R Ruemmler, M Renz, D Cana, SC Thal, EK Hartmann
PeerJ, 2020-12-09;8(0):e10471.
Species: Porcine
Sample Types: Whole Blood
-
Protective effects of new femoral reaming techniques (Reamer irrigator aspirator, RIA I and II) on pulmonary function and posttraumatic contusion (CT morphology) - results from a standardized large animal model
Authors: S Halvachiza, M Teuben, M Lempert, Y Kalbas, N Cesarovic, M Lipiski, E Benninger, P Cinelli, R Pfeifer, HC Pape
Injury, 2020-10-06;0(0):.
Species: Porcine
Sample Types: BALF
-
Effects of sustained inflation pressure during�neonatal cardiopulmonary resuscitation of asphyxiated piglets
Authors: GH Shim, SY Kim, PY Cheung, TF Lee, M O'Reilly, GM Schmölzer
PLoS ONE, 2020-06-23;15(6):e0228693.
Species: Porcine
Sample Types: Tissue Homogenates
-
Substitution of warthog NF-kappaB motifs into RELA of domestic pigs is not sufficient to confer resilience to African swine fever virus
Authors: S McCleary, R Strong, RR McCarthy, JC Edwards, EL Howes, LM Stevens, PJ Sánchez-Co, A Núñez, S Watson, AJ Mileham, SG Lillico, C Tait-Burka, C Proudfoot, M Ballantyne, CBA Whitelaw, F Steinbach, HR Crooke
Sci Rep, 2020-06-02;10(1):8951.
Species: Porcine
Sample Types: Serum
-
Low Protein-High Carbohydrate Diets Alter Energy Balance, Gut Microbiota Composition and Blood Metabolomics Profile in Young Pigs
Authors: S Spring, H Premathila, U DeSilva, C Shili, S Carter, A Pezeshki
Sci Rep, 2020-02-24;10(1):3318.
Species: Porcine
Sample Types: Serum
-
Sepsis causes right ventricular myocardial inflammation independent of pulmonary hypertension in a porcine sepsis model
Authors: SE Pischke, S Hestenes, HT Johansen, H Fure, JF Bugge, A Espinoza, H Skulstad, T Edvardsen, E Fosse, TE Mollnes, PS Halvorsen, EW Nielsen
PLoS ONE, 2019-06-27;14(6):e0218624.
Species: Porcine
Sample Types: Plasma
-
Modulation of the somatotropic axis, adiponectin and cytokine secretion during highly pathogenic porcine reproductive and respiratory syndrome virus type 1 (HP-PRRSV-1) infection
Authors: R Saleri, V Cavalli, L Ferrari, G Ogno, E Canelli, P Martelli, P Borghetti
Res. Vet. Sci., 2019-04-11;124(0):263-269.
Species: Porcine
Sample Types: Plasma
-
PO2 oscillations induce lung injury and inflammation
Authors: S Boehme, EK Hartmann, T Tripp, SC Thal, M David, D Abraham, JE Baumgardne, K Markstalle, KU Klein
Crit Care, 2019-03-27;23(1):102.
Species: Porcine
Sample Types: Plasma
-
Inactivating hepatitis C virus in donor lungs using light therapies during normothermic ex vivo lung perfusion
Authors: M Galasso, JJ Feld, Y Watanabe, M Pipkin, C Summers, A Ali, R Qaqish, M Chen, RVP Ribeiro, K Ramadan, L Pires, VS Bagnato, C Kurachi, V Cherepanov, G Moonen, A Gazzalle, TK Waddell, M Liu, S Keshavjee, BC Wilson, A Humar, M Cypel
Nat Commun, 2019-01-29;10(1):481.
Species: Porcine
Sample Types: Tissue Homogenates
-
Medium-Chain Triglycerides Attenuate Liver Injury in Lipopolysaccharide-Challenged Pigs by Inhibiting Necroptotic and Inflammatory Signaling Pathways
Authors: L Zhang, X Wang, S Chen, S Wang, Z Tu, G Zhang, H Zhu, X Li, J Xiong, Y Liu
Int J Mol Sci, 2018-11-21;19(11):.
