Human DC-SIGN/CD209 Antibody
Human DC-SIGN/CD209 Antibody Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of DC‑SIGN in Human DC‑SIGN Transfected 3T3 Mouse Cell Line by Flow Cytometry. Human DC-SIGN and DC-SIGN2 transfected 3T3 mouse embryonic fibroblast cell line were stained with Mouse Anti-Human DC-SIGN Monoclonal Antibody (Catalog # MAB161, filled histograms) or isotype control antibody (Catalog # MAB0041, open histogram), followed by Phycoerythrin-conjugated Anti-Mouse IgG F(ab')2Secondary Antibody (Catalog # F0102B).
 View Larger
View Larger
Detection of DC‑SIGN in Human Monocyte Derived Dendritic Cells by Flow Cytometry. Human monocyte derived dendritic cells were stained with Mouse Anti-Human DC-SIGN Monoclonal Antibody (Catalog # MAB161) followed by PE-conjugated anti-mouse IgG (Catalog # F0102B) and Anti-Human B7-2/CD86 Fluorescein-conjugated Monoclonal Antibody (Catalog # FAB141F). Quadrant markers were set based on control antibody staining (Catalog # MAB0041).
 View Larger
View Larger
Detection of Human DC-SIGN/CD209 by Flow Cytometry Infectivity of KSHV is enhanced in the presence of DC-SIGN and DC-SIGNR.A) 293T cells were transfected with empty pcDNA3 vector or expression constructs for DC-SIGN or DC-SIGNR. After 24 hours, cells were infected with 20 µl Bac16 delta K3 delta K5 or left uninfected as controls. Cells were harvested after additional 24 hours and surface stained with a DC-SIGN/R antibody (H-200) and analyzed by flow cytometry. Top three panels show transfected cells stained for DC-SIGN/R followed by PE- (DC-SIGN), FITC- (DC-SIGNR) or both (vector) conjugated secondary antibodies. Bottom panels shows KSHV infection of 293T cells transiently expressing DC-SIGN or DC-SIGNR. B, left panels) 293 cell lines stably expressing a vector construct, DC-SIGN or DC-SIGNR were fluorescently stained for surface expression of DC-SIGN or DC-SIGNR. The mean channel fluorescence is indicated in the upper right hand corner. Open histograms – secondary antibody alone; shaded histograms – DC-SIGN or DC-SIGNR staining. B, right panel) 293 pcDNA3, DC-SIGN or DC-SIGNR stable cell lines were pre-incubated with a control antibody (anti-ICAM1, 7 µg/ml), with mannan (100 µg/ml) or a monoclonal antibody specific for DC-SIGN (MAB161; 7 µg/ml) for 30 minutes on ice. These cells were then infected with wild type KSHV (Bac16 or rKSHV.219) at an MOI of 0.01. After 72 hours cells were harvested and evaluated for infection by flow cytometry measuring GFP