Human/Mouse Arginase 1/ARG1 APC-conjugated Antibody Summary
Met1-Lys322
Accession # P05089
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Arginase 1/ARG1 in HepG2 Human Cell Line by Flow Cytometry. HepG2 human hepatocellular carcinoma cell line was stained with Sheep Anti-Human/Mouse Arginase 1/ARG1 APC-conjugated Antigen Affinity-purified Polyclonal Antibody (Catalog # IC5868A, filled histogram) or isotype control antibody (Catalog # IC016A, open histogram). To facilitate intracellular staining, cells were fixed with Flow Cytometry Fixation Buffer (Catalog # FC004) and permeabilized with Flow Cytometry Permeabilization/Wash Buffer I (Catalog # FC005). View our protocol for Staining Intracellular Molecules.
 View Larger
View Larger
Detection of Arginase 1/ARG1 in Hepa 1-6 Mouse Cell Line by Flow Cytometry. Hepa 1-6 mouse hepatoma cell line was stained with Sheep Anti-Human/Mouse Arginase 1/ARG1 APC-conjugated Antigen Affinity-purified Polyclonal Antibody (Catalog # IC5868A, filled histogram) or isotype control antibody (Catalog # IC016A, open histogram). To facilitate intracellular staining, cells were fixed with Flow Cytometry Fixation Buffer (Catalog # FC004) and permeabilized with Flow Cytometry Permeabilization/Wash Buffer I (Catalog # FC005). View our protocol for Staining Intracellular Molecules.
 View Larger
View Larger
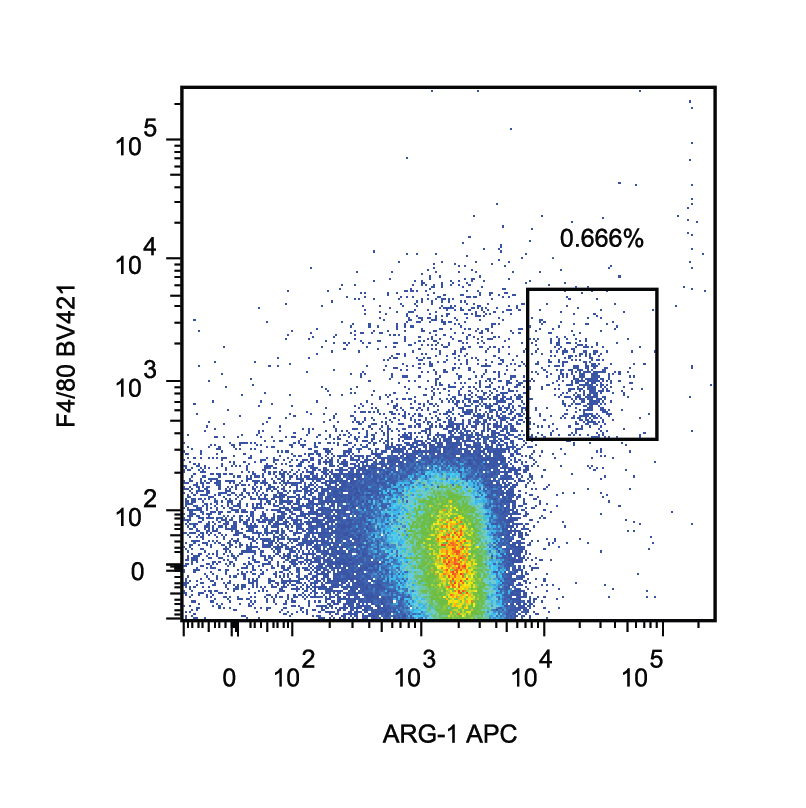
Detection of Mouse Arginase 1/ARG1/liver Arginase by Flow Cytometry ILC2 transfers to apoE−/− mice increase peritoneal B1 cells, eosinophils and AAMs. Flow cytometry gating strategies of B1 cells identified as CD19+B220lowCD11b+IgM+ (a), eosinophils as CD45+SiglecF+CD11b+ cells (d) and AAMs as CD45+CD11b+F480+Arg1+ cells (i) of apoE−/− mice for both control and ILC2 groups respectively as indicated. Percentages and numbers of peritoneal B1 cells (b, c), eosinophils (e, f) and AAMs (j, k). CD11c+ expression from gated eosinophils and respective cell numbers of activated eosinophils identified as CD45+SiglecF+CD11b+CD11c+cells (g, h). Mann Whitney U test, **P < 0.01, ***P < 0.001, ****P < 0.0001. Each data point represents one mouse Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31823769), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Arginase 1/ARG1/liver Arginase by Flow Cytometry Flow cytometry and qRT/PCR analyses of immune cell infiltrates. (A) Flow cytometry gating strategy for macrophages (CD11b+CD45hiLy6G-), microglia (CD11b+CD45loLy6G-), and neutrophils (CD11b+CD45hiLy6G+). (B) Macrophages or microglia were further characterized as alternatively activated (M2) CD206+Ym1+Arg1+ or (C) classically activated (M1) CD86+MHCII+iNOS+. (D) Flow cytometry gating for T cell subsets: Th1 = CD4+IFN-gamma +, Th17 = CD4+IL17+, Treg = CD4+CD25+FOXP3. (E, F, G) Flow cytometry of cells isolated from pooled brain and spinal cord showed that the only significant difference in total numbers of T cell subtypes isolated from the CNS at 21 dpi was a decrease in the ratio of IFN-gamma + to IL-17+ cells in the astroglial CXCL10 knockout mice (n = 3 mice/group, P = 0.0063). (H, I) No significant differences between astroglial CXCL10 knockout and control mice in total numbers of macrophages, microglia, and neutrophils (H) or M1 and M2 subtypes of macrophages and microglia (I) isolated from the CNS at 21 dpi (n = 3). (J) qRT/PCR of spinal cord tissue isolated at 14 dpi normalized to the housekeeping gene HSP90; CXCR3 expression was significantly upregulated in astroglial CXCL10 knockout mice compared to controls (P = 0.0157). IFN-gamma, FOXP3, ROR gamma t, iNOS, and arginase-1 mRNA levels were not significantly different between astroglial CXCL10 knockout and control mice at 14 dpi (n = 6 mice/group). Vertical bars = SEMs. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24924222), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Arginase 1/ARG1/liver Arginase by Flow Cytometry Tumor permissive microenvironment in Brca1 MT mammary glands.aS100a9/S100a8 mRNA expression in the subpopulations of luminal and stromal cells of WT 4-month mammary gland (WTV4MG) and MT 4-months-old virgin mammary gland (MTV4MG) (n = 3 mice). b Protein level of S100a9 in both WT (B477) and MT (G600) mammary epithelial cell lines and tumor tissues by Western blots (n = 3 individual experiment-up and n = 3 mice-down). c Co-staining of S100a9 (red) and CK18 (green) with antibodies on WTV4MG, MTV4MG, WTV6MG, and MTV6MG tissues (n = 3 pairs in each group, Scale bar: 20 μM). d The S100a9 and Arg1 positive cell populations by FACS analysis from the blood and mammary tissues of both WT and MT mice at 4-month and 6-month, respectively (FACS gating strategies see in Supplementary Fig. 8c, n = 3 mice/ group). e Co-staining with S100a9 (red) and CD206 (green) antibodies (left panel) and co-staining with S100a9 (red) and CK18 (green) antibodies (right panel) on tumor-adjacent tissues by IF (40X confocal microscope, Scale bar: 20 μM.) (n = 3 mice and 3 individual experiment). f Secreted S100a9 proteins (left) from both tumor cell and MDSC cells in tumor-adjacent mammary gland (n = 3 mice) and present in the supernatant of cultured cancer cells (right) (n = 3 individual experiment, Scale bar: 10 μM). g Protein levels of S100a9, TGF-beta, and Il-10 in mammary gland tissues of both WT and Brca1 MT mice at 4-month (n = 3 mice). h Protein levels of S100a9, TGF-beta, and IL-10 in mammary tissues of both WT and Brca1 MT mice at 6-month (n = 3 mice). The data are expressed as means ± SD (a) and P values determined by unpaired two-tailed Student’s t test. The experiments were independently repeated three times with similar results (a, b). Source data are provided as a Source data file. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35304461), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, 2 to 8 °C as supplied.