Species: Porcine
Sample Types: Serum
-
Neuroimmunological Implications of Subclinical Lipopolysaccharide from Salmonella Enteritidis
Authors: A Miko?ajczy, D Z?otkowska
Int J Mol Sci, 2018-10-22;19(10):.
Species: Porcine
Sample Types: Serum
-
PKD regulates actin polymerization, neutrophil deformability, and transendothelial migration in response to fMLP and trauma
Authors: C Wille, T Eiseler, ST Langenberg, J Richter, K Mizuno, P Radermache, U Knippschil, M Huber-Lang, T Seufferlei, S Paschke
J. Leukoc. Biol., 2018-04-14;0(0):.
Species: Porcine
Sample Types: Plasma
-
Inflammatory markers before and after farrowing in healthy sows and in sows affected with postpartum dysgalactia syndrome
Authors: M Kaiser, M Jacobson, PH Andersen, P Bækbo, JJ Cerón, J Dahl, D Escribano, S Jacobsen
BMC Vet. Res., 2018-03-12;14(1):83.
Species: Porcine
Sample Types: Serum
-
Sodium lactate improves renal microvascular thrombosis compared to sodium bicarbonate and 0.9% NaCl in a porcine model of endotoxic shock: an experimental randomized open label controlled study
Authors: T Duburcq, A Durand, A Tournoys, V Gnemmi, V Gmyr, F Pattou, M Jourdain, F Tamion, E Besnier, S Préau, E Parmentier, D Mathieu, J Poissy, R Favory
Ann Intensive Care, 2018-02-14;8(1):24.
Species: Porcine
Sample Types: Serum
-
Influence of zinc supplementation on immune parameters in weaned pigs
Authors: V Kloubert, K Blaabjerg, TS Dalgaard, HD Poulsen, L Rink, I Wessels
J Trace Elem Med Biol, 2018-01-31;0(0):.
Species: Porcine
Sample Types: Whole Blood
Applications: ELISA Capture -
Beneficial effects of inhaled nitric oxide with intravenous steroid in an ischemia-reperfusion model involving aortic clamping
Authors: W Gozdzik, S Zielinski, M Zielinska, K Ratajczak, P Skrzypczak, S Rodziewicz, A Kübler, K Löfström, P Dziegiel, M Olbromski, B Adamik, S Ryniak, P Harbut, J Albert, C Frostell
Int J Immunopathol Pharmacol, 2018-01-01;32(0):3946320177514.
Species: Porcine
Sample Types: Plasma
-
Septic porcine blood does not further activate coagulation during in vitro membrane oxygenation
Authors: Rolf Rossaint
Eur J Cardiothorac Surg, 2017-03-01;0(0):.
Species: Porcine
Sample Types: Plasma
-
Characterization of blunt chest trauma in a long-term porcine model of severe multiple trauma
Sci Rep, 2016-12-21;6(0):39659.
Species: Porcine
Sample Types: Serum
-
An Immunomodulatory Device Improves Insulin Resistance in Obese Porcine Model of Metabolic Syndrome
Authors: Angela J Westover
J Diabetes Res, 2016-10-13;2016(0):3486727.
Species: Porcine
Sample Types: Plasma
-
Soluble T cell immunoglobulin mucin domain 3 is shed from CD8+ T cells by the sheddase ADAM10, is increased in plasma during untreated HIV infection, and correlates with HIV disease progression.
Authors: Clayton K, Douglas-Vail M, Nur-ur Rahman A, Medcalf K, Xie I, Chew G, Tandon R, Lanteri M, Norris P, Deeks S, Ndhlovu L, Ostrowski M
J Virol, 2015-01-21;89(7):3723-36.
Species: Porcine
Sample Types: Plasma
-
Low tidal volume pressure support versus controlled ventilation in early experimental sepsis in pigs.