expression. Infection rates were normalized to 293 pcDNA3 cells treated with the control antibody. The fold increase in relative infectivity is indicated. Data are representative of four independent experiments with two performed in triplicate. C) 293 pcDNA3, DC-SIGN or DC-SIGNR stable lines were infected with 50 µl of concentrated Bac16 wildtype (wt), or mutants with deletion of K3 only ( delta K3), K5 only ( delta K5), or deletion of both K3 and K5 ( delta K3 delta K5) as indicated. At 72 hours post-infection, the cells were stained for surface expression of DC-SIGN, DC-SIGNR or MHC class I. GFP fluorescence was used as a marker for infection. MHC I staining is shown for infected 293 DC-SIGNR cells. Inset numbers indicate percentage of cells in each quadrant. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0058056), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Cynomolgus Monkey DC-SIGN/CD209 by Immunohistochemistry Rapid induction and persistence of DC-SIGN, S100 and CD68 post-vaccination in lymphoid tissue.Galleries of representative fields of A DC-SIGN and B CD68 staining in MLN at 3, 7, 10, 21, 125 d.p.i. C Representative fields of DC-SIGN signal tracking through the spleen 3–125 d.p.i. maximal from d3 in red pulp (rp) and marginal zones (mg) D Representative fields of splenic S100 levels illustrate similar staining intensity 3–125 d.p.i. predominantly in the germinal centres and red pulp. Staining intensities scoring were; pale yellow (no staining, −); yellow (very low, +); dark yellow (low, ++), red (medium, +++) and magenta (high, ++++). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25162725), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Cynomolgus Monkey DC-SIGN/CD209 by Immunohistochemistry Rapid induction and persistence of DC-SIGN, S100 and CD68 post-vaccination in lymphoid tissue.Galleries of representative fields of A DC-SIGN and B CD68 staining in MLN at 3, 7, 10, 21, 125 d.p.i. C Representative fields of DC-SIGN signal tracking through the spleen 3–125 d.p.i. maximal from d3 in red pulp (rp) and marginal zones (mg) D Representative fields of splenic S100 levels illustrate similar staining intensity 3–125 d.p.i. predominantly in the germinal centres and red pulp. Staining intensities scoring were; pale yellow (no staining, −); yellow (very low, +); dark yellow (low, ++), red (medium, +++) and magenta (high, ++++). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25162725), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