Background: Arginase 1/ARG1
Arginase 1 (ARG1) is a 35‑40 kDa member of the arginase family of enzymes. It is expressed in multiple cell types, including erythrocytes, hepatocytes, neutrophils, smooth muscle and macrophages. ARG1 demonstrates two distinct functions: in the hepatocyte cytoplasm, it catalyzes the conversion of arginine to ornithine and urea, while in multiple cells, it degrades arginine, thus indirectly downregulating NO synthase (NOS) activity by depriving this enzyme of its substrate. Human AGR1 is 322 amino acids (aa) in length. Its enzyme region comprises aa 9‑309 and contains two Mn atoms. ARG1 is modestly active as a monomer, but highly active as a 105 kDa homotrimer. Trimerization is promoted by nitrosylation of Cys303, creating a regulatory feedback loop with NOS. There are two isoform variants, one that shows an eight aa insertion after Gln43, and another that shows a deletion of aa 204‑289. Full-length human ARG1 shares 87% aa identity with mouse and rat ARG1.
Product Datasheets
Citations for Human/Mouse Arginase 1/ARG1 APC-conjugated Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
33
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Myeloid-intrinsic cell cycle-related kinase drives immunosuppression to promote tumorigenesis
Authors: Zhou J, Wang H, Shu T et al.
iScience
-
E2F3 renders an immunosuppressive tumor microenvironment in nasopharyngeal carcinoma: Involvements of the transcription activation of PRC1 and BIRC5
Authors: Qiang Wang, Qi Yu, Yueyang Liu
Immunity, Inflammation and Disease
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Functionally and Metabolically Divergent Melanoma-Associated Macrophages Originate from Common Bone-Marrow Precursors
Authors: Gabriela A. Pizzurro, Kate Bridges, Xiaodong Jiang, Aurobind Vidyarthi, Kathryn Miller-Jensen, Oscar R. Colegio
Cancers (Basel)
-
Targeting tumor-associated macrophages with STING agonism improves the antitumor efficacy of osimertinib in a mouse model of EGFR-mutant lung cancer
Authors: Ziying Lin, Qiwei Wang, Tao Jiang, Weihua Wang, Jean J. Zhao
Frontiers in Immunology
-
beta ig-h3-structured collagen alters macrophage phenotype and function in pancreatic cancer
Authors: Sophie Bachy, Zhichong Wu, Pia Gamradt, Kevin Thierry, Pascale Milani, Julien Chlasta et al.
iScience
-
Helminth resistance is mediated by differential activation of recruited monocyte-derived alveolar macrophages and arginine depletion
Authors: F Chen, DW El-Naccach, JJ Ponessa, A Lemenze, V Espinosa, W Wu, K Lothstein, L Jin, O Antao, JS Weinstein, P Damani-Yok, K Khanna, PJ Murray, A Rivera, MC Siracusa, WC Gause
Cell Reports, 2022-01-11;38(2):110215.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Flow Cytometry -
Ongoing Exposure to Peritoneal Dialysis Fluid Alters Resident Peritoneal Macrophage Phenotype and Activation Propensity
Authors: Tara E. Sutherland, Tovah N. Shaw, Rachel Lennon, Sarah E. Herrick, Dominik Rückerl
Frontiers in Immunology
-
Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke
Authors: Ligen Shi, Zeyu Sun, Wei Su, Fei Xu, Di Xie, Qingxiu Zhang et al.