Authors: Ziebart A, Hartmann E, Thomas R, Liu T, Duenges B, Schad A, Bodenstein M, Thal S, David M
Respir Res, 2014-09-06;15(0):101.
Species: Porcine
Sample Types: Plasma
-
Ex vivo adenoviral vector gene delivery results in decreased vector-associated inflammation pre- and post-lung transplantation in the pig.
Authors: Yeung JC, Wagnetz D, Cypel M, Rubacha M, Koike T, Chun YM, Hu J, Waddell TK, Hwang DM, Liu M, Keshavjee S
Mol. Ther., 2012-03-27;20(6):1204-11.
Species: Porcine
Sample Types: Plasma
-
Efficacy of linezolid compared to vancomycin in an experimental model of pneumonia induced by methicillin-resistant Staphylococcus aureus in ventilated pigs.
Authors: Martinez-Olondris P, Rigol M, Soy D, Guerrero L, Agusti C, Quera MA, Li Bassi G, Esperatti M, Luque N, Liapikou M, Filella X, Marco F, de la Bellacasa JP, Torres A
Crit. Care Med., 2012-01-01;40(1):162-8.
Species: Porcine
Sample Types: Serum
-
Dissecting the effects of lipopolysaccharides from nonlipopolysaccharide molecules in experimental porcine meningococcal sepsis.
Authors: Hellerud BC, Nielsen EW, Thorgersen EB, Lindstad JK, Pharo A, Tonnessen TI, Castellheim A, Mollnes TE, Brandtzaeg P
Crit. Care Med., 2010-06-01;38(6):1467-74.
Species: Porcine
Sample Types: Plasma
-
Comparative measurement of cell-mediated immune responses of swine to the M and N proteins of porcine reproductive and respiratory syndrome virus.
Authors: Jeong HJ, Song YJ, Lee SW
Clin. Vaccine Immunol., 2010-02-03;17(4):503-12.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Mutations in classical swine fever virus NS4B affect virulence in swine.
Authors: Fernandez-Sainz I, Gladue DP, Holinka LG
J. Virol., 2009-11-18;84(3):1536-49.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine.
Authors: McILwain RB, Timpa JG, Kurundkar AR
Lab. Invest., 2009-11-09;90(1):128-39.
Species: Porcine
Sample Types: Plasma
-
Cardiac Function and the Proinflammatory Cytokine Response After Recovery From Cardiac Arrest in Swine.
Authors: Niemann JT, Rosborough JP, Youngquist S, Shah AP, Lewis RJ, Phan QT, Filler SG
J. Interferon Cytokine Res., 2009-11-01;0(0):.
Species: Porcine
Sample Types: Plasma
-
CD14 inhibition efficiently attenuates early inflammatory and hemostatic responses in Escherichia coli sepsis in pigs.
Authors: Thorgersen EB, Hellerud BC, Nielsen EW, Barratt-Due A, Fure H, Lindstad JK, Pharo A, Fosse E, Tonnessen TI, Johansen HT, Castellheim A, Mollnes TE
FASEB J., 2009-10-19;24(3):712-22.
Species: Porcine
Sample Types: Plasma
-
Bbeta15-42 (FX06) reduces pulmonary, myocardial, liver, and small intestine damage in a pig model of hemorrhagic shock and reperfusion.
Authors: Roesner JP, Petzelbauer P, Koch A, Tran N, Iber T, Vagts DA, Scheeren TW, Vollmar B, Noldge-Schomburg GE, Zacharowski K
Crit. Care Med., 2009-02-01;37(2):598-605.
Species: Porcine
Sample Types: Plasma
-
Effect of linezolid compared with glycopeptides in methicillin-resistant Staphylococcus aureus severe pneumonia in piglets.
Authors: Luna CM, Bruno DA, Garcia-Morato J, Mann KC, Risso Patron J, Sagardia J, Absi R, Garcia Bottino M, Marchetti D, Famiglietti A, Baleztena M, Biancolini C
Chest, 2009-01-13;135(6):1564-71.
Species: Porcine
Sample Types: Serum
-
Inhibition of complement and CD14 attenuates the Escherichia coli-induced inflammatory response in porcine whole blood.
Authors: Thorgersen EB, Pharo A, Haverson K, Axelsen AK, Gaustad P, Kotwal GJ, Sfyroera G, Mollnes TE
Infect. Immun., 2008-12-01;77(2):725-32.
Species: Porcine
Sample Types: Whole Blood
-
Blood concentrations of the cytokines IL-1beta, IL-6, IL-10, TNF-alpha and IFN-gamma during experimentally induced swine dysentery.
Authors: Kruse R, Essen-Gustavsson B, Fossum C, Jensen-Waern M
Acta Vet. Scand., 2008-08-12;50(0):32.
Species: Porcine
Sample Types: Serum
-
Effects of glucocorticoids in ventilated piglets with severe pneumonia.
Authors: Sibila O, Luna CM, Agusti C, Baquero S, Gando S, Patron JR, Morato JG, Absi R, Bassi N, Torres A
Eur. Respir. J., 2008-05-28;32(4):1037-46.
Species: Porcine
Sample Types: Serum
-
Cytokine secretion depends on Galalpha(1,3)Gal expression in a pig-to-human whole blood model.
Authors: Saethre M, Schneider MK, Lambris JD, Magotti P, Haraldsen G, Seebach JD, Mollnes TE
J. Immunol., 2008-05-01;180(9):6346-53.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Experimental Pseudomonas aeruginosa pneumonia: evaluation of the associated inflammatory response.
Authors: Sibila O, Agusti C, Torres A, Baquero S, Gando S, Patron JR, Morato JG, Goffredo DH, Bassi N, Luna CM
Eur. Respir. J., 2007-09-05;30(6):1167-72.
Species: Porcine
Sample Types: Serum
-
Experimental severe Pseudomonas aeruginosa pneumonia and antibiotic therapy in piglets receiving mechanical ventilation.
Authors: Luna CM, Baquero S, Gando S, Patron JR, Morato JG, Sibila O, Absi R, Famiglietti A, Vay CA, Von Stecher F, Agusti C, Torres A
Chest, 2007-08-01;132(2):523-31.
Species: Porcine
Sample Types: Serum
-
The fibrin-derived peptide Bbeta15-42 is cardioprotective in a pig model of myocardial ischemia-reperfusion injury.
Authors: Roesner JP, Petzelbauer P, Koch A, Mersmann J, Zacharowski PA, Boehm O, Reingruber S, Pasteiner W, Mascher D, Wolzt M, Barthuber C, Noldge-Schomburg GE, Scheeren TW, Zacharowski K
Crit. Care Med., 2007-07-01;35(7):1730-5.
Species: Porcine
Sample Types: Plasma
-
Sex difference in link between interleukin-6 and stress.
Authors: Jankord R, Turk JR, Schadt JC, Casati J, Ganjam VK, Price EM, Keisler DH, Laughlin MH
Endocrinology, 2007-05-17;148(8):3758-64.
Species: Porcine
Sample Types: Plasma
-
Complete overlap of interleukin-31 receptor A and oncostatin M receptor beta in the adult dorsal root ganglia with distinct developmental expression patterns.
Authors: Bando T, Morikawa Y, Komori T, Senba E
Neuroscience, 2006-08-22;142(4):1263-71.
Species: Porcine
Sample Types: Plasma
-
Dietary fatty acid composition rather than vitamin E supplementation influence ex vivo cytokine and eicosanoid response of porcine alveolar macrophages.
Authors: Moller S, Lauridsen C
Cytokine, 2006-08-17;35(1):6-12.
Species: Porcine
Sample Types: Cell Culture Supernates
-
A novel antidote-controlled anticoagulant reduces thrombin generation and inflammation and improves cardiac function in cardiopulmonary bypass surgery.
Authors: Nimjee SM, Keys JR, Pitoc GA, Quick G, Rusconi CP, Sullenger BA
Mol. Ther., 2006-06-09;14(3):408-15.
Species: Porcine
Sample Types: Plasma
-
Effects of therapeutic bronchoalveolar lavage and partial liquid ventilation on meconium-aspirated newborn piglets.
Authors: Jeng MJ, Soong WJ, Lee YS, Chang HL, Shen CM, Wang CH, Yang SS, Hwang B
Crit. Care Med., 2006-04-01;34(4):1099-105.
Species: Porcine
Sample Types: Serum
-
Effects of heparin-binding protein (CAP37/azurocidin) in a porcine model of Actinobacillus pleuropneumoniae-induced pneumonia.
Authors: Lauritzen B, Lykkesfeldt J, Djurup R, Flodgaard H, Svendsen O
Pharmacol. Res., 2005-06-01;51(6):509-14.
Species: Porcine
Sample Types: Plasma
-
Protection against acute porcine lung ischemia/reperfusion injury by systemic preconditioning via hind limb ischemia.
Authors: Waldow T, Alexiou K, Witt W, Albrecht S, Wagner F, Knaut M, Matschke K
Transpl. Int., 2005-02-01;18(2):198-205.
Species: Porcine
Sample Types: Plasma
-
Conjugated linoleic acid attenuates the production and gene expression of proinflammatory cytokines in weaned pigs challenged with lipopolysaccharide.
Authors: Changhua L, Jindong Y, Defa L, Lidan Z, Shiyan Q, Jianjun X
J. Nutr., 2005-02-01;135(2):239-44.
Species: Porcine
Sample Types: Serum
-
Decreased protein accretion in pigs with viral and bacterial pneumonia is associated with increased myostatin expression in muscle.
Authors: Escobar J, Van Alstine WG, Baker DH
J. Nutr., 2004-11-01;134(11):3047-53.
Species: Porcine
Sample Types: Serum
-
Attenuation of reperfusion-induced systemic inflammation by preconditioning with nitric oxide in an in situ porcine model of normothermic lung ischemia.
Authors: Waldow T, Alexiou K, Witt W, Wagner FM, Gulielmos V, Matschke K, Knaut M
Chest, 2004-06-01;125(6):2253-9.
Species: Porcine
Sample Types: Plasma
-
Adiponectin differentially regulates cytokines in porcine macrophages.
Authors: Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME
Biochem. Biophys. Res. Commun., 2004-04-09;316(3):924-9.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Classical swine fever virus induces proinflammatory cytokines and tissue factor expression and inhibits apoptosis and interferon synthesis during the establishment of long-term infection of porcine vascular endothelial cells.
Authors: Bensaude E, Turner JL, Wakeley PR, Sweetman DA, Pardieu C, Drew TW, Wileman T, Powell PP
J. Gen. Virol., 2004-04-01;85(0):1029-37.
Species: Porcine
Sample Types: Cell Culture Supernates
-
Standardized lung recruitment during high frequency and conventional ventilation: similar pathophysiologic and inflammatory responses in an animal model of respiratory distress syndrome.
Authors: Krishnan RK, Meyers PA, Worwa C, Goertz R, Schauer G, Mammel MC
Intensive Care Med, 2004-03-02;30(6):1195-203.
Species: Porcine
Sample Types: Serum
-
Chronic porcine two-hit model with hemorrhagic shock and Pseudomonas aeruginosa sepsis.
Authors: Eissner B, Matz K, Smorodchenko A, Roschmann A, v Specht BU
Eur Surg Res, 2002-01-01;34(1):61-7.
Species: Porcine
Sample Types: Plasma
FAQs
-
Assay Diluent RD1-63 contains visible precipitate. Is this acceptable to use?
A small amount of precipitate is expected and acceptable in Assay Diluent RD1-63. Variability has been observed between vials and diluent lots. It may not fully resolubilize but this will not impact assay performance. It is not recommended to filter the precipitate.
Reviews for Porcine IL-6 Quantikine ELISA Kit
Average Rating: 5 (Based on 1 Review)
Have you used Porcine IL-6 Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: