Human CD36/SR-B3 Antibody Summary
Accession # P16671
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human CD36/SR‑B3 by Western Blot. Western blot shows lysates of human placenta tissue and human platelets. PVDF membrane was probed with 1 µg/mL of Rabbit Anti-Human CD36/SR-B3 Monoclonal Antibody (Catalog # MAB19554) followed by HRP-conjugated Anti-Rabbit IgG Secondary Antibody (Catalog # HAF008). A specific band was detected for CD36/SR-B3 at approximately 85 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
CD36/SR‑B3 in U937 Human Cell Line. CD36/SR-B3 was detected in immersion fixed U937 human histiocytic lymphoma cell line using Rabbit Anti-Human CD36/SR-B3 Monoclonal Antibody (Catalog # MAB19554) at 8 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Rabbit IgG Secondary Antibody (red; Catalog # NL004) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
CD36/SR‑B3 in Human Heart. CD36/SR-B3 was detected in immersion fixed paraffin-embedded sections of human heart using Rabbit Anti-Human CD36/SR-B3 Monoclonal Antibody (Catalog # MAB19554) at 3 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Rabbit IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC003). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cardiomyocyte membranes. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
CD36/SR‑B3 in Human Heart. CD36/SR-B3 was detected in immersion fixed paraffin-embedded sections of human heart using Rabbit Anti-Human CD36/SR-B3 Monoclonal Antibody (Catalog # MAB19554) at 3 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Rabbit IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC003). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to plasma membrane. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
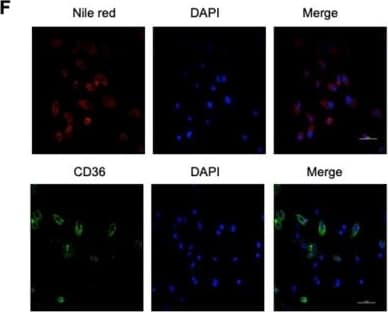
Detection of Mouse CD36/SR-B3 by Immunocytochemistry/Immunofluorescence High fat diets induced CD36 expression and promoted metastasis in mice. (A) SGC 7901 cells were injected into the tail vein of nude mice (n = 9 for each group) fed either a HFD or a normal chow diet. Photos of representative lung tissue samples in each group are shown. (B) Left: The histogram shows the proportion of mice with lung metastasis in each group. “Met” is short for “metastasis,” and “Met-free” represents “metastasis-free.” Right: Mann Whitney test was used to evaluate the number of metastatic nodes in the lungs of each group. (C) Representative CD36 staining of the metastatic nodes in mouse lungs from the HFD or normal chow diet groups. (D) CD36 mRNA levels in MKN-45 or SGC 7901 cells were assessed by real-time PCR after treatment with the indicated concentration of PA or control solvent for 24 h. The values shown are expressed as the means ± SD of three independent experiments. (E) Flow cytometry analysis of CD36 levels in MKN-45 or SGC 7901 cells after treatment with the indicated concentration of PA or control solvent for 24 h. K isotype IgG was used as a negative control. (F, G) Immunofluorescence staining showed the FA and CD36 levels in SGC 7901 cells without (F) or with (G) 0.4 µM PA treatment for 24 h. FAs were stained by Nile red. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31410220), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
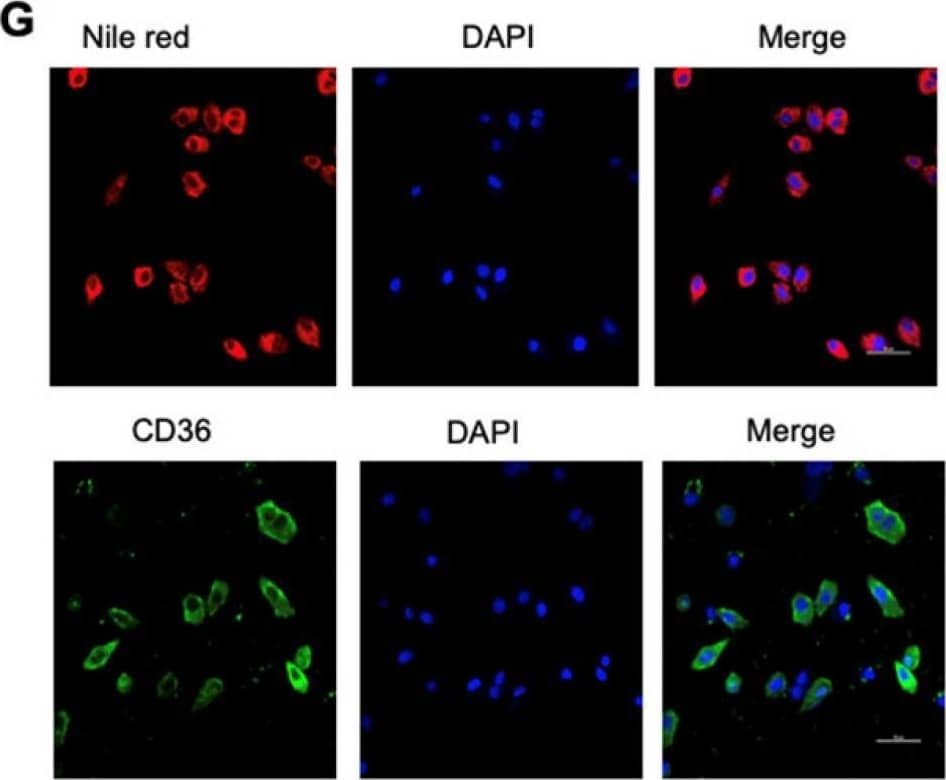
Detection of CD36/SR-B3 by Immunocytochemistry/ Immunofluorescence High fat diets induced CD36 expression and promoted metastasis in mice. (A) SGC 7901 cells were injected into the tail vein of nude mice (n = 9 for each group) fed either a HFD or a normal chow diet. Photos of representative lung tissue samples in each group are shown. (B) Left: The histogram shows the proportion of mice with lung metastasis in each group. “Met” is short for “metastasis,” and “Met-free” represents “metastasis-free.” Right: Mann Whitney test was used to evaluate the number of metastatic nodes in the lungs of each group. (C) Representative CD36 staining of the metastatic nodes in mouse lungs from the HFD or normal chow diet groups. (D) CD36 mRNA levels in MKN-45 or SGC 7901 cells were assessed by real-time PCR after treatment with the indicated concentration of PA or control solvent for 24 h. The values shown are expressed as the means ± SD of three independent experiments. (E) Flow cytometry analysis of CD36 levels in MKN-45 or SGC 7901 cells after treatment with the indicated concentration of PA or control solvent for 24 h. K isotype IgG was used as a negative control. (F, G) Immunofluorescence staining showed the FA and CD36 levels in SGC 7901 cells without (F) or with (G) 0.4 µM PA treatment for 24 h. FAs were stained by Nile red. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31410220), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD36/SR-B3 by Immunocytochemistry/ Immunofluorescence High fat diets induced CD36 expression and promoted metastasis in mice. (A) SGC 7901 cells were injected into the tail vein of nude mice (n = 9 for each group) fed either a HFD or a normal chow diet. Photos of representative lung tissue samples in each group are shown. (B) Left: The histogram shows the proportion of mice with lung metastasis in each group. “Met” is short for “metastasis,” and “Met-free” represents “metastasis-free.” Right: Mann Whitney test was used to evaluate the number of metastatic nodes in the lungs of each group. (C) Representative CD36 staining of the metastatic nodes in mouse lungs from the HFD or normal chow diet groups. (D) CD36 mRNA levels in MKN-45 or SGC 7901 cells were assessed by real-time PCR after treatment with the indicated concentration of PA or control solvent for 24 h. The values shown are expressed as the means ± SD of three independent experiments. (E) Flow cytometry analysis of CD36 levels in MKN-45 or SGC 7901 cells after treatment with the indicated concentration of PA or control solvent for 24 h. K isotype IgG was used as a negative control. (F, G) Immunofluorescence staining showed the FA and CD36 levels in SGC 7901 cells without (F) or with (G) 0.4 µM PA treatment for 24 h. FAs were stained by Nile red. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31410220), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of CD36/SR-B3 by Western Blot O-GlcNAcylation promoted CD36 transcription via activating the NF-kappa B pathway. (A) CD36 mRNA levels in MKN-45 or SGC 7901 cells were assessed by real-time PCR after a 24-hour treatment with TMG (10 μM) or isometric DMSO. (B) CD36 mRNA levels were assessed in MKN-45 or SGC 7901 OGT-knockout cells by real-time PCR. (C) CD36 mRNA levels in SGC 7901 cells with or without OGT knockout after treatment with the indicated concentration of PA for 24 h. (D) A luciferase reporter assay showed changes in the activity of 45 signal transduction pathways in SGC 7901 cells after a 24-hour treatment with TMG (10 μM). (E) A luciferase reporter assay showed the regulation of CD36 transcription by the indicated transcription factors in HEK 293T cells. (F) The levels of O-GlcNAcylation, RELA, phosphorylated RELA and CD36 in MKN-45 or SGC 7901 cells were assessed by western blotting after treatment with 10 μM TMG or isometric DMSO for the indicated times. beta -actin was used as a loading control. (G) A luciferase reporter assay showed the regulation of CD36 transcription by NF-kappa B after treatment with 10 μM TMG or isometric DMSO for 12 h. (H) CD36 mRNA levels in MKN-45 or SGC 7901 cells were assessed by real-time PCR after the indicated treatment. The values shown are expressed as the means ± SD of three independent experiments. Control: control solvent treatment for 24 h; PA: treatment with 0.4 μM of PA for 24 h; PA+PDTC: treatment with 0.4 μM of PA for 24 h and pretreatment with 50 µmol of pyrrolidine dithiocarbamate for 4 h; DMSO: isometric DMSO treatment for 24 h; TMG: treatment with 10 μM of TMG for 24 h; TMG+PDTC: treatment with 10 μM of TMG for 24 h and pretreatment with 50 µmol of pyrrolidine dithiocarbamate for 4 h. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31410220), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of CD36/SR-B3 by Western Blot PA treatment promoted metastasis and induced CD36 expression through activating the HBP. (A) GFAT and OGT mRNA levels in MKN-45 or SGC 7901 cells were assessed by real-time PCR after treatment with the indicated concentration of PA or control solvent for 3 h. The values shown are expressed as the means ± SD of three independent experiments. (B) GFAT, OGT and CD36 levels in MKN-45 or SGC 7901 cells were assessed by western blotting after treatment with the indicated concentration of PA or control solvent for 3 h. (C) The levels of O-GlcNAcylation in MKN-45 or SGC 7901 cells were assessed by western blotting after treatment with 10 μM TMG or isometric DMSO for 24 h. beta -actin was used as a loading control. (D) Transwell migration and invasion assay of MKN-45 and SGC 7901 cells after10 μM TMG or isometric DMSO treatment for 24 h. (E) Indicated cells were injected into nude mice (n = 10 for each group) via the tail vein and mice were fed with HFD. Animals were sacrificed at 6 weeks after the injections. Representative HE staining of the metastatic nodes in mouse lungs from each group. (F) Left: The histogram shows the proportion of mice with lung metastasis in each group. “Met” is short for “metastasis,” and “Met-free” represents “metastasis-free.” Right: Mann Whitney test was used to evaluate the number of metastatic nodes in the lungs of each group. * represents Mann Whitney test p < 0.05. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/31410220), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: CD36/SR-B3
CD36, alternatively known as platelet membrane glycoprotein IV (GPIV), GPIIIb, thrombospondin receptor, collagen receptor, fatty acid translocase (FAT), and scavenger receptor class B, member 3 (SR-B3), is an integral membrane glycoprotein that has multiple physiological functions (1). It is broadly expressed on a variety of cell types including microvascular endothelium, adipocytes, skeletal muscle, epithelial cells of the retina, breast, and intestine, smooth muscle cells, erythroid precursors, platelets, megakaryocytes, dendritic cells, monocytes/macrophages, and microglia (1, 2). As a member of the scavenger receptor family, CD36 is a multiligand pattern recognition receptor that interacts with a large number of structurally dissimilar ligands, including long chain fatty acid (LCFA), advanced glycation end products (AGE), thrombospondin-1, oxidized low-density lipoproteins (oxLDLs), high density lipoprotein (HDL), phosphatidylserine, apoptotic cells,
beta ‑amyloid fibrils (fA beta ), collagens I and IV, and Plasmodium falciparum-infected erythrocytes (3). CD36 is required for the anti-angiogenic effects of thrombospondin-1 in the corneal neovascularization assay (4). It plays a role in lipid metabolism and has been identified as a fatty acid translocase necessary for the binding and transport of LCFA in cells and tissues (5). CD36 has been implicated in the clearance of apoptotic cells and cell debris and has also been shown to mediate the internalization and degradation of a variety of its ligands such as oxLDL, AGE and fA beta (3). Upon ligand binding, CD36 transduces signals that mediate a wide range of pro-inflammatory cellular responses (2). CD36 plays a significant role in the initiation and pathogenesis of chronic inflammatory diseases such as Alzheimer’s disease and atherosclerosis (2, 3). The human CD36 gene encodes a single-chain 472 amino acid protein containing both an N- and a C-terminal cytoplasmic tail and an extracellular loop.
- Febbraio, M. et al. (2001) J. Clin. Invest. 108:785.
- Khoury, J. et al. (2003) J. Exp. Med. 197:1657.
- Husemann, J. et al. (2002) Glia 40:195.
- Armstrong, L and P. Bornstein (2003) Matrix. Biol. 22:63.
- Febbraio M. et al. (1999) J. Biol. Chem. 274:19055.
Product Datasheets
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human CD36/SR-B3 Antibody
There are currently no reviews for this product. Be the first to review Human CD36/SR-B3 Antibody and earn rewards!
Have you used Human CD36/SR-B3 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image