Immunity
-
Topical arginase inhibition decreases growth of cutaneous squamous cell carcinoma
Authors: A Mittal, M Wang, A Vidyarthi, D Yanez, G Pizzurro, D Thakral, E Tracy, OR Colegio
Scientific Reports, 2021-05-24;11(1):10731.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
High-salt diet downregulates TREM2 expression and blunts efferocytosis of macrophages after acute ischemic stroke
Authors: M Hu, Y Lin, X Men, S Wang, X Sun, Q Zhu, D Lu, S Liu, B Zhang, W Cai, Z Lu
Journal of Neuroinflammation, 2021-04-12;18(1):90.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Inhibition of leucine-rich repeats and calponin homology domain containing 1 accelerates microglia-mediated neuroinflammation in a rat traumatic spinal cord injury model
Authors: Wen-Kai Chen, Lin-Juan Feng, Qiao-Dan Liu, Qing-Feng Ke, Pei-Ya Cai, Pei-Ru Zhang et al.
Journal of Neuroinflammation
-
Midkine rewires the melanoma microenvironment toward a tolerogenic and immune-resistant state
Authors: D Cerezo-Wal, M Contreras-, K Troulé, X Catena, C Mucientes, TG Calvo, E Cañón, C Tejedo, PC Pennacchi, S Hogan, P Kölblinger, H Tejero, AX Chen, N Ibarz, O Graña-Cast, L Martinez, J Muñoz, P Ortiz-Rome, JL Rodriguez-, G Gómez-Lópe, F Al-Shahrou, R Rabadán, MP Levesque, D Olmeda, MS Soengas
Nat Med, 2020-10-19;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
DEK Expression in Breast Cancer Cells Leads to the Alternative Activation of Tumor Associated Macrophages
Authors: NA Pease, MS Shephard, M Sertorio, SE Waltz, LMP Vinnedge
Cancers (Basel), 2020-07-17;12(7):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
The Toxoplasma gondii virulence factor ROP16 acts in cis and trans, and suppresses T cell responses
Authors: Longfei Chen, David A. Christian, Joshua A. Kochanowsky, Anthony T. Phan, Joseph T. Clark, Shuai Wang et al.
Journal of Experimental Medicine
-
U3-1402 sensitizes HER3-expressing tumors to PD-1 blockade by immune activation
Authors: K Haratani, K Yonesaka, S Takamura, O Maenishi, R Kato, N Takegawa, H Kawakami, K Tanaka, H Hayashi, M Takeda, N Maeda, T Kagari, K Hirotani, J Tsurutani, K Nishio, K Doi, M Miyazawa, K Nakagawa
J. Clin. Invest., 2020-01-02;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
ILC2 transfers to apolipoprotein E deficient mice reduce the lipid content of atherosclerotic lesions
Authors: PT Mantani, P Dunér, I Ljungcrant, J Nilsson, H Björkbacka, GN Fredrikson
BMC Immunol., 2019-12-10;20(1):47.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Pro-inflammatory monocyte profile in patients with major depressive disorder and suicide behaviour and how ketamine induces anti-inflammatory M2 macrophages by NMDAR and mTOR
Authors: W Nowak, LN Grendas, LM Sanmarco, IG Estecho, ÁR Arena, N Eberhardt, DE Rodante, MP Aoki, FM Daray, EA Carrera Si, AE Errasti
EBioMedicine, 2019-11-18;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice
Authors: W Cai, X Dai, J Chen, J Zhao, M Xu, L Zhang, B Yang, W Zhang, M Rocha, T Nakao, J Kofler, Y Shi, RA Stetler, X Hu, J Chen
JCI Insight, 2019-10-17;4(20):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Function of CSF1 and IL34 in Macrophage Homeostasis, Inflammation, and Cancer
Authors: WeiYu Lin, Daqi Xu, Cary D. Austin, Patrick Caplazi, Kate Senger, Yonglian Sun et al.
Frontiers in Immunology
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
All trans-retinoic acid protects against acute ischemic stroke by modulating neutrophil functions through STAT1 signaling
Authors: W Cai, J Wang, M Hu, X Chen, Z Lu, JA Bellanti, SG Zheng
J Neuroinflammation, 2019-08-31;16(1):175.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Taenia crassiceps-Excreted/Secreted Products Induce a Defined MicroRNA Profile that Modulates Inflammatory Properties of Macrophages
Authors: D Martínez-S, JD Ruíz-Rosad, C Terrazas, BE Callejas, AR Satoskar, S Partida-Sá, LI Terrazas
J Immunol Res, 2019-05-14;2019(0):2946713.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
IL-6 mediates ER expansion during hyperpolarization of alternatively activated macrophages
Authors: EA Ayaub, K Tandon, M Padwal, J Imani, H Patel, A Dubey, O Mekhael, C Upagupta, A Ayoub, A Dvorkin-Gh, J Murphy, PS Kolb, S Lhotak, JG Dickhout, RC Austin, MR Kolb, CD Richards, K Ask
Immunol. Cell Biol., 2018-11-14;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Schwann Cell Transplantation Subdues the Pro-Inflammatory Innate Immune Cell Response after Spinal Cord Injury
Authors: DD Pearse, J Bastidas, SS Izabel, M Ghosh
Int J Mol Sci, 2018-08-28;19(9):.
Species: Rat
Sample Types: Whole Cells
Applications: Flow Cytometry -
CCR2-dependent monocytes/macrophages exacerbate acute brain injury but promote functional recovery after ischemic stroke in mice
Authors: W Fang, X Zhai, D Han, X Xiong, T Wang, X Zeng, S He, R Liu, M Miyata, B Xu, H Zhao
Theranostics, 2018-06-06;8(13):3530-3543.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Overexpression of OSM and IL-6 impacts the polarization of pro-fibrotic macrophages and the development of bleomycin-induced lung fibrosis
Authors: EA Ayaub, A Dubey, J Imani, F Botelho, MRJ Kolb, CD Richards, K Ask
Sci Rep, 2017-10-16;7(1):13281.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer
Authors: RY Huang, A Francois, AR McGray, A Miliotto, K Odunsi
Oncoimmunology, 2016-10-28;6(1):e1249561.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
A small-molecule inhibitor of SHIP1 reverses age- and diet-associated obesity and metabolic syndrome
Authors: Neetu Srivastava, Sonia Iyer, Raki Sudan, Christie Youngs, Robert W. Engelman, Kyle T. Howard et al.
JCI Insight
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Loss of SOCS3 in myeloid cells prolongs survival in a syngeneic model of glioma
Authors: Braden C. McFarland, Margaret P. Marks, Amber L. Rowse, Samuel C. Fehling, Magda Gerigk, Hongwei Qin et al.
Oncotarget
-
Tasquinimod Modulates Suppressive Myeloid Cells and Enhances Cancer Immunotherapies in Murine Models
Authors: Li Shen, Anette Sundstedt, Michael Ciesielski, Kiersten Marie Miles, Mona Celander, Remi Adelaiye et al.
Cancer Immunology Research
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model.
Authors: Mills Ko E, Ma J, Guo F, Miers L, Lee E, Bannerman P, Burns T, Ko D, Sohn J, Soulika A, Pleasure D
J Neuroinflammation, 2014-06-12;11(0):105.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE.
Authors: Moreno M, Bannerman P, Ma J, Guo F, Miers L, Soulika A, Pleasure D
J Neurosci, 2014-06-11;34(24):8175-85.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Targeting S1P1 Receptor Protects against Murine Immunological Hepatic Injury through Myeloid-Derived Suppressor Cells
Authors: Guangwei Liu, Yujing Bi, Ruoning Wang, Hui Yang, Yan Zhang, Xiao Wang et al.
The Journal of Immunology
-
CD47 restricts antiviral function of alveolar macrophages during influenza virus infection
Authors: Wenzek C, Steinbach P, WirsdOrfer F et al.
iScience
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse Arginase 1/ARG1 APC-conjugated Antibody
Average Rating: 4 (Based on 1 Review)
Have you used Human/Mouse Arginase 1/ARG1 APC-conjugated Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: