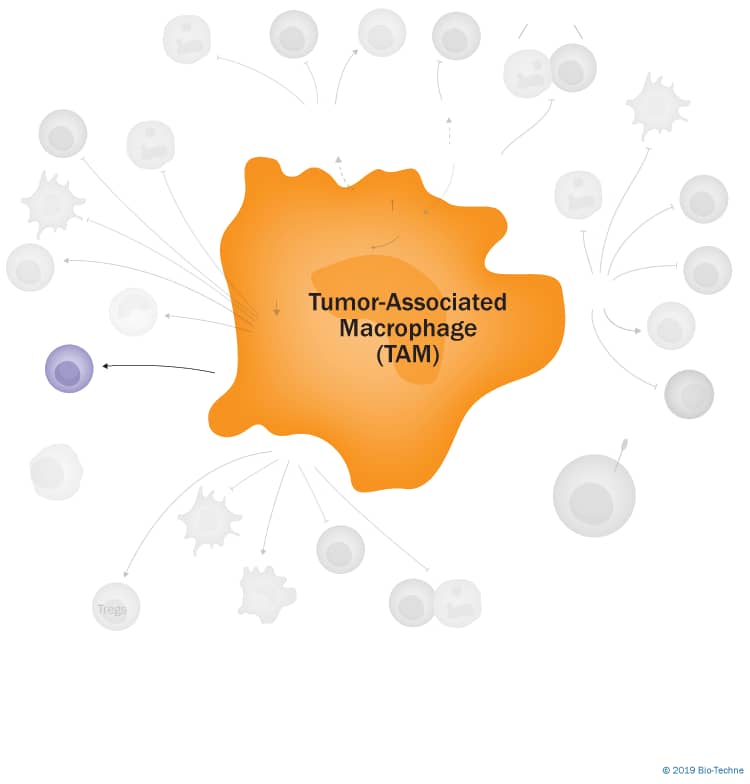
Mechanisms of Tumor-Associated Macrophage TAM-Mediated Immunosuppression
Click on the mechanisms of tumor-associated macrophage (TAM)-mediated immunosuppression listed on the left below under Explore Pathways to see the specific TAM-associated molecules that are involved in each mechanism and an explanation of how they negatively regulate the immune response.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
and derivatives
and derivatives
Cells
Cells
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Cells
Cells
T Cells
T Cells
Cells
Cells
Cells
Cells
T Cells
T Cells
T Cells
T Cells
Receptor
Receptor
T Cells
T Cells
Cells
Cells
T Cells
T Cells
T Cells
T Cells
Macrophages
Macrophages
Cells
Cells
Cells
Cells
CCL20, CCL22
CCL20, CCL22
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Use our Product Suggestion form to enter a request.
You will be notified once it becomes available.
Cells
Cells
T Cells
T Cells
Cells
Cells
Amino Acid Depletion
and Production of
Immunosuppressive
Metabolites
Amino Acid Depletion
and Production of
Immunosuppressive
Metabolites
Expression of Non-
Classical HLA Class
I Molecules
Expression of Non-
Classical HLA Class
I Molecules
Direct Engagement of
T Cell Inhibitory and
Apoptotic Receptors
Direct Engagement of
T Cell Inhibitory and
Apoptotic Receptors
Production of Inhibitory
Cytokines
Production of Inhibitory
Cytokines
Inhibition of Tumor
Cell Phagocytosis
Inhibition of Tumor
Cell Phagocytosis
Production of Treg-
Recruiting Chemokines
Production of Treg-
Recruiting Chemokines
Production of
PGE2
Production of
PGE2
Macrophage
Recruitment
to the TME
Macrophage
Recruitment
to the TME
Amino Amino Acid Depletion and Production of Immunosuppressive Metabolites
Natural Killer Cells• Inhibits proliferation and activity
T Cells
• Inhibits proliferation and activity
• Loss of TCR-CD3 zeta chain
Tregs
• Promotes development
Expression of Non-classical HLA Class I Molecules
HLA-ENatural Killer Cells, CD8+ T Cells
• Inhibits proliferation
• Inhibits cytotoxicity
HLA-G
Natural Killer Cells
• Inhibits MICA/NKG2D activation
• Inhibits IFN-gamma secretion
• Inhibits cytotoxicity
• Inhibits chemotaxis
Dendritic Cells
• Inhibits maturation
• Induces tolerogenic dendritic cells
CD8+ T Cells
• Inhibits proliferation
• Inhibits cytotoxicity
CD4+ T Cells
• Inhibits proliferation
• Induces the production of Th2-type cytokines
Tregs
• Promotes differentiation
B Cells
• Inhibits differentiation
• Inhibits Ig secretion
• Inhibits chemotaxis
Direct Engagement of T Cell Inhibitory and Apoptotic Receptors
Effector T CellsB7-H4-Unknown Receptor
• Suppresses activation of antigen-specific T cells
• Induces apoptosis of activated T cells
• Induces Treg development and proliferation
Fas L-Fas; TRAIL-TRAIL R2
• Induces CD8+ and CD4+ T cell apoptosis
PD-L1-PD1; B7-1 or B7-2-CTLA-4
• Inhibits T cell activation
• Induces T cell cycle arrest, anergy, and apoptosis
Production of Inhibitory Cytokines
Tregs• Promotes expansion
Dendritic Cells
• Inhibits IL-12 secretion and maturation
• Inhibits co-stimulatory molecule expression
• Promotes the production of immunosuppressive molecules
M2 Macrophages
• Promotes M2 polarization
Effector T Cells
• Inhibits activation and differentiation
• Inhibits cytokine production
CD8+ T Cells, Natural Killer Cells
• Down-regulates NKG2D and NKp30
• Inhibits NK cell proliferation and cytotoxicity
Production of Treg-Recruiting Chemokines
Tregs• Promotes recruitment
Inhibition of Tumor Cell Phagocytosis
Tumor Cells• Suppression of macrophage-mediated tumor cell detection and apoptosis
Macrophage Recruitment to the Tumor Microenvironment
• Promotes monocyte and macrophage migration toward inflamed tissues• Promotes M2 Polarization
• Stimulates angiogenesis
• Promotes further tumor development and metastasis
Production of PGE2
Natural Killer Cells• Inhibits IFN-gamma secretion
• Inhibits cytolytic activity
Effector T Cells
• Inhibits IL-2 synthesis and IL-2 R expression
• Inhibits Th1 differentiation
Dendritic Cells
• Inhibits IL-12 secretion
Tregs
• Promotes differentiation and accumulation
MDSCs
• Promotes expansion
• ↑ Suppressive activity

Tumor-Associated Macrophage (TAM)-Mediated Mechanisms of Immunosuppression
Tumor-associated macrophages (TAMs) are a heterogeneous population of cells that display a range of phenotypes depending on the type of tumor and their locations in the tumor microenvironment (TME). TAMs are commonly the most abundant infiltrating leukocyte in most tumors and are predominantly thought to have pro-tumor effects. These include both immunosuppressive effects in addition to pro-angiogenic and metastatic effects. The mechanisms by which TAMs have been described to mediate immunosuppression are detailed below.
Amino Acid Depletion and Production of Immunosuppressive Metabolites by Tumor-Associated Macrophages
Tumor-associated macrophages (TAMs) inhibit the anti-tumor immune response through multiple mechanisms. One mechanism is through the depletion of amino acids and the production of immunosuppressive metabolites. Like tumor cells and myeloid-derived suppressor cells (MDSCs), TAMs in several types of human tumors have been shown to overexpress indolamine-2,3-dioxygenase (IDO), which catalyzes the initial, rate-limiting step in L-tryptophan degradation by the kynurenine pathway. The degradation of L-tryptophan and the presence of kynurenine and its derivatives inhibit the proliferation and activity of CD4+ and CD8+ T cells and natural killer cells and promote the differentiation of regulatory T cells (Tregs). Additionally, murine TAMs also express arginase 1 (ARG1), leading to increased uptake of L-arginine, which reduces the expression of TCR-CD3 zeta chain on T cells and leads to proliferative arrest. Although TAMs appear to mediate immunosuppression through expression of ARG1 in murine tumor models, metabolism of L-arginine by ARG1 in human TAMs has not been conclusively demonstrated to be a mechanism by which T cell functions are suppressed in human tumors.
Expression of Non-Classical HLA Class I Molecules by Tumor-Associated Macrophages
A second mechanism by which TAMs inhibit the anti-tumor immune response is by expression of non-classical human leukocyte antigen (HLA) class I molecules such as HLA-E and HLA-G. Both HLA-E and HLA-G are HLA-class Ib molecules that inhibit the activities of different immune cell types by interacting with specific inhibitory receptors expressed on the surface of immune cells. HLA-E binds to the inhibitory CD94-NKG2A receptor expressed on natural killer (NK) cells or CD8+ T cells with six-fold higher affinity than it binds to the CD94-NKG2C activating receptor, and as a result, it can inhibit the proliferation and cytotoxicity of these cells. HLA-G can also inhibit IFN-gamma secretion and the cytotoxicity of NK and CD8+ T cells by interacting with either KIR2DL4 or the inhibitory leukocyte immunoglobulin receptor LILRB1/ILT2. Additionally, interaction of HLA-G with LILRB1/ILT2 on CD4+ T cells reduces CD4+ T cell proliferation and Th1 cytokine production, and induces Th2-type cytokine production, which further inhibits CD8+ T cell cytolytic functions and IFN-gamma expression. HLA-G also interacts with LILRB1/ILT2 or LILRB2/ILT4 expressed on dendritic cells and down-regulates the expression of co-stimulatory molecules, CCR7, and IL-12, and inactivates the MHC class II presentation pathway, resulting in suppression of dendritic cell maturation and induction of a tolerogenic DC phenotype. Tolerogenic DCs fail to promote T cell activation, and instead promote regulatory T cell (Treg) development, further promoting tumor immune escape. B cell responses can also be inhibited by interaction of HLA-G with the LILRB1/ILT2 on these cells. HLA-G inhibits B cell proliferation, differentiation, and Ig secretion.
Direct Engagement of T cell Inhibitory and Apoptotic Receptors by Tumor-Associated Macrophages
TAMs may also suppress T cell functions through the direct engagement of T cell inhibitory and apoptotic receptors. TAMs express B7-1/CD80 and B7-2/CD86 which can bind to the T cell-expressed co-inhibitory receptor CTLA-4, with higher affinity than they bind to the CD28 co-stimulatory receptor, resulting in suppression of T cell activation. PD-L1 and PD-L2 are also overexpressed on TAMs in multiple cancer tissues. Both of these ligands bind to PD-1 expressed on CD4+ and CD8+ effector T cells and can inhibit TCR signaling and promote T cell anergy and apoptosis. B7-H4, another B7 family member expressed by TAMs, binds to an unknown receptor expressed on activated T cells and inhibits IL-2 production, proliferation, and cytokine secretion. TAMs can also directly promote T cell apoptosis through their expression of Fas Ligand (Fas L) and TRAIL, which interact with their respective receptors, Fas/CD95 and TRAIL R2 on CD4+ and CD8+ effector T cells to mediate apoptosis.
Production of Inhibitory Cytokines by Tumor-Associated Macrophages
Similar to many other immunosuppressive cell types present in the tumor microenvironment, TAMs inhibit the anti-tumor immune response through the production of IL-10 and TGF-beta 1. Together these cytokines inhibit the activation, differentiation, proliferation, and functions of effector T cells. IL-10 and TGF-beta 1 also promote the expansion of suppressive T cells. TGF-beta 1 induces the expression of FoxP3 in CD4+CD25- conventional T cells thereby promoting the expansion of regulatory T cells, while IL-10 promotes the conversion of activated conventional T cells to IL-10-, TGF-beta 1-secreting Tr1 cells. In addition, TGF-beta 1 also reduces NK cell proliferation and cytotoxicity, down-regulates NKG2D and NKp30 expression on natural killer (NK) cells and CD8+ T cells, promotes the polarization of macrophages toward a M2 phenotype, and inhibits the expression of co-stimulatory molecules and IL-12 by dendritic cells (DCs), as well as DC maturation and migration.
Inhibition of Tumor Cell Phagocytosis by Tumor-Associated Macrophages
TAMs also promote tumor immune evasion through expression of signal regulatory protein alpha (SIRP alpha). SIRP alpha is a receptor for CD47, a cell surface protein that typically protects normal cells from phagocytosis by macrophages or dendritic cells. CD47 is frequently overexpressed on tumor cells and plays a key role in tumor escape by binding to SIRP alpha and sending macrophages a “don’t eat me” signal. Blockade of the CD47-SIRP alpha signal has been shown to stimulate phagocytosis, leading to tumor cell elimination.
Production of Treg-Recruiting Chemokines by Tumor-Associated Macrophages
TAMs may also indirectly inhibit the anti-tumor immune response by producing chemokines such as CCL2, CCL5, CCL20, and CCL22, which are involved in recruiting regulatory T cells (Tregs) to the tumor microenvironment. Tregs subsequently inhibit the activities of CD4+ and CD8+ effector T cells, natural killer (NK) cells, NKT cells, and antigen-presenting cells (APCs) through multiple mechanisms. These include the production of immunosuppressive cytokines and extracellular adenosine, growth factor depletion, induction of infectious tolerance and apoptosis, and CTLA-4- and LAG-3-mediated inhibition of dendritic cell maturation.
Production of PGE2 by Tumor-Associated Macrophages
TAMs also inhibit the anti-tumor immune response through production of prostaglandin E2 (PGE2).TAMs produce high levels of PGE2 through the up-regulated expression of the PGE2-producing enzymes, COX2 and PGES1. PGE2 inhibits IFN-gamma production and the cytolytic activity of natural killer (NK) cells. Additionally, it inhibits the early stages of dendritic cell differentiation, promotes MDSC expansion and suppressive activity, suppresses IL-2 production and responsiveness in T cells, and inhibits NK/Th1/CD8+ T cell-mediated immune responses while promoting Th2/Th17/Treg responses.
Monocyte/Macrophage Recruitment to the Tumor Microenvironment
The initial step in TAM-mediated immunosuppression in the TME is the recruitment of these cells to the tumor site. Monocytes are recruited to the tumor microenvironment (TME) by chemokines such as CSF-1, CCL2, and CCL5, which are secreted by tumor cells. Once present in the tumor tissue, monocytes differentiate into macrophages. As a result, macrophages accumulate in tumors, where they play a key role in promoting tumor progression. TAMs secrete growth factors, cytokines, chemokines, and other factors that not only inhibit the anti-tumor immune response, but also drive tumor cell proliferation, angiogenesis, invasion, and metastasis.
To learn more, explore the following macrophage-related resources:
Monocyte/Macrophage Research Area Page
Macrophage and Macrophage Activation State Cell Marker pages
The Complex Biology of Macrophages: Origins, Functions, & Activation States Poster
Get Print Copy of this Pathway