TGF-beta 1 Antibody Summary
2% cross-reactivity with recombinant human (rh) TGF‑ beta 3 and recombinant amphibian TGF‑ beta 5 and no cross-reactivity with recombinant porcine TGF-beta 2 or rhTGF-beta 2 is observed.
Applications
under non-reducing conditions only
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
TGF‑ beta 1 in HEK293 Human Cell Line. TGF-beta 1 was detected in immersion fixed HEK293 human embryonic kidney cell line using Mouse Anti-TGF-beta 1 Monoclonal Antibody (Catalog # MAB240) at 3 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 493-conjugated Anti-Mouse IgG Secondary Antibody (green; Catalog # NL009) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
TGF-beta 1 in Human Prostate Cancer Tissue. TGF-beta 1 was detected in immersion fixed paraffin-embedded sections of human prostate cancer tissue using Mouse Anti- TGF-beta 1 Monoclonal Antibody (Catalog # MAB240) at 25 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Mouse HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS002) and counterstained with hematoxylin (blue). Specific labeling was localized to the cytoplasm of epithelial cells in the prostate gland. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
Detection of TGF‑ beta 1 in PC‑3 Human Cell Line by Flow Cytometry. PC-3 human prostate cancer cell line was stained with Mouse Anti-TGF-beta 1 Monoclonal Antibody (Catalog # MAB240, filled histogram) or isotype control antibody (Catalog # MAB002, open histogram), followed by Allophycocyanin-conjugated Anti-Mouse IgG F(ab')2Secondary Antibody (Catalog # F0101B). To facilitate intracellular staining, cells were fixed with paraformaldehyde and permeabilized with saponin.
 View Larger
View Larger
TGF‑ beta 1 Inhibition of IL‑4-dependent Cell Proliferation and Neutralization by TGF‑ beta 1 Antibody. Recombinant Human TGF-beta 1 (Catalog # 240-B) inhibits Recombinant Mouse IL-4 (Catalog # 404-ML) induced proliferation in the HT-2 mouse T cell line in a dose-dependent manner (orange line). Inhibition of Recombinant Mouse IL-4 (7.5 ng/mL) activity elicited by Recombinant Human TGF-beta 1 (0.25 ng/mL) is neutralized (green line) by increasing concentrations of Mouse Anti-TGF-beta 1 Monoclonal Antibody (Catalog # MAB240). The ND50 is typically 0.3-1.0 µg/mL.
 View Larger
View Larger
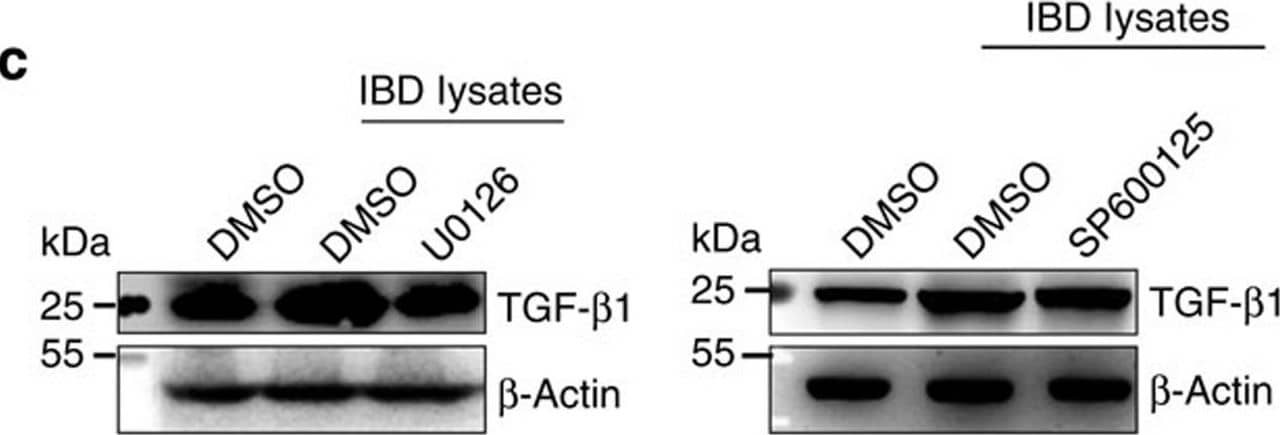
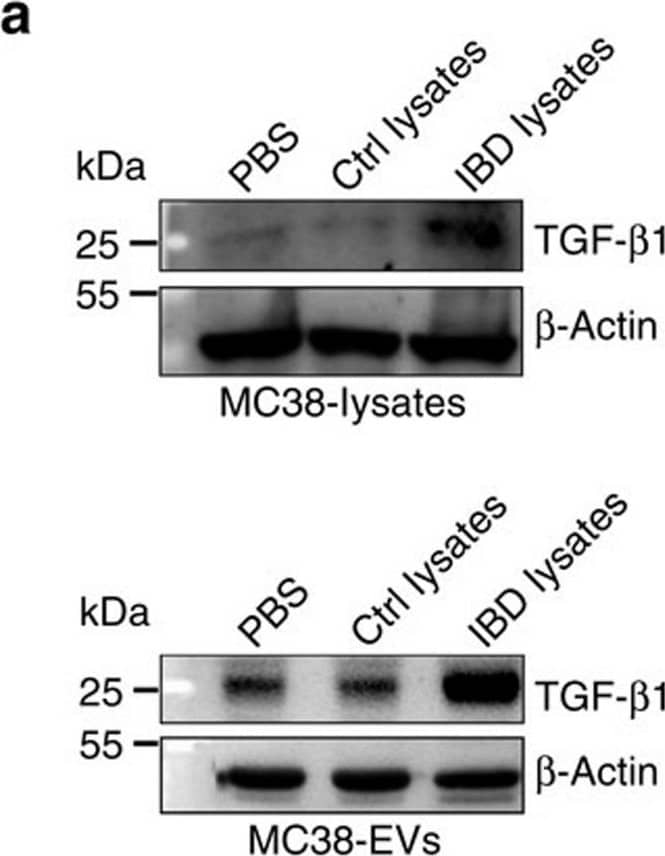
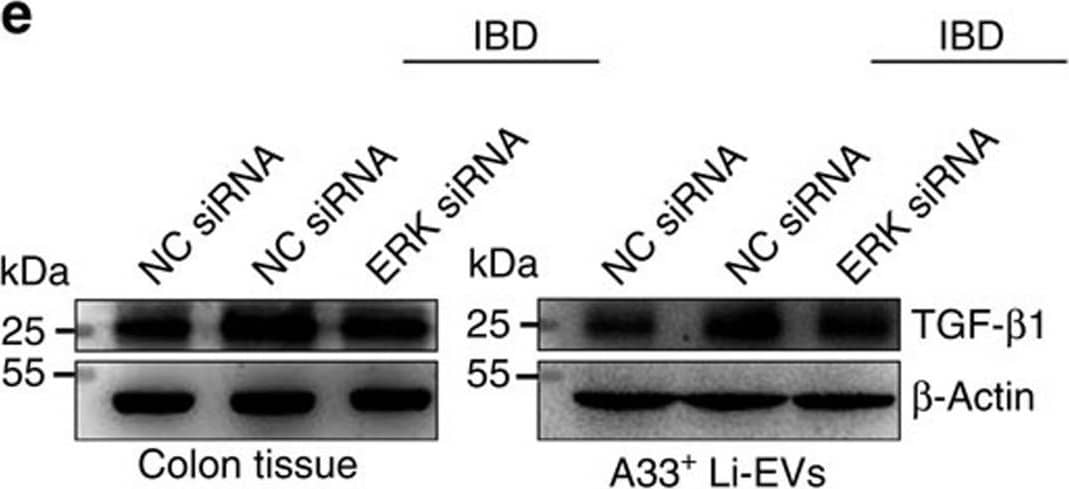
Detection of Mouse TGF-beta 1 by Western Blot ERK and TGF-beta 1 levels in IBD-A33+ Li-EVs.(a) MC38 cells were treated with 1 mg ml−1 IBD lysates or Ctrl lysates for 24 h. TGF-beta 1 in MC38 cells or MC38-EVs was measured using western blot analysis. (b) MC38 cells were treated with 1 mg ml−1 IBD lysates or Ctrl lysates for the indicated times. Activation of ERK, JNK, p38 and ATK in MC38 cells was measured using western blot analysis. (c) MC38 cells were pretreated with DMSO, 10 μM ERK-specific inhibitor U0126 or JNK-specific inhibitor SP600125 for 30 min and then treated with 1 mg ml−1 IBD lysates or Ctrl lysates for 24 h. TGF-beta 1 in MC38-EVs was measured by western blot analysis. (d) Activation of ERK, JNK, p38 and ATK in colon tissues from healthy control or IBD mice was measured using western blot analysis. (e,f) Mice were fed with 2% DSS solution on day 0. Mice received intrarectal injection with 20 μg cholesterol-conjugated ERK or NC siRNA every other day on days 0–11. Twenty-four hours after the last injection, mice were killed. TGF-beta 1 in colon tissues and A33+ Li-EVs was measured using western blot analysis (e). The body weights of mice were measured daily (f). Ctrl group, mice that received normal drinking water. (a–e) Data are representative of three independent experiments; (f) data are presented as the mean±s.d. from one of the two independent experiments (n=5 per group). P values were generated by two-way ANOVA, followed by Newman–Keuls multiple comparison test using GraphPad Prism 5 (**P<0.01, ***P<0.001), IBD+NC siRNA versus IBD+ERK siRNA. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27721471), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse TGF-beta 1 by Western Blot ERK and TGF-beta 1 levels in IBD-A33+ Li-EVs.(a) MC38 cells were treated with 1 mg ml−1 IBD lysates or Ctrl lysates for 24 h. TGF-beta 1 in MC38 cells or MC38-EVs was measured using western blot analysis. (b) MC38 cells were treated with 1 mg ml−1 IBD lysates or Ctrl lysates for the indicated times. Activation of ERK, JNK, p38 and ATK in MC38 cells was measured using western blot analysis. (c) MC38 cells were pretreated with DMSO, 10 μM ERK-specific inhibitor U0126 or JNK-specific inhibitor SP600125 for 30 min and then treated with 1 mg ml−1 IBD lysates or Ctrl lysates for 24 h. TGF-beta 1 in MC38-EVs was measured by western blot analysis. (d) Activation of ERK, JNK, p38 and ATK in colon tissues from healthy control or IBD mice was measured using western blot analysis. (e,f) Mice were fed with 2% DSS solution on day 0. Mice received intrarectal injection with 20 μg cholesterol-conjugated ERK or NC siRNA every other day on days 0–11. Twenty-four hours after the last injection, mice were killed. TGF-beta 1 in colon tissues and A33+ Li-EVs was measured using western blot analysis (e). The body weights of mice were measured daily (f). Ctrl group, mice that received normal drinking water. (a–e) Data are representative of three independent experiments; (f) data are presented as the mean±s.d. from one of the two independent experiments (n=5 per group). P values were generated by two-way ANOVA, followed by Newman–Keuls multiple comparison test using GraphPad Prism 5 (**P<0.01, ***P<0.001), IBD+NC siRNA versus IBD+ERK siRNA. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27721471), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
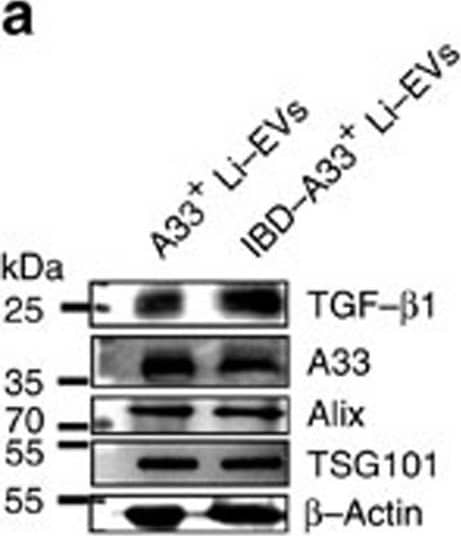
Detection of Mouse TGF-beta 1 by Western Blot A33+ Li-EVs alleviate IBD through TGF-beta 1 signalling.(a) A33+ Li-EVs were isolated from healthy control or 2% DSS-induced IBD mice. TGF-beta 1, A33 and beta -Actin were detected by western blot analysis. (b) The CD4+ T-cell proliferation assay was performed as described above in the presence of 30 μg ml−1 Ctrl or IBD-A33+ Li-EVs, and the results were statistically analysed (n=9). (c–f) Mice were fed with drinking water containing 2% DSS on day 0. On days −2 and 2, the mice were intravenously treated with the indicated dose of A33+ Li-EVs. The body weights were measured daily (c). Appearance (left) and statistical analysis (right) of colonic length on days 11 (d). Histological appearance on day 11. Representative colonic sections stained with haematoxylin and eosine (H&E). In the PBS group, the right image is a magnified region of the left image (e). IL-1 beta, IL-6, TNF-alpha, IL-10, IL-22 levels and MPO activity in colon tissue were measured on day 11 (f). (g) IBD mice were treated with 100 μg A33+ Li-EVs, IBD-A33+ Li-EVs and Sp-EVs on days −2 and 2. The body weights were measured daily. (h) TGF-beta 1 in A33+ Li-EVs and Sp-EVs was detected using western blot analysis. (i) IBD was introduced into Smad3+/− mice and treated with 100 μg A33+ Li-EVs and IBD-A33+ Li-EVs on days −2 and 2. The body weights were measured daily. Ctrl group, mice received normal drinking water; PBS group, mice with drinking water containing 2% DSS and intravenously treated with PBS on days −2 and 2. (a,b,d–f) Data are representative of three independent experiments or shown as mean values±s.e.m. pooled from three independent experiments; (c,g,i) data are presented as the mean±s.d. from one of the three independent experiments (n=5 per group). P values were generated by one-way ANOVA in b,d,f, or two-way ANOVA in c,g,i, followed by Newman–Keuls multiple comparison test using GraphPad Prism 5 (*P<0.05, **P<0.01, ***P<0.001), corresponding colour indicating the relevant group versus PBS group in c, versus PBS group in d,f; IBD-A33+ Li-EVs versus A33+ Li-EVs in g. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27721471), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
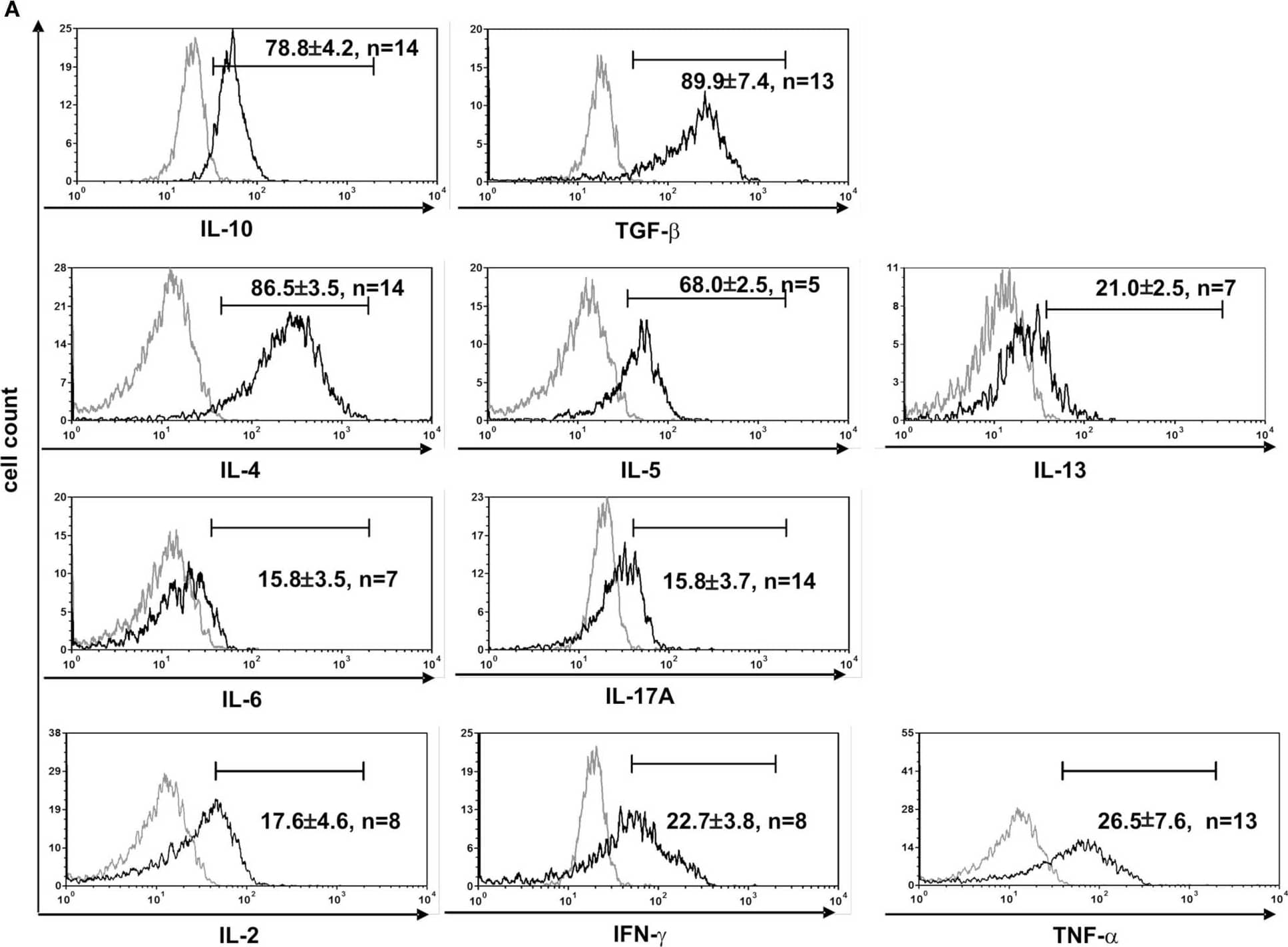
Detection of Human TGF-beta 1 by Flow Cytometry CD4+ Vδ1+ T-cell clones have a TH0-cytokine profile and are effector-memory cells. (A) CD4+ Vδ1+ clones produce regulatory IL-10 and TGF-beta, TH2 cytokines IL-4, -5, and -13, and to a lesser extent proinflammatory IL-17A and IL-6, and the TH1-cytokines IL-2, IFN-gamma, and TNF-alpha after stimulation with PMA/ionomycin. (B) CD4+ Vδ1+ clones were not naïve but belonged to the effector memory; they were CD45RA−, CD27−, CCR7−, and predominantly CD62L− and CD28±. Image collected and cropped by CiteAb from the following publication (https://journal.frontiersin.org/article/10.3389/fimmu.2014.00645/abstract), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse TGF-beta 1 by Western Blot ERK and TGF-beta 1 levels in IBD-A33+ Li-EVs.(a) MC38 cells were treated with 1 mg ml−1 IBD lysates or Ctrl lysates for 24 h. TGF-beta 1 in MC38 cells or MC38-EVs was measured using western blot analysis. (b) MC38 cells were treated with 1 mg ml−1 IBD lysates or Ctrl lysates for the indicated times. Activation of ERK, JNK, p38 and ATK in MC38 cells was measured using western blot analysis. (c) MC38 cells were pretreated with DMSO, 10 μM ERK-specific inhibitor U0126 or JNK-specific inhibitor SP600125 for 30 min and then treated with 1 mg ml−1 IBD lysates or Ctrl lysates for 24 h. TGF-beta 1 in MC38-EVs was measured by western blot analysis. (d) Activation of ERK, JNK, p38 and ATK in colon tissues from healthy control or IBD mice was measured using western blot analysis. (e,f) Mice were fed with 2% DSS solution on day 0. Mice received intrarectal injection with 20 μg cholesterol-conjugated ERK or NC siRNA every other day on days 0–11. Twenty-four hours after the last injection, mice were killed. TGF-beta 1 in colon tissues and A33+ Li-EVs was measured using western blot analysis (e). The body weights of mice were measured daily (f). Ctrl group, mice that received normal drinking water. (a–e) Data are representative of three independent experiments; (f) data are presented as the mean±s.d. from one of the two independent experiments (n=5 per group). P values were generated by two-way ANOVA, followed by Newman–Keuls multiple comparison test using GraphPad Prism 5 (**P<0.01, ***P<0.001), IBD+NC siRNA versus IBD+ERK siRNA. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27721471), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: TGF-beta 1
TGF-beta 1, -2, and -3 are a closely related group of proteins (70-80% sequence homology) that are produced by many cell types and function as growth and differentiation factors. The active forms of TGF-beta 1, -2, and -3 are disulfide-linked homodimers.
Product Datasheets
Citations for TGF-beta 1 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
90
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Matrix-producing neutrophils populate and shield the skin
Authors: Vicanolo, T;Özcan, A;Li, JL;Huerta-López, C;Ballesteros, I;Rubio-Ponce, A;Dumitru, AC;Nicolás-Ávila, JÁ;Molina-Moreno, M;Reyes-Gutierrez, P;Johnston, AD;Martone, C;Greto, E;Quílez-Alvarez, A;Calvo, E;Bonzon-Kulichenko, E;Álvarez-Velez, R;Chooi, MY;Kwok, I;González-Bermúdez, B;Malleret, B;Espinosa, FM;Zhang, M;Wang, YL;Sun, D;Zhen Chong, S;El-Armouche, A;Kim, KK;Udalova, IA;Greco, V;Garcia, R;Vázquez, J;Dopazo, A;Plaza, GR;Alegre-Cebollada, J;Uderhardt, S;Ng, LG;Hidalgo, A;
Nature
Species: Mouse, Transgenic Mouse
Sample Types: In Vivo
Applications: In vivo assay -
Effects of Nitric Oxide on Bladder Detrusor Overactivity through the NRF2 and HIF-1? Pathways: A Rat Model Induced by Metabolic Syndrome and Ovarian Hormone Deficiency
Authors: Lin, HY;Lu, JH;Lin, RJ;Chueh, KS;Juan, TJ;Mao, JW;Lee, YC;Chuang, SM;Shen, MC;Sun, TW;Juan, YS;
International journal of molecular sciences
Species: Rat
Sample Types: Tissue Homogenates
Applications: Western Blot -
ZBTB18 restricts chromatin accessibility and prevents transcriptional adaptations that drive metastasis
Authors: R Wang, AB Bhatt, BA Minden-Bir, OK Travis, S Tiwari, H Jia, W Rosikiewic, O Martinot, E Childs, R Loesch, G Tossou, S Jamieson, D Finkelstei, B Xu, M Labelle
Science Advances, 2023-01-06;9(1):eabq3951.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Intermittent compressive force regulates dentin matrix protein 1 expression in human periodontal ligament stem cells
Authors: J Manokawinc, S Chareonvit, V Trachoo, P Limraksasi, H Egusa, T Osathanon
Journal of dental sciences, 2022-07-15;18(1):105-111.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Cardiac fibroblasts regulate the development of heart failure via Htra3-TGF-beta-IGFBP7 axis
Authors: T Ko, S Nomura, S Yamada, K Fujita, T Fujita, M Satoh, C Oka, M Katoh, M Ito, M Katagiri, T Sassa, B Zhang, S Hatsuse, T Yamada, M Harada, H Toko, E Amiya, M Hatano, O Kinoshita, K Nawata, H Abe, T Ushiku, M Ono, M Ikeuchi, H Morita, H Aburatani, I Komuro
Nature Communications, 2022-06-07;13(1):3275.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Tumor-infiltrating lymphocytes are functionally inactivated by CD90+ stromal cells and reactivated by combined Ibrutinib and Rapamycin in human pleural mesothelioma
Authors: H Yang, S Berezowska, P Dorn, P Zens, P Chen, RW Peng, TM Marti, GJ Kocher, RA Schmid, SRR Hall
Theranostics, 2022-01-01;12(1):167-185.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Targeting Cpt1a-Bcl-2 interaction modulates apoptosis resistance and fibrotic remodeling
Authors: L Gu, R Surolia, JL Larson-Cas, C He, D Davis, J Kang, VB Antony, AB Carter
Cell Death and Differentiation, 2021-08-20;0(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
&alphaSMA+ fibroblasts suppress Lgr5+ cancer stem cells and restrain colorectal cancer progression
Authors: KM McAndrews, K Vázquez-Ar, C Kwak, H Sugimoto, X Zheng, B Li, ML Kirtley, VS LeBleu, R Kalluri
Oncogene, 2021-06-09;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
TGF-&beta loaded exosome enhances ischemic wound healing in vitro and in vivo
Authors: A Shi, J Li, X Qiu, M Sabbah, S Boroumand, TC Huang, C Zhao, A Terzic, A Behfar, SL Moran
Theranostics, 2021-04-30;11(13):6616-6631.
Species: Rabbit
Sample Types: Whole Tissue
Applications: IHC -
Active food ingredients production from cold pressed processing residues of Camellia oleifera and Camellia sinensis seeds for regulation of blood pressure and vascular function
Authors: SS Chiang, LS Chen, CY Chu
Chemosphere, 2020-12-11;267(0):129267.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Assessment of the functional efficacy of root canal treatment with high-frequency waves in rats
Authors: S Matsui, N Yoneda, H Maezono, K Kuremoto, T Ishimoto, T Nakano, H Yumoto, S Ebisu, Y Noiri, M Hayashi
PLoS ONE, 2020-09-29;15(9):e0239660.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Immune modulation by complement receptor 3-dependent human monocyte TGF-beta1-transporting vesicles
Authors: LD Halder, EAH Jo, MZ Hasan, M Ferreira-G, T Krüger, M Westermann, DI Palme, G Rambach, N Beyersdorf, C Speth, ID Jacobsen, O Kniemeyer, B Jungnickel, PF Zipfel, C Skerka
Nat Commun, 2020-05-11;11(1):2331.
Species: Human
Sample Types: Whole Cells
Applications: SEM -
Neutrophils from severe asthmatic patients induce epithelial to mesenchymal transition in healthy bronchial epithelial cells
Authors: A Haddad, M Gaudet, M Plesa, Z Allakhverd, AK Mogas, S Audusseau, CJ Baglole, DH Eidelman, R Olivenstei, MS Ludwig, Q Hamid
Respir. Res., 2019-10-29;20(1):234.
Species: Human
Sample Types: Whole Cells
Applications: ICC, Neutralization -
Response gene to complement 32 expression in macrophages augments paracrine stimulation-mediated colon cancer progression
Authors: P Zhao, B Wang, Z Zhang, W Zhang, Y Liu
Cell Death Dis, 2019-10-10;10(10):776.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Intermittent compressive force promotes osteogenic differentiation in human periodontal ligament cells by regulating the transforming growth factor-beta pathway
Authors: J Manokawinc, P Pavasant, C Sawangmake, N Limjeeraja, CN Limjeeraja, H Egusa, T Osathanon
Cell Death Dis, 2019-10-07;10(10):761.
Species: Human
Sample Types: Whole Cells
Applications: ICC, Neutralization -
Adipose-derived stem cells prevent the onset of bisphosphonate-related osteonecrosis of the jaw through transforming growth factor ?-1-mediated gingival wound healing
Authors: X Zang, L He, L Zhao, Y He, E Xiao, Y Zhang
Stem Cell Res Ther, 2019-06-13;10(1):169.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
A KDM6A-KLF10 reinforcing feedback mechanism aggravates diabetic podocyte dysfunction
Authors: CL Lin, YC Hsu, YT Huang, YH Shih, CJ Wang, WC Chiang, PJ Chang
EMBO Mol Med, 2019-05-01;11(5):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Elevated circulating TGF?1 during acute liver failure activates TGF?R2 on cortical neurons and exacerbates neuroinflammation and hepatic encephalopathy in mice
Authors: M McMillin, S Grant, G Frampton, AD Petrescu, E Williams, B Jefferson, A Thomas, A Brahmarout, S DeMorrow
J Neuroinflammation, 2019-04-02;16(1):69.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Cryptotanshinone Ameliorates Radiation-Induced Lung Injury in Rats
Authors: Y Jiang, F You, J Zhu, C Zheng, R Yan, J Zeng
Evid Based Complement Alternat Med, 2019-02-20;2019(0):1908416.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC-P -
Association between FOXP3+ regulatory T-cells and occurrence of peritoneal lesions in women with ovarian endometrioma and dermoid cysts
Authors: KN Khan, K Yamamoto, A Fujishita, A Koshiba, H Kuroboshi, S Sakabayash, S Teramukai, M Nakashima, J Kitawaki
Reprod. Biomed. Online, 2019-01-31;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Spatio-temporal overview of neuroinflammation in an experimental mouse stroke model
Authors: L Buscemi, M Price, P Bezzi, L Hirt
Sci Rep, 2019-01-24;9(1):507.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Eosinophil recruitment is dynamically regulated by interplay among lung dendritic cell subsets after allergen challenge
Authors: S Yi, J Zhai, R Niu, G Zhu, M Wang, J Liu, H Huang, Y Wang, X Jing, L Kang, W Song, Y Shi, H Tang
Nat Commun, 2018-09-24;9(1):3879.
-
Targeted deletion of T cell S1P receptor 1 ameliorates cardiac fibrosis in streptozotocin-induced diabetic mice
Authors: CS Abdullah, ZQ Jin
FASEB J., 2018-04-26;0(0):fj201800231R.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Specific Inhibitor of Smad3 (SIS3) Attenuates Fibrosis, Apoptosis, and Inflammation in Unilateral Ureteral Obstruction Kidneys by Inhibition of Transforming Growth Factor ? (TGF-?)/Smad3 Signaling
Authors: X Ji, H Wang, Z Wu, X Zhong, M Zhu, Y Zhang, R Tan, Y Liu, J Li, L Wang
Med. Sci. Monit., 2018-03-20;24(0):1633-1641.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
CD8?+CD11c+ Extracellular Vesicles in the Lungs Control Immune Homeostasis of the Respiratory Tract via TGF-?1 and IL-10
Authors: S Wan, S Wang, L Weng, G Zhang, Z Lin, X Fei, F Zhang, F Yang, J Wang, Z Cai
J. Immunol., 2018-01-22;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Functional Assay, Neutralization -
Human amniotic epithelial cells regulate osteoblast differentiation through the secretion of TGF?1 and microRNA-34a-5p
Authors: G Wang, F Zhao, D Yang, J Wang, L Qiu, X Pang
Int. J. Mol. Med., 2017-11-17;41(2):791-799.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Neutralization -
Fibroblast deletion of ROCK2 attenuates cardiac hypertrophy, fibrosis, and diastolic dysfunction
Authors: T Shimizu, N Narang, P Chen, B Yu, M Knapp, J Janardanan, J Blair, JK Liao
JCI Insight, 2017-07-06;2(13):.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
Suppression of Hepatic Epithelial-to-Mesenchymal Transition by Melittin via Blocking of TGF?/Smad and MAPK-JNK Signaling Pathways
Authors: JH Park, B Park, KK Park
Toxins (Basel), 2017-04-13;9(4):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
FOXO1 expression in keratinocytes promotes connective tissue healing
Authors: C Zhang, J Lim, J Liu, B Ponugoti, S Alsadun, C Tian, R Vafa, DT Graves
Sci Rep, 2017-02-21;7(0):42834.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
The Use of Platelet-Rich and Platelet-Poor Plasma to Enhance Differentiation of Skeletal Myoblasts: Implications for the Use of Autologous Blood Products for Muscle Regeneration
Authors: O Miroshnych, WT Chang, JL Dragoo
Am J Sports Med, 2016-12-27;45(4):945-953.
Species: Human
Sample Types: Protein
Applications: Western Blot -
A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood
Nat Commun, 2016-11-22;7(0):13363.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Role of urotensin�II in advanced glycation end product-induced extracellular matrix synthesis in rat proximal tubular epithelial cells
Authors: Jiping Qi
Int. J. Mol. Med., 2016-10-26;0(0):.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
Platelet-Derived Ectosomes Reduce NK Cell Function
J Immunol, 2016-07-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Macrophage TGF-?1 and the Proapoptotic Extracellular Matrix Protein BIGH3 Induce Renal Cell Apoptosis in Prediabetic and Diabetic Conditions
Authors: RJ Moritz, RG LeBaron, CF Phelix, R Rupaimoole, HS Kim, A Tsin, R Asmis
Int J Clin Med, 2016-07-21;7(7):496-510.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Western Blot -
A novel immunomodulatory function of neutrophils on rhinovirus-activated monocytes in vitro
Authors: Brian G Oliver
Thorax, 2016-06-10;71(11):1039-1049.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Differential TGFbeta pathway targeting by miR-122 in humans and mice affects liver cancer metastasis.
Authors: Yin S, Fan Y, Zhang H, Zhao Z, Hao Y, Li J, Sun C, Yang J, Yang Z, Yang X, Lu J, Xi J
Nat Commun, 2016-03-18;7(0):11012.
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot -
Manual therapy as an effective treatment for fibrosis in a rat model of upper extremity overuse injury
Authors: GM Bove, MY Harris, H Zhao, MF Barbe
J. Neurol. Sci, 2015-12-24;361(0):168-80.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC-P -
Regulatory Dendritic Cells Restrain NK Cell IFN-gamma Production through Mechanisms Involving NKp46, IL-10, and MHC Class I-Specific Inhibitory Receptors.
Authors: Spallanzani R, Torres N, Avila D, Ziblat A, Iraolagoitia X, Rossi L, Domaica C, Fuertes M, Rabinovich G, Zwirner N
J Immunol, 2015-07-31;195(5):2141-8.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Vitamin D regulates cytokine patterns secreted by dendritic cells to promote differentiation of IL-22-producing T cells.
Authors: Sommer A, Fabri M
PLoS ONE, 2015-06-24;10(6):e0130395.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Hypoxia down-regulates expression of secretory leukocyte protease inhibitor in bronchial epithelial cells via TGF-beta1.
Authors: Pahlman L, Jogi A, Gram M, Mori M, Egesten A
BMC Pulm Med, 2015-03-07;15(0):19.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
TGF-beta3-expressing CD4+CD25(-)LAG3+ regulatory T cells control humoral immune responses.
Authors: Okamura T, Sumitomo S, Morita K, Iwasaki Y, Inoue M, Nakachi S, Komai T, Shoda H, Miyazaki J, Fujio K, Yamamoto K
Nat Commun, 2015-02-19;6(0):6329.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Dendritic cell SIRT1-HIF1alpha axis programs the differentiation of CD4+ T cells through IL-12 and TGF-beta1.
Authors: Liu G, Bi Y, Xue L, Zhang Y, Yang H, Chen X, Lu Y, Zhang Z, Liu H, Wang X, Wang R, Chu Y, Yang R
Proc Natl Acad Sci U S A, 2015-02-17;112(9):E957-65.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Th17-related cytokines contribute to recall-like expansion/effector function of HMBPP-specific Vgamma2Vdelta2 T cells after Mycobacterium tuberculosis infection or vaccination.
Authors: Shen H, Wang Y, Chen C, Frencher J, Huang D, Yang E, Ryan-Payseur B, Chen Z
Eur J Immunol, 2015-02-01;45(2):442-51.
Species: Primate - Macaca fascicularis (Crab-eating Monkey or Cynomolgus Macaque)
Sample Types: Whole Cells
Applications: Neutralization -
Surfactant protein D attenuates sub-epithelial fibrosis in allergic airways disease through TGF-ss
Authors: Ogawa H, Ledford J, Mukherjee S, Aono Y, Nishioka Y, Lee J, Izumi K, Hollingsworth J
Respir Res, 2014-11-29;15(1):143.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Oncogenic mutations in intestinal adenomas regulate Bim-mediated apoptosis induced by TGF-beta.
Authors: Wiener Z, Band A, Kallio P, Hogstrom J, Hyvonen V, Kaijalainen S, Ritvos O, Haglund C, Kruuna O, Robine S, Louvard D, Ben-Neriah Y, Alitalo K
Proc Natl Acad Sci U S A, 2014-05-13;111(21):E2229-36.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Immunoprecipitation -
Mesenchymal stem cells cancel azoxymethane-induced tumor initiation.
Authors: Nasuno M, Arimura Y, Nagaishi K, Isshiki H, Onodera K, Nakagaki S, Watanabe S, Idogawa M, Yamashita K, Naishiro Y, Adachi Y, Suzuki H, Fujimiya M, Imai K, Shinomura Y
Stem Cells, 2014-04-01;32(4):913-25.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
Regulation of renal fibrosis by Smad3 Thr388 phosphorylation.
Authors: Qu X, Li X, Zheng Y, Ren Y, Puelles V, Caruana G, Nikolic-Paterson D, Li J
Am J Pathol, 2014-01-30;184(4):944-52.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
CD4+CD25+ T regulatory cells activated during feline immunodeficiency virus infection convert T helper cells into functional suppressors through a membrane-bound TGFbeta / GARP-mediated mechanism.
Authors: Miller M, Petty C, Tompkins M, Fogle J
Virol J, 2014-01-18;11(0):7.
Species: Feline
Sample Types: Whole Cells
Applications: Neutralization -
Oral Escherichia coli colonization factor antigen I fimbriae ameliorate arthritis via IL-35, not IL-27.
Authors: Kochetkova I, Thornburg T, Callis G, Holderness K, Maddaloni M, Pascual D
J Immunol, 2013-12-11;192(2):804-16.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement.
Authors: Bialas A, Stevens B
Nat Neurosci, 2013-10-27;16(12):1773-82.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: Immunodepletion -
Cancer-associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor-beta signaling.
Authors: Hasegawa T, Yashiro M, Nishii T, Matsuoka J, Fuyuhiro Y, Morisaki T, Fukuoka T, Shimizu K, Shimizu T, Miwa A, Hirakawa K
Int J Cancer, 2013-10-24;134(8):1785-95.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Exosomes with membrane-associated TGF-beta1 from gene-modified dendritic cells inhibit murine EAE independently of MHC restriction.
Authors: Yu L, Yang F, Jiang L, Chen Y, Wang K, Xu F, Wei Y, Cao X, Wang J, Cai Z
Eur J Immunol, 2013-06-21;43(9):2461-72.
Species: Mouse
Sample Types: Exosomes
Applications: Neutralization -
Reduction of methylglyoxal-induced glycation by pyridoxamine improves adipose tissue microvascular lesions.
Authors: Rodrigues T, Matafome P, Santos-Silva D, Sena C, Seica R
J Diabetes Res, 2013-04-07;2013(0):690650.
Species: Rat
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
A dynamic dual role of IL-2 signaling in the two-step differentiation process of adaptive regulatory T cells.
Authors: Guo Z, Khattar M, Schroder P, Miyahara Y, Wang G, He X, Chen W, Stepkowski S
J Immunol, 2013-02-20;190(7):3153-62.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Transforming growth factor beta induces sensory neuronal hyperexcitability, and contributes to pancreatic pain and hyperalgesia in rats with chronic pancreatitis.
Authors: Zhu Y, Colak T, Shenoy M, Liu L, Mehta K, Pai R, Zou B, Xie X, Pasricha P
Mol Pain, 2012-09-11;8(0):65.
Species: Rat
Sample Types: In Vivo
Applications: Neutralization -
HIV-1 promotes intake of Leishmania parasites by enhancing phosphatidylserine-mediated, CD91/LRP-1-dependent phagocytosis in human macrophages.
Authors: Lodge R, Ouellet M, Barat C, Andreani G, Kumar P, Tremblay MJ
PLoS ONE, 2012-03-06;7(3):e32761.
Species: Human
Sample Types: Recombinant Protein
Applications: Neutralization -
Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease.
Authors: Ma W, Han W, Greer PA, Tuder RM, Toque HA, Wang KK, Caldwell RW, Su Y
J. Clin. Invest., 2011-10-17;121(11):4548-66.
Species: Human
Sample Types: Recombinant Protein
Applications: Western Blot -
C-reactive protein promotes diabetic kidney disease in a mouse model of type 1 diabetes.
Authors: Liu F, Chen HY, Huang XR, Chung AC, Zhou L, Fu P, Szalai AJ, Lan HY
Diabetologia, 2011-07-09;54(10):2713-23.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition.
Authors: Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman GJ, Shannon MF, Drew PA, Khew-Goodall Y, Goodall GJ
Mol. Biol. Cell, 2011-03-16;22(10):1686-98.
Species: Canine
Sample Types: Whole Cells
Applications: Neutralization -
Mechanical Stretch Induces Epithelial-Mesenchymal Transition in Alveolar Epithelia via Hyaluronan Activation of Innate Immunity.
Authors: Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S
J. Biol. Chem., 2011-03-11;286(20):17435-44.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Overexpression of TGF-ß 1 gene induces cell surface localized glucose-regulated protein 78-associated latency-associated peptide/TGF-ß.
Authors: Oida T, Weiner HL
J. Immunol., 2010-08-18;185(6):3529-35.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
AIDS progression is associated with the emergence of IL-17-producing cells early after simian immunodeficiency virus infection.
Authors: Campillo-Gimenez L, Cumont MC, Fay M, Kared H, Monceaux V, Diop O, Muller-Trutwin M, Hurtrel B, Levy Y, Zaunders J, Dy M, Leite-de-Moraes MC, Elbim C, Estaquier J
J. Immunol., 2009-12-16;184(2):984-92.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Tissue
Applications: IHC-Fr -
Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells.
Authors: Pot C, Jin H, Awasthi A
J. Immunol., 2009-07-01;183(2):797-801.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Intrastromal keratotomy with femtosecond laser avoids profibrotic TGF-beta1 induction.
Authors: Meltendorf C, Burbach GJ, Ohrloff C, Ghebremedhin E, Deller T
Invest. Ophthalmol. Vis. Sci., 2009-04-22;50(8):3688-95.
Species: Rabbit
Sample Types: Whole Tissue
Applications: IHC-Fr -
Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-{beta} and STAT3 activation.
Authors: Hosui A, Kimura A, Yamaji D, Zhu BM, Na R, Hennighausen L
J. Exp. Med., 2009-03-30;206(4):819-31.
Species: Mouse
Sample Types: Cell Culture Supernates, Whole Cells
Applications: ICC, Western Blot -
Vaccination route that induces transforming growth factor beta production fails to elicit protective immunity against Leishmania donovani infection.
Authors: Bhowmick S, Mazumdar T, Ali N
Infect. Immun., 2009-01-21;77(4):1514-23.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Neutralization -
Detuning CD8+ T lymphocytes by down-regulation of the activating receptor NKG2D: role of NKG2D ligands released by activated T cells.
Authors: Cerboni C, Ardolino M, Santoni A, Zingoni A
Blood, 2009-01-05;113(13):2955-64.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Activation of Toll-like receptor 3 augments myofibroblast differentiation.
Authors: Sugiura H, Ichikawa T, Koarai A, Yanagisawa S, Minakata Y, Matsunaga K, Hirano T, Akamatsu K, Ichinose M
Am. J. Respir. Cell Mol. Biol., 2008-11-06;40(6):654-62.
Species: Human
Sample Types: Cell Culture Supernates, Whole Cells
Applications: ELISA Development, Neutralization -
Transforming growth factor-beta stimulates intestinal epithelial focal adhesion kinase synthesis via smad- and p38-dependent mechanisms.
Authors: Walsh MF, Ampasala DR, Hatfield J, Vander Heide R, Suer S, Rishi AK, Basson MD
Am. J. Pathol., 2008-06-26;173(2):385-99.
Species: Human, Rat
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-P -
The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury.
Authors: Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH
Stem Cells, 2008-01-10;26(4):1047-55.
Species: Rat
Sample Types: Tissue Homogenates
Applications: ELISA Development -
Bystander central memory but not effector memory CD8+ T cells suppress allograft rejection.
Authors: Wan N, Dai H, Wang T, Moore Y, Zheng XX, Dai Z
J. Immunol., 2008-01-01;180(1):113-21.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells.
Authors: Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F
Nat. Immunol., 2007-08-05;8(9):942-9.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells.
Authors: Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Yokota SJ, Bankert RB
J. Immunol., 2007-05-01;178(9):5552-62.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Vascular endothelial growth factor can signal through platelet-derived growth factor receptors.
Authors: Ball SG, Shuttleworth CA, Kielty CM
J. Cell Biol., 2007-04-30;177(3):489-500.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells.
Authors: Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA
J. Immunol., 2007-02-15;178(4):2018-27.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Increased frequency of CD4+ cells expressing CD161 in cancer patients.
Authors: Iliopoulou EG, Karamouzis MV, Missitzis I, Ardavanis A, Sotiriadou NN, Baxevanis CN, Rigatos G, Papamichail M, Perez SA
Clin. Cancer Res., 2006-12-01;12(23):6901-9.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
A GMCSF and IL-15 fusokine leads to paradoxical immunosuppression in vivo via asymmetrical JAK/STAT signaling through the IL-15 receptor complex.
Authors: Rafei M, Wu JH, Annabi B, Lejeune L, Francois M, Galipeau J
Blood, 2006-11-02;109(5):2234-42.
Species: Mouse
Sample Types: Cell Culture Supernates
Applications: Array Development -
Inhibition of p38 mitogen-activated protein kinase and transforming growth factor-beta1/Smad signaling pathways modulates the development of fibrosis in adriamycin-induced nephropathy.
Authors: Li J, Campanale NV, Liang RJ, Deane JA, Bertram JF, Ricardo SD
Am. J. Pathol., 2006-11-01;169(5):1527-40.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Human intestinal intraepithelial lymphocytes keep TNF alpha levels low by cell uptake and feedback inhibition of transcription.
Authors: Ebert EC, Mehta V
Cell. Immunol., 2006-08-17;241(1):7-13.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma.
Authors: Yang XH, Yamagiwa S, Ichida T, Matsuda Y, Sugahara S, Watanabe H, Sato Y, Abo T, Horwitz DA, Aoyagi Y
J. Hepatol., 2006-03-09;45(2):254-62.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Transforming growth factor beta 1(TGF-beta1) down-regulates TNFalpha-induced RANTES production in rheumatoid synovial fibroblasts through NF-kappaB-mediated transcriptional repression.
Authors: Cho ML, Min SY, Chang SH, Kim KW, Heo SB, Lee SH, Park SH, Cho CS, Kim HY
Immunol. Lett., 2006-03-03;105(2):159-66.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
trans fatty acids and systemic inflammation in heart failure.
Authors: Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC
Am. J. Clin. Nutr., 2004-12-01;80(6):1521-5.
Species: Human
Sample Types: Plasma
Applications: ELISA Development -
Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses.
Authors: Lim HW, Hillsamer P, Kim CH
J. Clin. Invest., 2004-12-01;114(11):1640-9.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function.
Authors: Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, Levine BL, June CH, Blazar BR, Porter SB
Blood, 2004-09-16;105(2):750-8.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Neutralization -
Rapid analysis of inflammatory cytokines in cerebrospinal fluid using chip-based immunoaffinity electrophoresis.
Authors: Phillips TM
Electrophoresis, 2004-06-01;25(10):1652-9.
Species: Human
Sample Types: Serum
Applications: Affinity Chromatography -
Transforming growth factor-beta -Smad signaling pathway cooperates with NF-kappa B to mediate nontypeable Haemophilus influenzae-induced MUC2 mucin transcription.
Authors: Jono H, Shuto T, Xu H, Kai H, Lim DJ, Gum JR, Kim YS, Yamaoka S, Feng XH, Li JD
J. Biol. Chem., 2002-09-16;277(47):45547-57.
Species: Human
Sample Types: Cell Lysates
Applications: Neutralization -
Th2 cytokine regulation of type I collagen gel contraction mediated by human lung mesenchymal cells.
Authors: Liu X, Kohyama T, Wang H, Zhu YK, Wen FQ, Kim HJ, Romberger DJ, Rennard SI
Am. J. Physiol. Lung Cell Mol. Physiol., 2002-05-01;282(5):L1049-56.
Species: Human
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Induction of a hypertrophic growth status of coronary smooth muscle cells is associated with an overexpression of TGF-beta.
Authors: Schmidt A, 2019, Gopfert C, Vlodavsky I, Volker W, Buddecke E
101211, 2002-03-01;81(3):138-44.
Species: Bovine
Sample Types: Whole Cells
Applications: Neutralization -
Repeated exposure induces periportal fibrosis in Schistosoma mansoni-infected baboons: role of TGF-beta and IL-4.
Authors: Farah IO, Mola PW, Kariuki TM, Nyindo M, Blanton RE, King CL
J. Immunol., 2000-05-15;164(10):5337-43.
Species: Primate - Papio anubis (Olive Baboon)
Sample Types: Cell Culture Supernates
Applications: ELISA Development -
Isoform specificity of commercially-available anti-TGF-beta antibodies.
Authors: Mozes MM, Hodics T, Kopp JB
J. Immunol. Methods, 1999-05-27;225(1):87-93.
Species: Human
Sample Types: Recombinant Protein
Applications: Western Blot
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for TGF-beta 1 Antibody
Average Rating: 4.7 (Based on 9 Reviews)
Have you used TGF-beta 1 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
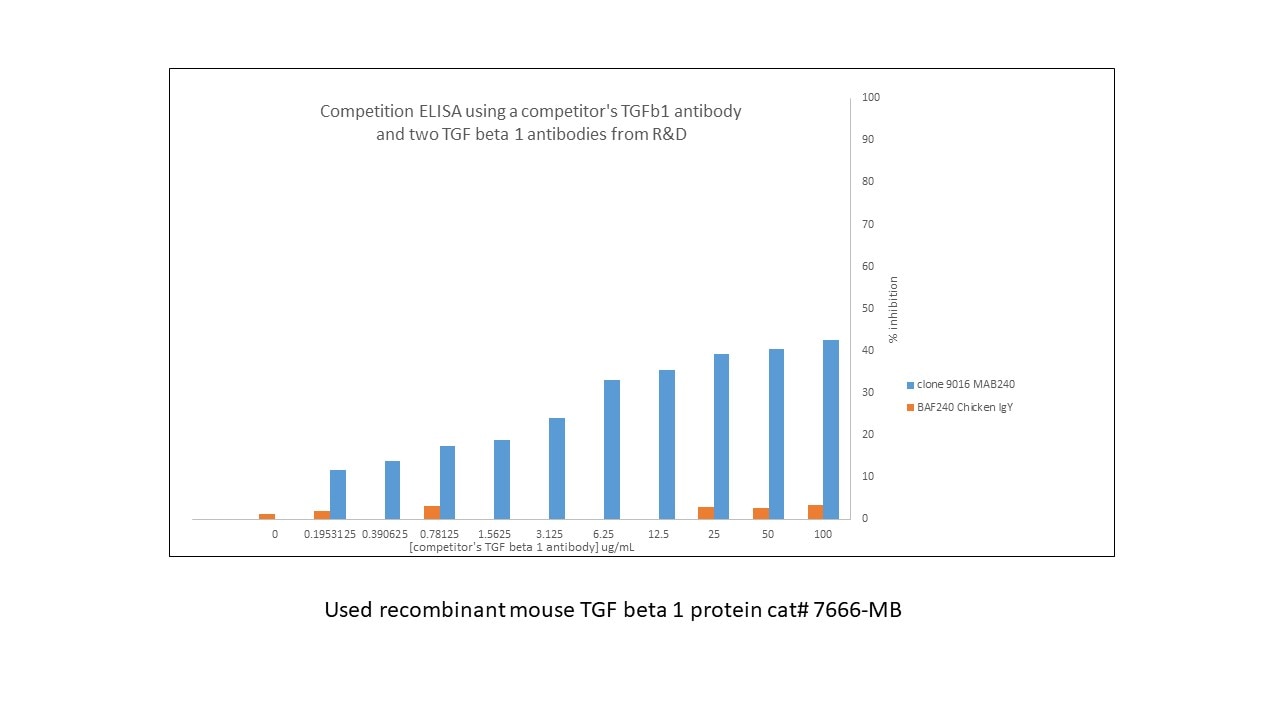
I performed a competition ELISA to see if another company's TGFb1 had a similar epitope to two of R&D's antibodies . I coated my plate with 50ng/well of recombinant mouse TGF beta 1 protein (cat# 7666-MB) overnight. I washed and blocked with reagent diluent 2 DY995 for 2hr, washed and added another company's TGFb1 antibody at different concentrations 0.2-100ug/mL in reagent diluent 2 and incubated 1hr. I did not wash my wells but add the two antibodies MAB240-biotinylated it in-house and BAF at a final concentration of 2ug/mL and incubated 1hr diluted in reagent diluent 2. I washed and added streptavidin-HRP DY998 (1:200) and incubated 30 mins, washed and developed with the substrate pack DY999. I found MAB240 competed for binding to the mouse TGF beta 1 protein when the competitor's antibody was present but the chicken polyclonal was not inhibited.
10.1093/cercor/bhy164
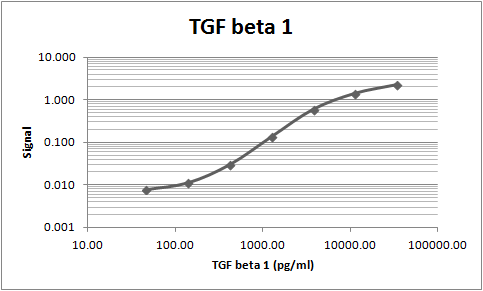
This antibody was used as the capture to build an ELISA for TGF-beta 1. BAF240 was used as the detection.
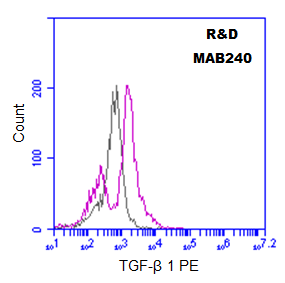
10^6 of MDA-MB-231 cells were stained for 30 minutes at 4C with 2.5 ug of Mouse TGF-β 1 antibody (red), or control antibody (black). Secondary: PE-Goat anti-Mouse IgG 1:1,000. Blocking: 1% BSA. Unfixed cells.