ProDots Recombinant Human IL-2 GMP Protein
ProDots Recombinant Human IL-2 GMP Protein Summary
Product Specifications
The specific activity of ProDots Recombinant Human IL-2 GMP is >5.0 x 106 IU/mg, which is calibrated against the human IL-2 WHO International Standard (NIBSC code: 86/500).
Ala21-Thr153, with an N-terminal Met
Produced using non-animal reagents in an animal-free laboratory.
Manufactured and tested under cGMP guidelines.
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
PRD202-GMP
| Formulation | Lyophilized from an acetonitrile-based formulation using proprietary excipients. |
| Reconstitution | Reconstitute immediately prior to use with up to 25 mL of cell culture media. |
| Shipping | The product is shipped with polar packs. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Store material as supplied, unopened, and unreconstituted at 2-8 °C until use. Stability is a minimum of 8 weeks when stored at 2-8 °C as supplied. Refer to lot specific COA for the Use by Date. |
Scientific Data
 View Larger
View Larger
GMP-grade ProDots®Recombinant Human IL-2 (Catalog # PRD202-GMP) stimulates cell proliferation of the CTLL‑2 mouse cytotoxic T cell line. The ED50 for this effect is 0.05-0.25 ng/mL. Three independent lots were tested for activity and plotted on the same graph to show lot-to-lot consistency of GMP IL-2.
 View Larger
View Larger
Comparative tests for function within biologically relevant model systems show equivalence of GMP ProDot™ Proteins and GMP Proteins. Activation of PBMCs after 9 days in culture with ExCellerate Human T Cell Media CCM030, GMP Cloudz Human T Cell Activation Kit CLD001-GMP and GMP IL-2 ProDot (PRD202-GMP) or GMP IL-2 202-GMP shows comparable A. T cell fold expansion and B. phenotype.
Reconstitution Calculator
Background: IL-2
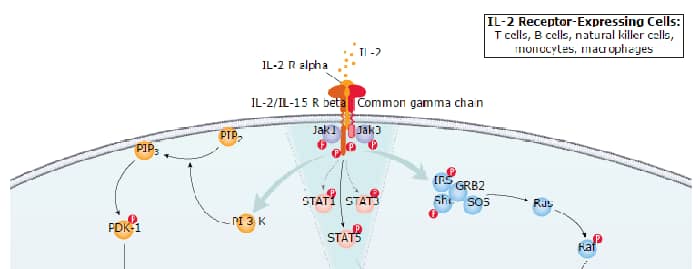
Interleukin-2 (IL-2) is a O-glycosylated, four alpha -helix bundle cytokine that has potent stimulatory activity for antigen-activated T cells. It is expressed by CD4+ and CD8+ T cells, gamma δ T cells, B cells, dendritic cells, and eosinophils (1-3). Mature human IL-2 shares 56% and 66% aa sequence identity with mouse and rat IL-2, respectively. Human and mouse IL-2 exhibit cross-species activity (4). The receptor for IL-2 consists of three subunits that are present on the cell surface in varying preformed complexes (5-7). The 55 kDa IL-2 R alpha is specific for IL-2 and binds with low affinity. The 75 kDa IL-2 R beta, which is also a component of the IL-15 receptor, binds IL-2 with intermediate affinity. The 64 kDa common gamma chain gamma c/IL-2 R gamma, which is shared with the receptors for IL-4, -7, -9, -15, and -21, does not independently interact with IL-2. Upon ligand binding, signal transduction is performed by both IL-2 R beta and gamma c. IL-2 is best known for its autocrine and paracrine activity on T cells. It drives resting T cells to proliferate and induces IL-2 and IL-2 R alpha synthesis (1, 2). It contributes to T cell homeostasis by promoting the Fas-induced death of naïve CD4+ T cells but not activated CD4+ memory lymphocytes (8). IL-2 plays a central role in the expansion and maintenance of regulatory T cells, although it inhibits the development of Th17 polarized cells (9-11). Thus, IL-2 may be a key cytokine in the natural suppression of autoimmunity (12, 13).
- Ma, A. et al. (2006) Annu. Rev. Immunol. 24:657.
- Gaffen, S.L. and K.D. Liu (2004) Cytokine 28:109.
- Taniguchi, T. et al. (1983) Nature 302:305.
- Mosmann, T.R. et al. (1987) J. Immunol. 138:1813.
- Liparoto, S.F. et al. (2002) Biochemistry 41:2543.
- Wang, X. et al. (2005) Science 310:1159.
- Bodnar, A. et al. (2008) Immunol. Lett. 116:117.
- Jaleco, S. et al. (2003) J. Immunol. 171:61.
- Malek, T.R. (2003) J. Leukoc. Biol. 74:961.
- Laurence, A. et al. (2007) Immunity 26:371.
- Kryczek, I. et al. (2007) J. Immunol. 178:6730.
- Afzali, B. et al. (2007) Clin. Exp. Immunol. 148:32.
- Fehervari, Z. et al. (2006) Trends Immunol. 27:109.
Manufacturing Specifications
GMP ProteinsR&D Systems, a Bio-Techne Brand's GMP proteins are produced according to relevant sections of the following documents: WHO TRS, No. 822, 1992 Annex 1, Good Manufacturing Practices for Biological Products; USP Chapter 1043, Ancillary Materials for Cell, Gene and Tissue-Engineered Products and USP Chapter 92, Growth Factors and Cytokines Used in Cell Therapy Manufacturing.
R&D Systems' quality focus includes:
- Manufactured and tested under an ISO 9001:2015 and ISO 13485:2016 certified quality system
- Documented processes and QA control of documentation and process changes
- Personnel training programs
- Raw material testing and vendor qualification/monitoring
- Fully validated equipment, processes and test methods
- Equipment calibration schedules using a computerized calibration program
- Facility maintenance, safety programs and pest control
- Material review process for variances
- Monitoring of stability over product shelf-life
R&D Systems strives to provide our customers with the analytical characteristics of each product so that customers may determine whether our products are appropriate for their research. The Certificate of Analysis provided contains the following lot specific information:
- N-terminal amino acid analysis, SDS-PAGE analysis, and endotoxin level (as determined by LAL assay) performed on each bulk QC lot, not on individual bottlings of each QC lot
- Post-bottling lot-specific bioassay results (compliance with an established range) and results of microbial bioburden testing (using broth culture, Sabourand's dextrose and blood agar plates with results reported at 3 days and at 7 days)
- Host Cell Protein testing performed by ELISA
- Mycoplasma testing by ribosomal RNA hybridization assay
Additional testing and documentation requested by the customer can be arranged at an additional cost. Testing may include, but is not limited to, USP< 61> bioburden testing, positive identity testing, testing for adventitious agents and testing for residual host cell content.
Production records and facilities are available for examination by appropriate personnel on-site at R&D Systems in Minneapolis, Minnesota USA.
R&D Systems sells GMP grade products for preclinical or clinical ex vivo use. They are not for in vivo use. Please read the following End User Terms prior to using this product.
Animal-Free Manufacturing Conditions
Our dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers in a dedicated cold storage room.
Quality Assurance
- Low Endotoxin Level.
- No impairment of biological activity.
- High quality product obtained under stringent conditions.
Product Specific Notices
The GMP ProDot bag(s) should be used after proper inspection. Assess the bags after media is added to the bag either through the weldable port or needless valve. Inspect the bag to ensure complete dissolution of the GMP ProDot, and visually inspect seals and corners for a complete seal.
GMP ProDots are fragile. Please handle with care. If breakage of a ProDot is observed, there is no integrity lost, and can be used as indicated.
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for ProDots Recombinant Human IL-2 GMP Protein
There are currently no reviews for this product. Be the first to review ProDots Recombinant Human IL-2 GMP Protein and earn rewards!
Have you used ProDots Recombinant Human IL-2 GMP Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image