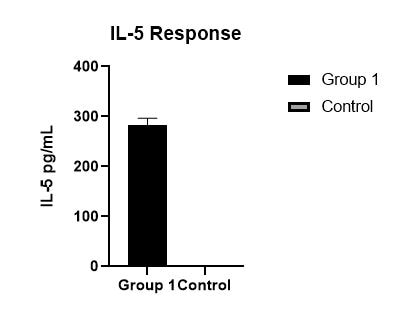
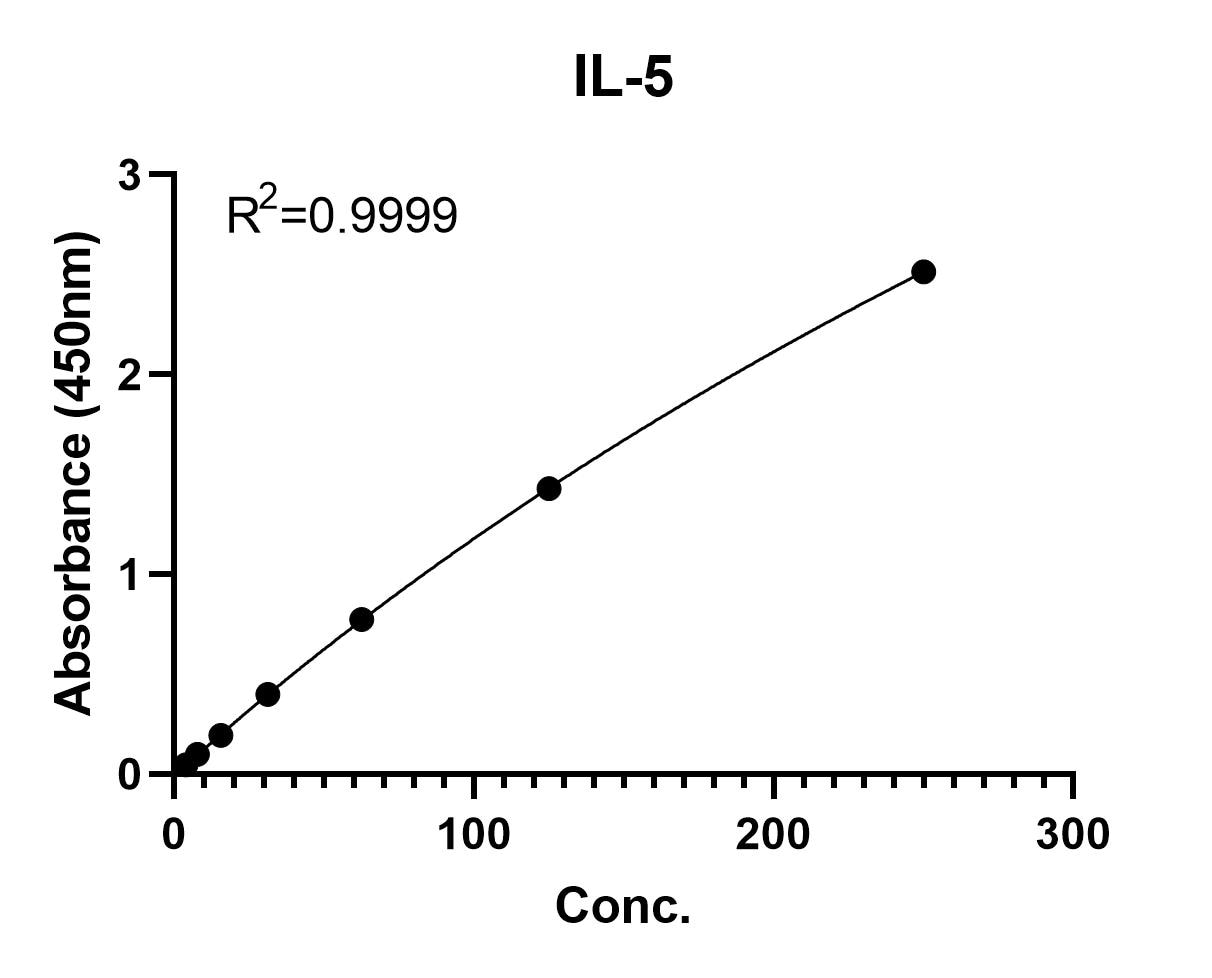
Human IL-5 Quantikine ELISA Kit Summary
Product Summary
Precision
Cell Culture Supernates, Urine
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 28.1 | 89.3 | 170 | 31.2 | 92.6 | 174 |
| Standard Deviation | 0.89 | 2 | 7.27 | 2.5 | 4.2 | 8.7 |
| CV% | 3.2 | 2.2 | 4.2 | 8 | 4.5 | 5 |
Serum, EDTA Plasma, Heparin Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 31.3 | 95.6 | 173 | 32.9 | 97.9 | 180 |
| Standard Deviation | 1.4 | 3.5 | 6.5 | 4 | 6.7 | 8.4 |
| CV% | 4.5 | 3.7 | 3.8 | 12.2 | 6.8 | 4.7 |
Recovery
The recovery of human IL-5 spiked to different levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=4) | 101 | 95-107 |
| EDTA Plasma (n=4) | 104 | 86-118 |
| Heparin Plasma (n=4) | 101 | 87-112 |
| Serum (n=4) | 104 | 88-116 |
| Urine (n=4) | 96 | 91-106 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-5
IL-5 (Interleukin-5) is a secreted cytokine that is expressed as a covalent dimer. It is produced by activated Th2 cells, eosinophils, mast cells, and iNKT cells and promotes eosinophilic and basophilic differentiation and activation. IL-5 signals through a receptor complex containing IL-5 R alpha and the Common beta Chain/CD131 on eosinophils and basophils. Naturally occurring soluble forms of IL-5 R alpha retain the ability to bind IL-5 and limit its bioavailability.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 µL of Assay Diluent to each well.
- Add 100 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours on a horizontal orbital microplate shaker.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes.
- Add 200 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours on the shaker.
- Aspirate and wash 4 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes on the benchtop. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Human IL-5 Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
44
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Cytoimmunological Profile of Lower Airways in Post-COVID-19 Syndrome (PCS): Predictive Value of Bronchoalveolar Lavage
Authors: Dolna-Michno, J;Kopi?ski, P;Przybylski, G;Wypasek, E;Szyma?ska, M;W?drowska, E;Miko?ajczyk, K;Senderek, T;Gagat, M;
Journal of clinical medicine
Species: Human
Sample Types: BALF
-
A Comparative Analysis of Innate Immune Responses and the Structural Characterization of Spike from SARS-CoV-2 Gamma Variants and Subvariants
Authors: Scovino, AM;Dahab, EC;Diniz-Lima, I;de Senna Silveira, E;Barroso, SPC;Cardoso, KM;Nico, D;Makhoul, GJ;da Silva-Junior, EB;Freire-de-Lima, CG;Freire-de-Lima, L;Fonseca, LMD;Valente, N;Nacife, V;Machado, A;Araújo, M;Vieira, GF;Pauvolid-Corrêa, A;Siqueira, M;Morrot, A;
Microorganisms
Species: Human
Sample Types: Cell Culture Supernates
-
Annexin A1 is a cell-intrinsic metalloregulator of zinc in human ILC2s
Authors: Irie, M;Kabata, H;Sasahara, K;Kurihara, M;Shirasaki, Y;Kamatani, T;Baba, R;Matsusaka, M;Koga, S;Masaki, K;Miyata, J;Araki, Y;Kikawada, T;Kabe, Y;Suematsu, M;Yamagishi, M;Uemura, S;Moro, K;Fukunaga, K;
Cell reports
Species: Human
Sample Types: Cell Culture Supernates
-
IL-17 cytokines preferentially act on na�ve CD4+ T cells with the IL-17AF heterodimer inducing the greatest functional changes
Authors: MP Crawford, N Borcherdin, NJ Karandikar
PLoS ONE, 2023-04-28;18(4):e0285166.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-17 cytokines preferentially act on na�ve CD4+ T cells with the IL-17AF heterodimer inducing the greatest functional changes
Authors: MP Crawford, N Borcherdin, NJ Karandikar
PLoS ONE, 2023;18(4):e0285166.
Species: Human
Sample Types: Cell Culture Supernates
-
Heterogeneous Response of Airway Eosinophilia to Anti-IL-5 Biologics in Severe Asthma Patients
Authors: MK Šoki?, M Rijavec, P Korošec, U Bidovec-St, I Kern, R Vantur, S Škrgat
Journal of personalized medicine, 2022-01-07;12(1):.
Species: Human
Sample Types: Serum
-
Socioeconomic status, financial stress, and glucocorticoid resistance among youth with asthma: Testing the moderation effects of maternal involvement and warmth
Authors: Y Jiang, AK Farrell, ET Tobin, HE Mair-Meije, DE Wildman, F Luca, RB Slatcher, S Zilioli
Brain, Behavior, and Immunity, 2021-05-17;0(0):.
Species: Human
Sample Types: Plasma
-
Clinical characteristics of patients with not well-controlled severe asthma in Japan: Analysis of the Keio Severe Asthma Research Program in Japanese population (KEIO-SARP) registry
Authors: T Tanosaki, H Kabata, M Matsusaka, J Miyata, K Masaki, T Mochimaru, S Okuzumi, M Kuwae, R Watanabe, Y Suzuki, K Sayama, K Izuhara, K Asano, K Fukunaga
Allergol Int, 2020-07-08;0(0):.
Species: Human
Sample Types: Serum
-
New participant stratification and combination of urinary biomarkers and confounders could improve diagnostic accuracy for overactive bladder
Authors: S Firouzmand, L Ajori, JS Young
Sci Rep, 2020-02-20;10(1):3085.
Species: Human
Sample Types: Urine
-
Obesity and childhood asthma in male schoolchildren in Saudi Arabia: Is there a role for leptin, interleukin-4, interleukin-5, and interleukin-21?
Authors: M Al-Ayed, K Alshaybari, D Alshehri, A Jamaan, I Nasser, H Alaamri, W Alaseeri, AA Mahfouz, S Ali Alsare, AM Asaad, A Ali Magzou, MA Qureshi, MH Shalayel
Ann Saudi Med, 2019-10-03;39(5):295-301.
Species: Human
Sample Types: Serum
-
Serum sST2 levels predict severe exacerbation of asthma
Authors: M Watanabe, K Nakamoto, T Inui, M Sada, K Honda, M Tamura, Y Ogawa, T Yokoyama, T Saraya, D Kurai, H Ishii, H Takizawa
Respir. Res., 2018-09-03;19(1):169.
Species: Human
Sample Types: Serum
-
Live attenuated B. pertussis BPZE1 rescues the immune functions of Respiratory Syncytial virus infected human dendritic cells by promoting Th1/Th17 responses.
Authors: Schiavoni I, Fedele G, Quattrini A, Bianco M, Schnoeller C, Openshaw P, Locht C, Ausiello C
PLoS ONE, 2014-06-26;9(6):e100166.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunoepidemiological profiling of onchocerciasis patients reveals associations with microfilaria loads and ivermectin intake on both individual and community levels.
Authors: Arndts, Kathrin, Specht, Sabine, Debrah, Alexande, Tamarozzi, Francesc, Klarmann Schulz, Ute, Mand, Sabine, Batsa, Linda, Kwarteng, Alexande, Taylor, Mark, Adjei, Ohene, Martin, Coralie, Layland, Laura E, Hoerauf, Achim
PLoS Negl Trop Dis, 2014-02-20;8(2):e2679.
Species: Human
Sample Types: Plasma
-
Absence of a role for interleukin-13 in inflammatory bowel disease.
Authors: Biancheri P, Di Sabatino A, Ammoscato F, Facciotti F, Caprioli F, Curciarello R, Hoque S, Ghanbari A, Joe-Njoku I, Giuffrida P, Rovedatti L, Geginat J, Corazza G, MacDonald T
Eur J Immunol, 2014-02-01;44(2):370-85.
Species: Human
Sample Types: Cell Culture Supernates
-
Anti-interleukin-5 and multiple autoantibodies are associated with human atherosclerotic diseases and serum interleukin-5 levels.
Authors: Ishigami, Tomoaki, Abe, Kaito, Aoki, Ichiro, Minegishi, Shintaro, Ryo, Akihide, Matsunaga, Satoko, Matsuoka, Kazuhiro, Takeda, Hiroyuki, Sawasaki, Tatsuya, Umemura, Satoshi, Endo, Yaeta
FASEB J, 2013-05-22;27(9):3437-45.
Species: Human
Sample Types: Serum
-
Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma.
Authors: Bachert C, Zhang N, Holtappels G, De Lobel L, Van Cauwenberge P, Liu S, Lin P, Bousquet J, Van Steen K
J. Allergy Clin. Immunol., 2010-11-01;126(5):962.
Species: Human
Sample Types: Tissue Homogenates
-
Staphylococcus aureus in nasal lavage and biopsy of patients with chronic rhinosinusitis.
Authors: Niederfuhr A, Kirsche H, Deutschle T, Poppert S, Riechelmann H, Wellinghausen N
Allergy, 2008-10-01;63(10):1359-67.
Species: Human
Sample Types: Nasal Lavage Fluid
-
Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma.
Authors: Hothersall EJ, Chaudhuri R, McSharry C, Donnelly I, Lafferty J, McMahon AD, Weir CJ, Meiklejohn J, Sattar N, McInnes I, Wood S, Thomson NC
Thorax, 2008-08-29;63(12):1070-5.
Species: Human
Sample Types: Serum
-
Mycobacterium bovis Bacillus Calmette-Guerin suppresses inflammatory Th2 responses by inducing functional alteration of TSLP-activated dendritic cells.
Authors: Yokoi T, Amakawa R, Tanijiri T, Sugimoto H, Torii Y, Amuro H, Son Y, Tajima K, Liu YJ, Ito T, Fukuhara S
Int. Immunol., 2008-08-13;20(10):1321-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Detection of pancreatic cancer using antibody microarray-based serum protein profiling.
Authors: Ingvarsson J, Wingren C, Carlsson A, Ellmark P, Wahren B, Engstrom G, Harmenberg U, Krogh M, Peterson C, Borrebaeck CA
Proteomics, 2008-06-01;8(11):2211-9.
Species: Human
Sample Types: Serum
-
Distinct subsets of human invariant NKT cells differentially regulate T helper responses via dendritic cells.
Authors: Liu TY, Uemura Y, Suzuki M, Narita Y, Hirata S, Ohyama H, Ishihara O, Matsushita S
Eur. J. Immunol., 2008-04-01;38(4):1012-23.
Species: Human
Sample Types: Cell Culture Supernates
-
Systemic responses after bronchial aspirin challenge in sensitive patients with asthma.
Authors: Makowska JS, Grzegorczyk J, Bienkiewicz B, Wozniak M, Kowalski ML
J. Allergy Clin. Immunol., 2007-12-20;121(2):348-54.
Species: Human
Sample Types: Serum
-
Computed tomographic scan-diagnosed chronic obstructive pulmonary disease-emphysema: eotaxin-1 is associated with bronchodilator response and extent of emphysema.
Authors: Miller M, Ramsdell J, Friedman PJ, Cho JY, Renvall M, Broide DH
J. Allergy Clin. Immunol., 2007-11-01;120(5):1118-25.
Species: Human
Sample Types: BALF
-
1, 25 Dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis.
Authors: Vidyarani M, Selvaraj P, Jawahar MS, Narayanan PR
Cytokine, 2007-10-29;40(2):128-34.
Species: Human
Sample Types: Cell Culture Supernates
-
Placental growth factor down-regulates type 1 T helper immune response by modulating the function of dendritic cells.
Authors: Lin YL, Liang YC, Chiang BL
J. Leukoc. Biol., 2007-08-30;82(6):1473-80.
Species: Human
Sample Types: Cell Culture Supernates
-
Relationship between eosinophilia and levels of chemokines (CCL5 and CCL11) and IL-5 in bronchoalveolar lavage fluid of patients with mustard gas-induced pulmonary fibrosis.
Authors: Emad A, Emad Y
J. Clin. Immunol., 2007-07-10;27(6):605-12.
Species: Human
Sample Types: BALF
-
T-lymphocyte activation and the levels of eosinophilic cationic protein and interleukin-5 in asthmatic children with acute exacerbation and effect of glucocorticoid treatment.
Authors: Kocak AK, Bor O, Yildiz B, Erdogan L, Us T
Allergy Asthma Proc, 2006-07-01;27(4):371-7.
Species: Human
Sample Types: Serum
-
A network-based analysis of the late-phase reaction of the skin.
Authors: Benson M, Langston MA, Adner M, Andersson B, Torinssson-Naluai A, Cardell LO
J. Allergy Clin. Immunol., 2006-05-11;118(1):220-5.
Species: Human
Sample Types: Cell Culture Supernates
-
Chemokines indicate allergic bronchopulmonary aspergillosis in patients with cystic fibrosis.
Authors: Hartl D, Latzin P, Zissel G, Krane M, Krauss-Etschmann S, Griese M
Am. J. Respir. Crit. Care Med., 2006-03-16;173(12):1370-6.
Species: Human
Sample Types: Serum
-
Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response.
Authors: Kristjansson S, Bjarnarson SP, Wennergren G, Palsdottir AH, Arnadottir T, Haraldsson A, Jonsdottir I
J. Allergy Clin. Immunol., 2005-10-01;116(4):805-11.
Species: Human
Sample Types: Tissue Secretion
-
Eotaxin-3 and interleukin-5 pleural fluid levels are associated with pleural fluid eosinophilia in post-coronary artery bypass grafting pleural effusions.
Authors: Kalomenidis I, Stathopoulos GT, Barnette R, Guo Y, Peebles RS, Blackwell TS, Light RW
Chest, 2005-06-01;127(6):2094-100.
Species: Human
Sample Types: Pleural Effusion
-
Relationships between allergic inflammation and nasal airflow in children with seasonal allergic rhinitis.
Authors: Ciprandi G, Tosca MA, Marseglia GL, Klersy C
Ann. Allergy Asthma Immunol., 2005-02-01;94(2):258-61.
Species: Human
Sample Types: Nasal Lavage Fluid
-
Antifungal immune reactivity in nasal polyposis.
Authors: Pitzurra L, Bellocchio S, Nocentini A, Bonifazi P, Scardazza R, Gallucci L, Stracci F, Simoncelli C, Bistoni F, Romani L
Infect. Immun., 2004-12-01;72(12):7275-81.
Species: Human
Sample Types: Nasal Lavage Fluid
-
Effects of antiasthmatic agents on the functions of peripheral blood monocyte-derived dendritic cells from atopic patients.
Authors: Saeki S, Matsuse H, Kondo Y, Machida I, Kawano T, Tomari S, Obase Y, Fukushima C, Kohno S
J. Allergy Clin. Immunol., 2004-09-01;114(3):538-44.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of ozone exposure on airway responses to inhaled allergen in asthmatic subjects.
Authors: Chen LL, Tager IB, Peden DB, Christian DL, Ferrando RE, Welch BS, Balmes JR
Chest, 2004-06-01;125(6):2328-35.
Species: Human
Sample Types: BALF
-
Regulation of the interleukin-5 receptor alpha-subunit on peripheral blood eosinophils from healthy subjects.
Authors: Hellman C, 107334, Hallden G, Hylander B, Lundahl J
Lagging strand gap suppression connects BRCA-mediated fork protection to nucleosome assembly through PCNA-dependent CAF-1 recycling, 2003-01-01;131(1):75-81.
Species: Human
Sample Types: Peritoneal Fluid
-
T lymphocyte-mediated changes in airway smooth muscle responsiveness are attributed to induced autocrine release and actions of IL-5 and IL-1beta.
Authors: Hakonarson H, Whelan R, Leiter J, Kim C, Chen M, Campbell D, Grunstein MM
J. Allergy Clin. Immunol., 2002-10-01;110(4):624-33.
Species: Human
Sample Types: Cell Culture Supernates
-
Relationship between the perception of dyspnoea and airway inflammatory markers.
Authors: Jang AS, Choi IS
Respir Med, 2002-03-01;96(3):150-4.
Species: Human
Sample Types: Saliva
-
Structural features of peptide analogs of human histocompatibility leukocyte antigen class I epitopes that are more potent and immunogenic than wild-type peptide.
Authors: Tangri S, Ishioka GY, Huang X, Sidney J, Southwood S, Fikes J, Sette A
J. Exp. Med., 2001-09-17;194(6):833-46.
Species: Human
Sample Types: Cell Culture Supernates
-
Bleomycin stimulates lung fibroblast and epithelial cell lines to release eosinophil chemotactic activity.
Authors: Sato E, Koyama S
Eur. Respir. J., 2000-11-01;16(5):951-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Natural interferon alpha/beta-producing cells link innate and adaptive immunity.
Authors: Kadowaki N, Antonenko S, Lau JY, Liu YJ
J. Exp. Med., 2000-07-17;192(2):219-26.
Species: Human
Sample Types: Cell Culture Supernates
-
Airway inflammation in asthma and perennial allergic rhinitis. Relationship with nonspecific bronchial responsiveness and maximal airway narrowing.
Authors: Alvarez MJ, Olaguibel JM, Garcia BE, Rodriquez A, Tabar AI, Urbiola E
Allergy, 2000-04-01;55(4):355-62.
Species: Human
Sample Types: Sputum
-
Cytokine profiles in parotid saliva from HIV-1-infected individuals: changes associated with opportunistic infections in the oral cavity.
Authors: Black, K P, Merrill, K W, Jackson, S, Katz, J
Oral Microbiol Immunol, 2000-04-01;15(2):74-81.
Species: Human
Sample Types: Saliva
-
Low levels of interferon-gamma in nasal fluid accompany raised levels of T-helper 2 cytokines in children with ongoing allergic rhinitis.
Authors: Benson M, Strannegard IL, Wennergren G, Strannegard O
Pediatr Allergy Immunol, 2000-02-01;11(1):20-8.
Species: Human
Sample Types: Nasal Lavage Fluid
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human IL-5 Quantikine ELISA Kit
Average Rating: 5 (Based on 3 Reviews)
Have you used Human IL-5 Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Easy to use, Good results
Kit worked well, however human plasma had undetectable levels of IL-5