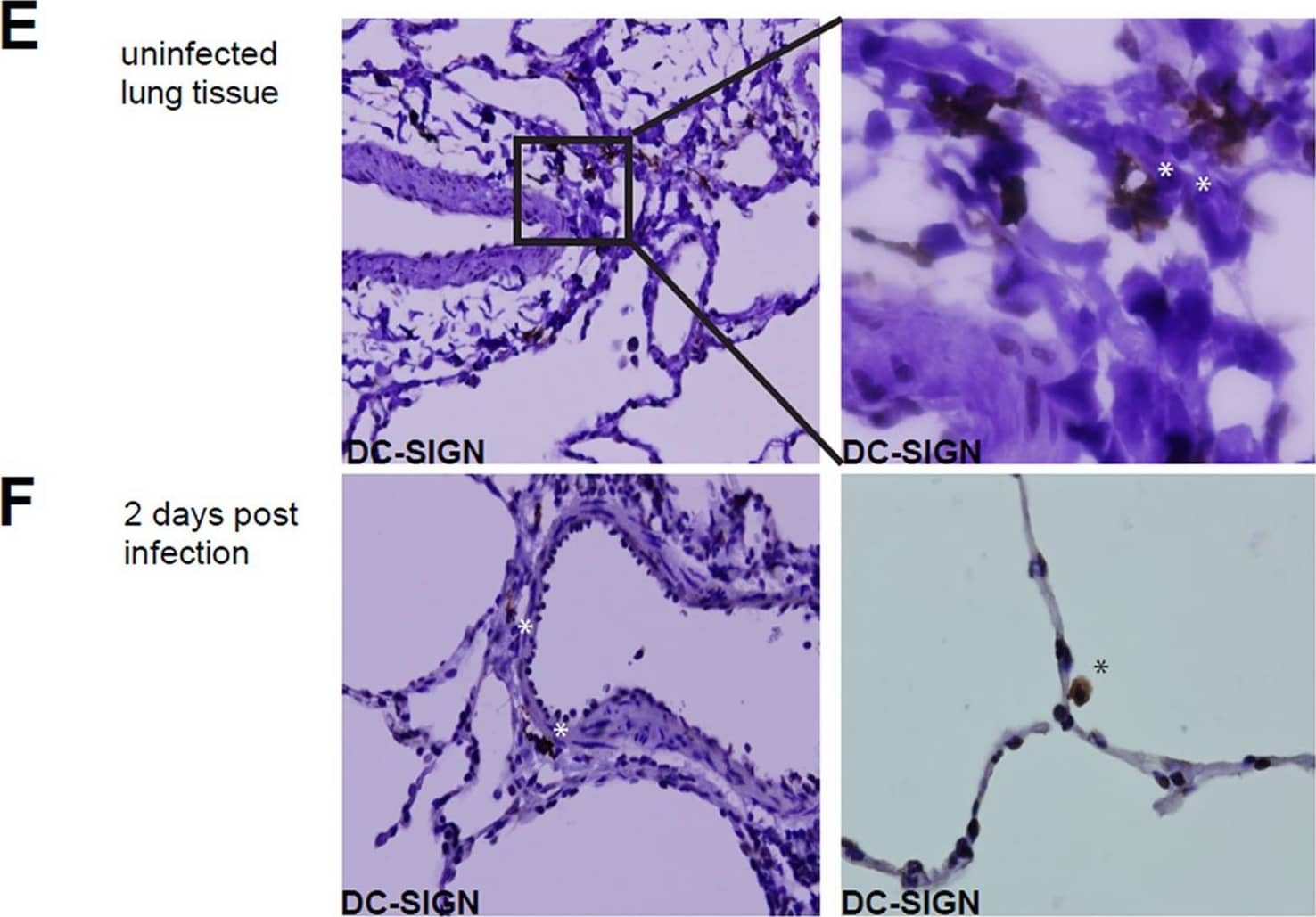
Detection of DC-SIGN/CD209 by Immunohistochemistry Infection of DC-SIGN+ cells in the lung.(A) Infection of the DC-SIGNhi, DC-SIGNlo and DC-SIGN- cells in BAL from MV-infected macaques, determined by detection of EGFP in flow cytometry at day 2–5 d.p.i. Each dot represents an individual animal. Lines indicate geometric means. (B) Macroscopic images from EGFP+ lung slices collected 3 d.p.i., cultured for additional 3,5,7 or 10 days. (C) Phenotype of cells migrating from the ex vivo cultured lung slice, collected from supernatant after 5 days of culturing (D) Phenotype of EGFP+ cells collected from lung slice medium. (E-F) DC-SIGN expression on lung sections from uninfected macaques (E) or 2.d.p.i. (F) Asterisks indicate DC-SIGN reactivity. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/23227146), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
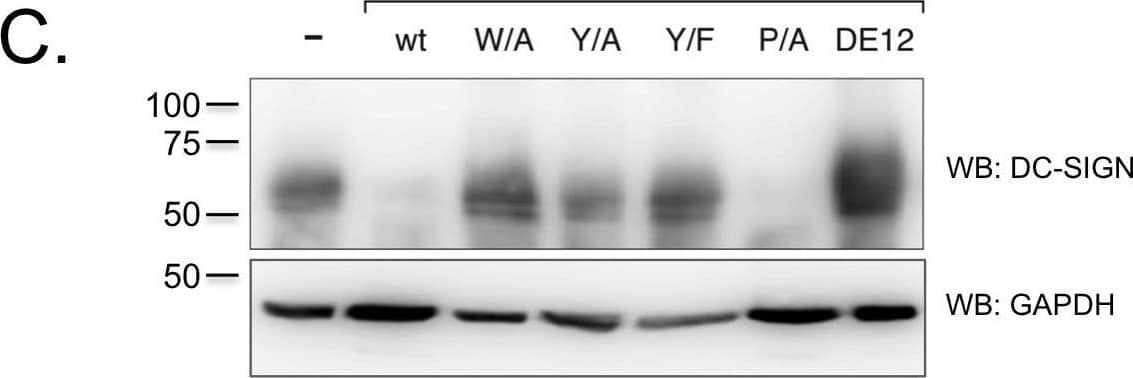
Detection of DC-SIGN/CD209 by Western Blot K3 and K5 affect the stability of DC-SIGN and DC-SIGNR in a RING-CH domain-dependent mechanism.A) 293 cells stably expressing wild-type K3 or K5, or the RING-CH mutant of either viral protein were transiently transfected with 2 µg DC-SIGN or DC-SIGNR constructs. At ∼48 hpt,cells were lysed in RIPA buffer and 30 µg of normalized lysate were loaded per sample. Protein levels of DC-SIGN or DC-SIGNR were determined by WB, and then blots were reprobed for lamin B as a loading control. Data is representative of at least three independent experiments. B) THP-1 cell stably expressing the indicated K5 constructs or empty vector were stained for cell surface levels of DC-SIGN. Solid histogram, empty vector; grey histogram, K5 construct; dotted histogram, isotype control. C) The same THP-1 cell lines used in Panel B, were lysed and subjected to western blotting with either a DC-SIGN (H-200) or GAPDH (O411) antibody, as loading control. Data is representative of at least three independent experiments. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/23460925), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
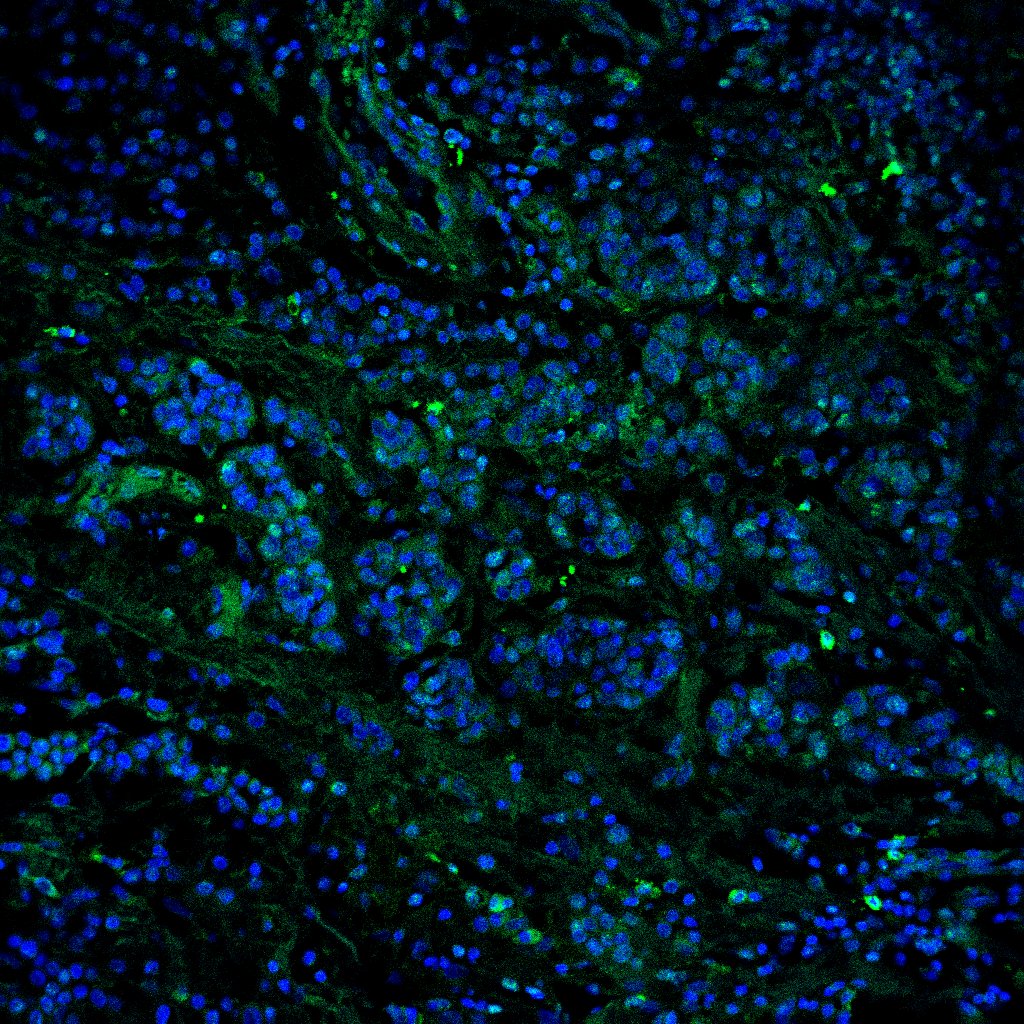
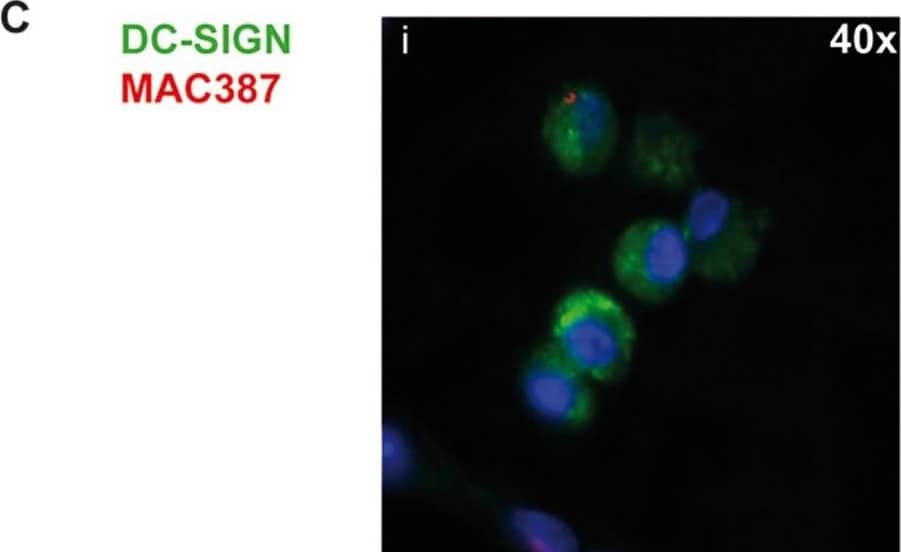
Detection of DC-SIGN/CD209 by Immunocytochemistry/ Immunofluorescence DC-SIGN is expressed by DCs and a subset of macrophages in lymph nodes.(A) TBLN cells were stained for DC-SIGN in combination with DC and macrophage markers and analyzed by flow cytometry. Gray areas show negative controls (DC-SIGN single staining). Percentages of positive cells expressing the markers are annotated in the upper right corner. (B) DC-SIGN expression of CD11c+ and CD83+ cells in TBLNs. (C-D) Dual immunofluorescence staining of DC-SIGN (green) and MAC387 (red) in lung sections 2 or 3 d.p.i. (C i-iv) and axillary lymphoid tissue 4 d.p.i. (D). Nuclei are stained blue with Hoechst. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/23227146), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: DC-SIGN/CD209
Human DC-Sign (dendritic cell-specific ICAM-3 grabbing nonintegrin; also CD209) is a member of the chromosome 19 C-type lectin family that includes DC-SIGN, DC-SIGN-related protein, CD23 and LSECtin (1). DC-SIGN was initially reported to be a 46 kDa, 404 amino acid (aa) type II transmembrane protein that contained a 40 aa cytoplasmic N-terminus, a 21 aa transmembrane segment, and a 343 aa extracellular C-terminus (2). The extracellular region contains a distal, 115 aa Ca++-dependent carbohydrate-binding lectin domain and a membrane-proximal linker segment that is composed of seven 23 aa repeats (2, 3). The lectin domain is believed to preferably bind mannose, either within the context of ICAM-3 (on T cells) or ICAM-2 (on endothelial cells) (2, 4, 5). DC-SIGN expression appears to be limited to dendritic cells (DC) and macrophages (6), and DC interaction with the ICAMs both aids DC cell trafficking and immunological synapse formation (7). Since the original report on DC-SIGN, multiple splice forms have been discovered, generating both membrane-bound and soluble forms (3). There are eight type A isoforms, all of which begin with the same 15 aa of exon 1a. Four contain the transmembrane region of exon II, and four do not (i.e., are soluble). Among these eight type A isoforms, only three retain the entire 343 aa found in the full length form described in reference #2 (the full length form is referred to as type I mDC-SIGN1A) (3). Five additional isoforms utilize an alternate start site, and these are referred to as type B isoforms. These all show a 35 aa cytoplasmic domain. One also has a transmembrane segment; four do not. Two of the five contain full, unspliced extracellular regions (3). All of this suggests enormous complexity in DC-SIGN biology. DC-SIGN is not well conserved across species. Human and mouse show little overall aa identity. In the lectin domain, however, human DC-SIGN shares 68% aa identity with mouse DC-SIGN (8). Human and rhesus monkey DC-SIGN share 91% aa identity over the entire extracellular region (8). A detailed description of the additional properties of this monoclonal antibody (MAB161) have been published (9, 10).
- Liu, W. et al. (2004) J. Biol. Chem. 279:18748.
- Curtis, B.M. et al. (1992) Proc. Natl. Acad. Sci. USA 89:8356.
- Mummidi, S. et al. (2001) J. Biol. Chem. 276:33196.
- Su, S.V. et al. (2004) J. Biol. Chem. 279:19122.
- Cambi, A. et al. (2005) Cell. Microbiol. 7:481.
- Serrano-Gomez, D. et al. (2004) J. Immunol. 173:5635.
- Geijtenbeek, T.B.H. and Y. van Kooyk (2003) Curr. Top. Microbiol. Immunol. 276:32.
- Baribaud, F. et al. (2001) J. Virol. 75:10281.
- Wu, L. et al. (2002) J. Virol. 76:5905.
- Baribaud, F. et al. (2002) J. Virol.76:9135.
Product Datasheets
Citations for Human DC-SIGN/CD209 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
48
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Lactobacillus crispatus S-layer proteins modulate innate immune response and inflammation in the lower female reproductive tract
Authors: Decout, A;Krasias, I;Roberts, L;Molina, B;Charenton, C;Romero, D;Tee, Q;Marchesi, J;Ng, S;Sykes, L;Bennett, P;MacIntyre, D;
Nature communications
Species: Human
Sample Types: Cervical Fluid
Applications: Western Blot -
N-glycosylation of viral glycoprotein is a novel determinant for the tropism and virulence of highly pathogenic tick-borne bunyaviruses
Authors: Shimojima, M;Sugimoto, S;Taniguchi, S;Maeki, T;Yoshikawa, T;Kurosu, T;Tajima, S;Lim, CK;Ebihara, H;
PLoS pathogens
Species: Human, Primate - Cercopithecus aethiops (African Green Monkey)
Sample Types: Whole Cells
Applications: Flow Cytometry -
Epigenetic glycosylation of SARS-CoV-2 impact viral infection through DC&L-SIGN receptors
Authors: Lei Guo, Yan Liang, Heng Li, Huiwen Zheng, Zening Yang, Yanli Chen et al.
iScience
Species: N/A
Sample Types: Recombinant Protein
Applications: ELISA Detection -
Suppression of DC-SIGN and gH Reveals Complex, Subset-Specific Mechanisms for KSHV Entry in Primary B Lymphocytes
Authors: N Palmerin, F Aalam, R Nabiee, M Muniraju, JG Ogembo, J Totonchy
Viruses, 2021-07-31;13(8):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Expression of the human or porcine C-type lectins DC-SIGN/L-SIGN confers susceptibility to porcine epidemic diarrhea virus entry and infection in otherwise refractory cell lines
Authors: P Zhao, LD Xu, Y Zhang, H Cao, R Chen, B Wang, YW Huang
Microbial pathogenesis, 2021-05-19;157(0):104956.
Species: Hamster
Sample Types: Whole Cells
Applications: IHC -
Term Human Placental Trophoblasts Express SARS-CoV-2 Entry Factors ACE2, TMPRSS2, and Furin
Authors: Yingshi Ouyang, Tarique Bagalkot, Wendy Fitzgerald, Elena Sadovsky, Tianjiao Chu, Ana Martínez-Marchal et al.
mSphere
-
Early Colorectal Responses to HIV-1 and Modulation by Antiretroviral Drugs
Authors: C Herrera, MD McRaven, KG Laing, J Dennis, TJ Hope, RJ Shattock
Vaccines, 2021-03-08;9(3):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Blockade of DC-SIGN+ tumor-associated macrophages reactivates anti-tumor immunity and improves immunotherapy in muscle-invasive bladder cancer
Authors: B Hu, Z Wang, H Zeng, Y Qi, Y Chen, T Wang, J Wang, Y Chang, Q Bai, Y Xia, Y Wang, L Liu, Y Zhu, B Dai, J Guo, L Xu, W Zhang, J Xu
Cancer Res., 2020-02-14;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Dengue viruses infect human megakaryocytes, with probable clinical consequences
Authors: MB Vogt, A Lahon, RP Arya, JL Spencer Cl, R Rico-Hesse
PLoS Negl Trop Dis, 2019-11-25;13(11):e0007837.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Cell-to-Cell Spreading of HIV-1 in Myeloid Target Cells Escapes SAMHD1 Restriction
Authors: M Xie, H Leroy, R Mascarau, M Woottum, M Dupont, C Ciccone, A Schmitt, B Raynaud-Me, C Vérollet, J Bouchet, L Bracq, S Benichou
MBio, 2019-11-19;10(6):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Tricarboxylic Acid Cycle Activity and Remodeling of Glycerophosphocholine Lipids Support Cytokine Induction in Response to Fungal Patterns
Authors: S Márquez, JJ Fernández, C Mancebo, C Herrero-Sá, S Alonso, TA Sandoval, M Rodríguez, JR Cubillos-R, O Montero, N Fernández, M Sánchez Cr
Cell Rep, 2019-04-09;27(2):525-536.e4.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
DC-SIGN promotes Japanese encephalitis virus transmission from dendritic cells to T cells via virological synapses
Authors: Ping Wang, Mei Li, Wei Lu, Di Zhang, Qinxue Hu, Yalan Liu
Virologica Sinica
Species: Insect - Mosquito
Sample Types: Whole Cells
Applications: Neutralization -
Properdin and factor H production by human dendritic cells modulates their T-cell stimulatory capacity and is regulated by IFN-?
Authors: KO Dixon, J O'Flynn, N Klar-Moham, MR Daha, C van Kooten
Eur. J. Immunol, 2017-03-01;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Kluyveromyces marxianus and Saccharomyces boulardii Induce Distinct Levels of Dendritic Cell Cytokine Secretion and Significantly Different T Cell Responses In Vitro
PLoS ONE, 2016-11-29;11(11):e0167410.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Metformin Uniquely Prevents Thrombosis by Inhibiting Platelet Activation and mtDNA Release
Sci Rep, 2016-11-02;6(0):36222.
Species: Rat
Sample Types: Whole Cells
Applications: Neutralization -
Clearance of autophagy-associated dying retinal pigment epithelial cells - a possible source for inflammation in age-related macular degeneration
Cell Death Dis, 2016-09-08;7(9):e2367.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
HCV RNA Activates APCs via TLR7/TLR8 While Virus Selectively Stimulates Macrophages Without Inducing Antiviral Responses
Sci Rep, 2016-07-07;6(0):29447.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Analysis of the entry mechanism of Crimean-Congo hemorrhagic fever virus, using a vesicular stomatitis virus pseudotyping system
Authors: Yuto Suda, Shuetsu Fukushi, Hideki Tani, Shin Murakami, Masayuki Saijo, Taisuke Horimoto et al.
Archives of Virology
-
Paracoccidioides brasiliensis interferes on dendritic cells maturation by inhibiting PGE2 production.
Authors: Fernandes R, Bachiega T, Rodrigues D, Golim M, Dias-Melicio L, Balderramas H, Kaneno R, Soares A
PLoS ONE, 2015-03-20;10(3):e0120948.
Species: Human
Sample Types: Whole Cells
Applications: Blocking -
Porphyromonas gingivalis evasion of autophagy and intracellular killing by human myeloid dendritic cells involves DC-SIGN-TLR2 crosstalk.
Authors: El-Awady, Ahmed R, Miles, Brodie, Scisci, Elizabet, Kurago, Zoya B, Palani, Chithra, Arce, Roger M, Waller, Jennifer, Genco, Caroline, Slocum, Connie, Manning, Matthew, Schoenlein, Patricia, Cutler, Christop
PLoS Pathog, 2015-02-13;10(2):e1004647.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Selective susceptibility of human skin antigen presenting cells to productive dengue virus infection.
