Human Anti-Dengue Virus IgG ELISA Kit
Human Anti-Dengue Virus IgG ELISA Kit Summary
Kit Summary
The Human Anti-Dengue Virus IgG ELISA Kit is a 3.5 hour solid phase ELISA designed to measure Dengue Virus IgG antibodies in human serum and EDTA plasma.
Key Benefits
- High specificity with minimal cross-reactivity with Zika Virus IgG antibodies
- High sensitivity
- Detection of Dengue Virus IgG antibodies later in the infection when IgM antibodies may be undetectable
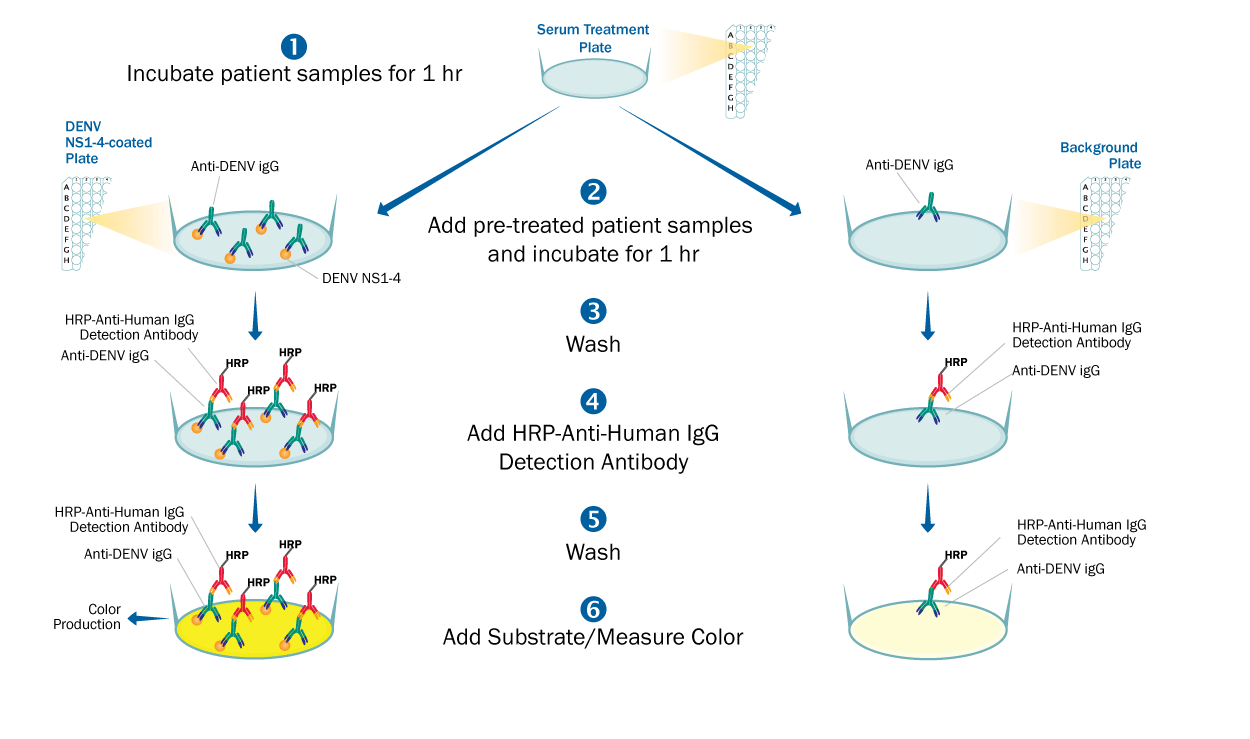
This is an antigen-down enzyme immunoassay where recombinant Dengue Virus strains 1, 2, 3, & 4 NS1 antigens are pre-coated onto a 96-well microplate and used to bind antibodies found in the sample. When the sample is added (such as human serum or EDTA plasma), antibodies found in the sample that recognize Dengue Virus NS1 antigens bind the antigen coated plate and are retained in the well. After washing away unbound substances, an enzyme linked polyclonal antibody specific for human IgG is added to the wells. Following a wash to remove any unbound enzyme linked antibody, a substrate is added to the wells and color develops in proportion to the amount of IgG antibodies in the sample bound to the Dengue Virus NS1 antigens. The color development is stopped, and the intensity of the color is measured.
The potential for false positives due to Dengue Virus NS1 antigen cross-reactive antibodies to related flaviviruses, such as Zika Virus, is minimized by treatment of the samples. Samples are treated with a propriety treatment reagent prior to being added to the Dengue Virus NS1 antigen coated plate. Sample specific background is determined by adding identically treated samples to an uncoated background plate and measuring the amount of IgG antibodies non-specifically bound to the well. To interpret results, net sample OD readings are calculated by subtracting each sample background plate reading from the Dengue Virus NS1 antigen plate reading.
Precision
Intra-Assay Precision (Precision within an assay)
Three Anti-Dengue Virus IgG positive samples with low, middle, and high net O.D. were tested twenty-four times on one plate to assess intra-assay precision.
Inter-assay Precision (Precision between assays)
Three Anti-Dengue Virus IgG positive samples with low, middle, and high net O.D. were tested in twenty separate assays to assess inter-assay precision. Assays were performed by at least three technicians.
| Intra-Assay Precision | Inter-Assay Precision | |||||
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 24 | 24 | 24 | 20 | 20 | 20 |
| Net O.D. | 0.838 | 1.776 | 2.811 | 0.709 | 1.556 | 2.756 |
| Standard deviation | 0.019 | 0.033 | 0.039 | 0.079 | 0.151 | 0.276 |
| CV (%) | 2.3 | 1.9 | 1.4 | 11.1 | 9.7 | 10 |
Specificity
This assay recognizes Dengue Virus specific human IgG antibodies with minimal cross-reactivity of human Zika IgG antibodies.
Dengue fever is a mosquito-borne tropical disease caused by the Dengue Virus (DENV), a member of the flavivirus genus. Symptoms of Dengue fever include a high fever, headache, vomiting, muscle and joint pains and a characteristic skin rash. In a small proportion of cases, the disease develops into the life-threatening Dengue hemorrhagic fever, which results in bleeding, low levels of blood platelets and blood plasma leakage, or into Dengue shock syndrome, where dangerously low blood pressure occurs (1). The incidence of Dengue Virus infections has grown dramatically around the world in recent decades. The actual numbers of Dengue cases are underreported, and many cases are misclassified. One recent estimate indicates 390 million Dengue infections per year of which 96 million manifest a level of disease severity (2). Serological diagnosis of Dengue infection is complicated by cross-reactivity among other flaviviruses, such as Zika Virus (ZIKV) (3). Because DENV, ZIKV, and other flavivirus co-circulate in endemic regions and share high sequence similarity, there is a high possibility of IgM and IgG cross-reactivity in immunoassays (4). There is a need for a simple serological test that displays high Dengue specificity with minimal cross-reactivity with other flaviviruses.
- Kularatne, S.A. (2015) BMJ, 351:h4661.
- Bhatt, S. et al. (2013) Nature, 496:504.
- Musso, D. et al. (2016) Clin. Microbiol. Rev. 29:487.
- Cabral-Castro, M.J. et al. (2016) J. Clin. Virol. 82:108.
Specifications
Product Datasheets
FAQs
-
What would cause the Calculated Net O.D. Value for the Treatment Control to be greater than 0.170?
The Treatment Control is a Flavivirus antibody control for cross-reactivity. A high reading may indicate error in preparation of the Treatment Control, or the Treatment Reagent is expired.
-
Does the kit contain live Virus?
Purified Dengue Virus or other Flaviviruses are not components in the kit. Some components in this kit contain human source materials and have been tested negative for antibodies to HIV 1 & HIV 2, Hepatitis C and Hepatitis B surface antigen. However, material should be handled as potentially infectious.
-
Can the Human Anti-Dengue Virus IgG ELISA Kit be used for diagnostic purposes?
No, it is not approved for diagnostic use. The Human Dengue Virus IgG ELISA Kit is for Research Use Only.
-
Does this Human Anti-Dengue Virus IgG ELISA Kit show cross-reaction to Zika, West Nile, Yellow Fever, etc.?
Sample treatment minimizes cross reaction to Zika Virus. Cross-reaction with other Flavivirus such as West Nile or Yellow Fever is likely to be minimal, however has not been fully tested
-
What is the formulation of the Treatment Reagent in Catalog # DENG00, Human Anti-Dengue Virus IgG ELISA Kit?
This is a propriety formulation designed to reduce cross-reactivity caused by IgG antibodies to Zika, West Nile, Yellow Fever, and other related Flavivirus.
-
What is the advantage of the Human Anti-Dengue Virus IgG ELISA Kit when compared to PCR?
The viral load of Dengue Virus can be of low intensity and short duration, so detection via real-time RT-PCR has a very limited timeframe, and typically should be performed early (up to 10 days) after onset of illness.
Our Human Anti-Dengue Virus IgG ELISA Kit can detect neutralizing IgG antibodies to Dengue Virus as early as 5 days after the onset of illness, and the IgG antibodies formed as immune response are expected to persist for many years, possibly lifelong, after infection.
-
Why are the background OD readings high with some patient samples when using the Human Anti-Dengue Virus IgG ELISA kit?
It is normal to see high background OD readings with some patient samples when using the Human Anti-Dengue Virus IgG ELISA kit. It is not known why this is observed. This is one reason specific background OD readings are determined for each patient sample in each assay, which is a unique feature, to this kit to reduce the likelihood of false positives from high background.
Reviews for Human Anti-Dengue Virus IgG ELISA Kit
There are currently no reviews for this product. Be the first to review Human Anti-Dengue Virus IgG ELISA Kit and earn rewards!
Have you used Human Anti-Dengue Virus IgG ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image