Authors: Cerny D, Haniffa M, Shin A, Bigliardi P, Tan B, Lee B, Poidinger M, Tan E, Ginhoux F, Fink K
PLoS Pathog, 2014-12-04;10(12):e1004548.
Species: Human
Sample Types: Whole Cells
Applications: Blocking -
Live-attenuated measles virus vaccine targets dendritic cells and macrophages in muscle of nonhuman primates.
Authors: Rennick L, de Vries R, Carsillo T, Lemon K, van Amerongen G, Ludlow M, Nguyen D, Yuksel S, Verburgh R, Haddock P, McQuaid S, Duprex W, de Swart R
J Virol, 2014-12-03;89(4):2192-200.
Species: Primate - Macaca fascicularis (Crab-eating Monkey or Cynomolgus Macaque)
Sample Types: Whole Tissue
Applications: IHC -
Early biodistribution and persistence of a protective live attenuated SIV vaccine elicits localised innate responses in multiple lymphoid tissues.
Authors: Ferguson D, Mattiuzzo G, Ham C, Stebbings R, Li B, Rose N, Mee E, Smith D, Page M, Cranage M, Almond N, Towers G, Berry N
PLoS ONE, 2014-08-27;9(8):e104390.
Species: Primate - Macaca fascicularis (Crab-eating Monkey or Cynomolgus Macaque)
Sample Types: Whole Tissue
Applications: IHC-P -
Platelets and erythrocyte-bound platelets bind infectious HIV-1 in plasma of chronically infected patients.
Authors: Beck, Zoltan, Jagodzinski, Linda L, Eller, Michael, Thelian, Doris, Matyas, Gary R, Kunz, Anjali N, Alving, Carl R
PLoS ONE, 2013-11-25;8(11):e81002.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
In vivo targeting of human DC-SIGN drastically enhances CD8(+) T-cell-mediated protective immunity.
Authors: Hesse C, Ginter W, Forg T, Mayer C, Baru A, Arnold-Schrauf C, Unger W, Kalay H, van Kooyk Y, Berod L, Sparwasser T
Eur J Immunol, 2013-07-22;43(10):2543-53.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Kaposi’s Sarcoma-Associated Herpesvirus K3 and K5 Proteins Down Regulate Both DC-SIGN and DC-SIGNR
Authors: Sabine M. Lang, Meisha O. F. Bynoe, Roshan Karki, Michael A. Tartell, Robert E. Means
PLoS ONE
-
A Prominent Role for DC-SIGN+ Dendritic Cells in Initiation and Dissemination of Measles Virus Infection in Non-Human Primates
Authors: Annelies W. Mesman, Rory D. de Vries, Stephen McQuaid, W. Paul Duprex, Rik L. de Swart, Teunis B. H. Geijtenbeek
PLoS ONE
Species: Macaca fascicularis fascicularis
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages.
Authors: Wu M, Chen S, Yang A, Lin W, Lin Y, Chen N, Tsai I, Li L, Hsieh S
Blood, 2012-11-14;121(1):95-106.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Retagging identifies dendritic cell-specific intercellular adhesion molecule-3 (ICAM3)-grabbing non-integrin (DC-SIGN) protein as a novel receptor for a major allergen from house dust mite.
Authors: Emara M, Royer PJ, Mahdavi J
J. Biol. Chem., 2011-12-28;287(8):5756-63.
Species: Human
Sample Types: Recombinant Protein
Applications: ELISA Development -
Respiratory syncytial virus glycoprotein G interacts with DC-SIGN and L-SIGN to activate ERK1 and ERK2.
Authors: Johnson TR, McLellan JS, Graham BS
J. Virol., 2011-11-16;86(3):1339-47.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Semen clusterin is a novel DC-SIGN ligand.
Authors: Sabatte J, Faigle W, Ceballos A, Morelle W, RodrÃgues CR, Lenicov FR, Thepaut M, Fieschi F, Malchiodi E, Fernandez M, Arenzana-Seisdedos F, Lortat-Jacob H, Michalski JC, Geffner J, Amigorena S
J. Immunol., 2011-10-17;187(10):5299-309.
Species: Human
Sample Types: Seminal Plasma, Whole Cells
Applications: Flow Cytometry, Neutralization -
In situ distribution of HIV-binding CCR5 and C-type lectin receptors in the human endocervical mucosa.
Authors: Hirbod T, Kaldensjo T, Broliden K
PLoS ONE, 2011-09-30;6(9):e25551.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-Fr -
Plasmacytoid dendritic cells infiltrate the skin in positive tuberculin skin test indurations.
Authors: Bond E, Liang F, Sandgren KJ, Smed-Sorensen A, Bergman P, Brighenti S, Adams WC, Betemariam SA, Rangaka MX, Lange C, Wilkinson RJ, Andersson J, Lore K
J. Invest. Dermatol., 2011-08-18;132(1):114-23.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-Fr -
DC-SIGN mediates cell-free infection and transmission of human T-cell lymphotropic virus type 1 by dendritic cells.
Authors: Jain P, Manuel SL, Khan ZK, Ahuja J, Quann K, Wigdahl B
J. Virol., 2009-08-19;83(21):10908-21.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC, Neutralization -
Gram-positive bacteria enhance HIV-1 susceptibility in Langerhans cells, but not in dendritic cells, via Toll-like receptor activation.
Authors: Ogawa Y, Kawamura T, Kimura T, Ito M, Blauvelt A, Shimada S
Blood, 2009-03-11;113(21):5157-66.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
A role of the Ca2+ binding site of DC-SIGN in the phagocytosis of E. coli.
Authors: Iyori M, Ohtani M, Hasebe A, Totsuka Y, Shibata K
Biochem. Biophys. Res. Commun., 2008-10-10;377(2):367-72.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, ICC, Western Blot -
Evolving immunosuppressive microenvironment during human cervical carcinogenesis.
Authors: Kobayashi A, Weinberg V, Darragh T, Smith-McCune K
Mucosal Immunol, 2008-07-02;1(5):412-20.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Glycosylation-dependent interactions of C-type lectin DC-SIGN with colorectal tumor-associated Lewis glycans impair the function and differentiation of monocyte-derived dendritic cells.
Authors: Nonaka M, Ma BY, Murai R, Nakamura N, Baba M, Kawasaki N, Hodohara K, Asano S, Kawasaki T
J. Immunol., 2008-03-01;180(5):3347-56.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Lewis X oligosaccharides targeting to DC-SIGN enhanced antigen-specific immune response.
Authors: Wang J, Zhang Y, Wei J, Zhang X, Zhang B, Zhu Z, Zou W, Wang Y, Mou Z, Ni B, Wu Y
Immunology, 2007-03-20;121(2):174-82.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Expression of DC-SIGN and DC-SIGNR on human sinusoidal endothelium: a role for capturing hepatitis C virus particles.
Authors: Lai WK, Sun PJ, Zhang J, Jennings A, Lalor PF, Hubscher S, McKeating JA, Adams DH
Am. J. Pathol., 2006-07-01;169(1):200-8.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Recombinant vesicular stomatitis viruses encoding simian immunodeficiency virus receptors target infected cells and control infection.
Authors: Okuma K, Boritz E, Walker J, Sarkar A, Alexander L, Rose JK
Virology, 2005-12-01;346(1):86-97.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Cell Lysates
Applications: Western Blot -
BCR/ABL promotes dendritic cell-mediated natural killer cell activation.
Authors: Terme M, Borg C, Guilhot F, Masurier C, Flament C, Wagner EF, Caillat-Zucman S, Bernheim A, Turhan AG, Caignard A, Zitvogel L
Cancer Res., 2005-07-15;65(14):6409-17.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Urine dendritic cells: a noninvasive probe for immune activity in bladder cancer?
Authors: Beatty JD, Islam S, North ME, Knight SC, Ogden CW
BJU Int., 2004-12-01;94(9):1377-83.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells.
Authors: Arrighi JF, Pion M, Garcia E, Escola JM, van Kooyk Y, Geijtenbeek TB, Piguet V
J. Exp. Med., 2004-11-15;200(10):1279-88.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs.
Authors: Borg C, Jalil A, Laderach D, Maruyama K, Wakasugi H, Charrier S, Ryffel B, Cambi A, Figdor C, Vainchenker W, Galy A, Caignard A, Zitvogel L
Blood, 2004-07-08;104(10):3267-75.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Dendritic cells in the human decidua.
Authors: Gardner L, Moffett A
Biol. Reprod., 2003-06-25;69(4):1438-46.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr -
DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells.
Authors: Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman JC, Lagrange PH, Gicquel B, Neyrolles O
J. Exp. Med., 2003-01-06;197(1):121-7.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN.
Authors: Wu L, 107253, Bashirova AA, Martin TD, Villamide L, Mehlhop E, Chertov AO, Unutmaz D, Pope M, Carrington M, KewalRamani VN
IL-6 signalling biomarkers in hospitalised patients with moderate to severe SARS-CoV-2 infection in a single centre study in Sweden, 2002-01-29;99(3):1568-73.
Species: Human, Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Flow Cytometry, Neutralization
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human DC-SIGN/CD209 Antibody
Average Rating: 4 (Based on 3 Reviews)
Have you used Human DC-SIGN/CD209 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: