Human/Mouse Oct-3/4 Antibody Summary
Met1-Asn265 (Met262Leu)
Accession # Q01860
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
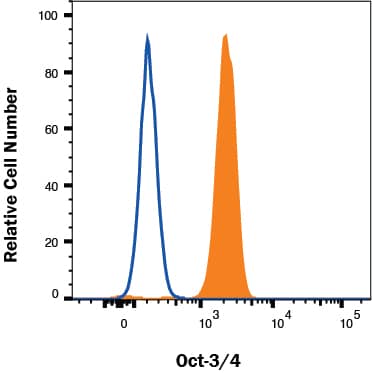
Detection of Oct‑3/4 in NTERA-2 cells by Flow Cytometry NTERA-2 cells were stained with Rat Anti-Human/Mouse Oct‑3/4 Monoclonal Antibody (Catalog # MAB1759, filled histogram) or isotype control antibody (Catalog # MAB0061, open histogram) followed by Allophycocyanin-conjugated Anti-Rat IgG Secondary Antibody (Catalog # F0113). To facilitate intracellular staining, cells were fixed and permeabilized with FlowX FoxP3 Fixation & Permeabilization Buffer Kit (Catalog # FC012). View our protocol for Staining Intracellular Molecules.
 View Larger
View Larger
Detection of Human Oct-3/4 by Western Blot. Western blot shows lysates of BG01V human embryonic stem cells and NTera-2 human testicular embryonic carcinoma cell line. PVDF membrane was probed with 1 µg/mL of Rat Anti-Human/Mouse Oct-3/4 Monoclonal Antibody (Catalog # MAB1759) followed by HRP-conjugated Anti-Rat IgG Secondary Antibody (Catalog # HAF005). A specific band was detected for Oct-3/4 at approximately 46 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Oct‑3/4 in BG01V Human Embryonic Stem Cells. Oct‑3/4 was detected in immersion fixed BG01V human embryonic stem cells using Rat Anti-Human/Mouse Oct‑3/4 Monoclonal Antibody (Catalog # MAB1759) at 10 µg/mL for 3 hours at room temperature. Cells were stained using Rhodamine Red-coupled anti-rat IgG (red) and counterstained with DAPI (blue). Specific staining was localized to nuclei. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
Detection of Human Oct-3/4 by Immunocytochemistry/Immunofluorescence Pertussis toxin does not affect the maintenance of pluripotency pluripotent stem cell colonies or the ability to form all three embryonic germ layers.(A) Pertussis toxin does not affect the maintenance of pluripotency of hES or hiPS cells as assessed by the expression of the pluripotency markers Nanog, Oct4, Sox2, TDGF1, and ZFP42 by quantitative real-time PCR. (B) Immunocytochemistry of TRA-1-81 (green) and Oct-3/4 (red) also did not reveal differences between hES or hiPS cells treated with pertussis toxin and control treatments in pluripotent stem cell colonies. Nuclei were stained with Hoechst (blue). Scale bar, 200 µm. (C) hES or iPS cells treated with pertussis toxin formed embryoid bodies and differentiated into cell types of the all three embryonic germ layers as assessed by immunocytochemistry for alpha -fetoprotein (a marker of endoderm), alpha -smooth muscle actin (mesoderm) and beta III-tubulin (ectoderm). Scale bar, 100 µm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/19936228), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Oct-3/4 by Western Blot Effect of DFX on spherogenicity of human cancer cell lines and treatment with Nanog siRNA in vitro. (A) After treatment with 0.2% DMSO or 50 μM DFX, a single suspension of HSC-2 cells or OE33 cells was used for the sphere formation assay in a 96-well ultra-low attachment plate. DFX suppressed the spherogenicity of HSC-2 cells and OE33 cells. (B) A single suspension of HSC-2 cells or OE33 cells as described above was used for the spheroid colony assay in a 24-well ultra-low attachment plate. The number of spheres over 50 μm in diameter was counted. The experiments were performed in triplicate, and means ± S.E.M. of each group are shown. DFX significantly suppressed the number of spheres. * p < 0.05. (C) HSC-2 cells were transfected with control or si-Nanog for 48 h, and the expression of stemness markers (Nanog, Sox2, Oct3/4, Klf4, c-Myc) was determined with western blot analysis. beta -actin was used as a loading control. siRNA suppressed the expression of Nanog, Oct3/4, and Klf4. (D) HSC-2 cells were transfected with control or si-Nanog for 48 h, and the sphere formation assay was performed. No differences were found in spherogenicity between the control and si-Nanog cultures. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30717462), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Oct-3/4 by Immunocytochemistry/Immunofluorescence Undifferentiated H7 hESC colonies (on passage 15) 3 days after passaging on hFF feeder cells (a) and after 55 passages on MEF feeder cells (b). H7 cells passaged 11 times on hFF feeder cells expressed Nanog (c and d), Oct4 (e and f), SSEA4 (g and h), and TRA-1-60 (i and j). H7 cells on passage 45 on MEF feeder cells expressed Nanog (k and l), Oct4 (m and n), and SSEA4 (o and p). Scale bars 200 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/22315618), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Oct-3/4 by Immunocytochemistry/Immunofluorescence Undifferentiated H7 hESC colonies (on passage 15) 3 days after passaging on hFF feeder cells (a) and after 55 passages on MEF feeder cells (b). H7 cells passaged 11 times on hFF feeder cells expressed Nanog (c and d), Oct4 (e and f), SSEA4 (g and h), and TRA-1-60 (i and j). H7 cells on passage 45 on MEF feeder cells expressed Nanog (k and l), Oct4 (m and n), and SSEA4 (o and p). Scale bars 200 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/22315618), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Oct-3/4 by Western Blot The effect of DFX against stemness of miPS cells in vitro and cytotoxicity analysis. (A) miPS cells were treated with the indicated dose of DFX (0, 1, 10, 50, 100 μM) and subjected to western blot analysis with antibodies to stemness markers (Nanog, Sox2, Oct3/4, Klf4, c-Myc) or beta -actin (loading control). Stemness markers were suppressed by DFX at concentrations over 50 μM. (B) miPS cells treated with 50 μM DFX were cultured in suspension for 72 h. DFX treatment of miPS cells suppressed spherogenesis and GFP expression, which indicates suppression of Nanog. (C) Micrographs of the fluorescence-based Live/Dead assay showing live and dead miPS cells following treatment with 0.2% DMSO (control) or 50 μM DFX (magnification ×40). The morphology of miPS cells after treatment with DFX changed from round to spindle shaped. Almost all cells were stained green, which indicates live cells. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30717462), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Oct-3/4
Oct-3/4, also termed Oct-3 or Oct-4, is a POU transcription factor that is expressed in totipotent embryonic stem and germ cells.(2, 3) Oct-3/4 is required to sustain stem cell self-renewal and pluripotency.(4) It is considered a master regulator of pluripotency that controls lineage commitment and is the most widely recognized marker of totipotent embryonic stem cells.
-
Takeda, J. et al. (1992) Nucleic Acids Res. 20:4613.
-
Scholer, H.R. et al. (1989) EMBO J. 8:2543.
-
Rosner, M.H. et al. (1990) Nature 345:686.
- Niwa, H. et al. (2000) Nat. Genet. 24:372.
Product Datasheets
Citations for Human/Mouse Oct-3/4 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
50
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
CIBZ Regulates Mesodermal and Cardiac Differentiation of by Suppressing T and Mesp1 Expression in Mouse Embryonic Stem Cells
Authors: Tomomi Kotoku, Koji Kosaka, Miki Nishio, Yasumasa Ishida, Masashi Kawaichi, Eishou Matsuda
Scientific Reports
-
CD133-positive cancer stem cells from colo205 human colon adenocarcinoma cell line show resistance to chemotherapy and display a specific metabolomic profile
Authors: Zangiacomi Vincent, Kenichi Urakami, Koji Maruyama, Ken Yamaguchi, Masatoshi Kusuhara
Genes & Cancer
-
In vitro Differentiation of TERT-Transfected Multi-Lineage Progenitor Cells (MLPC) into Immortalized Hepatocyte-Like Cells
Authors: Collins D, Hapke J, Aravalli R, Steer C
HMER
-
Heterozygous loss of Zbtb38 leads to early embryonic lethality via the suppression of Nanog and Sox2 expression
Authors: Miki Nishio, Takuya Matsuura, Shunya Hibi, Shiomi Ohta, Chio Oka, Noriaki Sasai et al.
Cell Proliferation
-
Establishment of a TRPV2 knockout human embryonic stem cell line (WAe009-A-1Y) using episomal vector-based CRISPR/Cas9
Authors: Bai, R;Zhang, S;Gu, X;You, Y;Liu, X;
Stem cell research
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Delayed forebrain excitatory and inhibitory neurogenesis in STRADA-related megalencephaly via mTOR hyperactivity
Authors: Pan, T;Lin, G;Li, X;VanHeyningen, D;Walker, JC;Kohli, S;Saravanan, A;Kondur, A;Jaklic, DC;Pantoja-Gutierrez, S;Vaid, S;Sturza, J;Inoki, K;Imamichi, T;Chang, W;Dang, LT;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Generation of a human embryonic stem cell line (WAe009-A-3B) carrying homozygous TNNT2 gene knockout by CRISPR/Cas9 editing
Authors: Talanaite, D;Wang, H;Qi, M;Lan, F;
Stem cell research
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
PCDH12 loss results in premature neuronal differentiation and impeded migration in a cortical organoid model
Authors: Rakotomamonjy, J;Rylaarsdam, L;Fares-Taie, L;McDermott, S;Davies, D;Yang, G;Fagbemi, F;Epstein, M;Fairbanks-Santana, M;Rozet, JM;Guemez-Gamboa, A;
Cell reports
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Generation of a PPP1CA knockout human pluripotent stem cell line via CRISPR/Cas9
Authors: X Wang, J Sun, C Fu, C Liu
Stem Cell Research, 2023-03-21;69(0):103077.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Establishment of a PPP2CA homozygous knockout human embryonic stem cell line via CRISPR/Cas9 system
Authors: T Ding, Z Gao, H Liu, Y Li, S Liu, L Jian
Stem Cell Research, 2023-01-16;67(0):103029.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Generation of a homozygous TAZ knockout hESCs line by CRISPR/Cas9 system
Authors: M Zhou, P Huang, R Bai, X Liu
Stem Cell Research, 2022-09-20;64(0):102923.
Species: Human
Sample Types: Transfected Whole Cells
Applications: ICC -
The Use of Trichostatin A during Pluripotent Stem Cell Generation Does Not Affect MHC Expression Level
Authors: S Farahi, S Hosseini, H Ghanbarian, SM Hashemi, M Salehi, S Hosseini
Stem Cells International, 2022-02-15;2022(0):9346767.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC/IF -
Brahma safeguards canalization of cardiac mesoderm differentiation
Authors: SK Hota, KS Rao, AP Blair, A Khalilimey, KM Hu, R Thomas, K So, V Kameswaran, J Xu, BJ Polacco, RV Desai, N Chatterjee, A Hsu, JM Muncie, AM Blotnick, SAB Winchester, LS Weinberger, R Hüttenhain, IS Kathiriya, NJ Krogan, JJ Saucerman, BG Bruneau
Nature, 2022-01-26;602(7895):129-134.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
LncCDH5-3:3 Regulates Apoptosis, Proliferation, and Aggressiveness in Human Lung Cancer Cells
Authors: K Kwa?niak, J Czarnik-Kw, K Malysheva, K Pogoda, O Korchynsky, P Rybojad, B Karczmarek, J Tabarkiewi
Cells, 2022-01-23;11(3):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Generation of a homozygous S100A1 knockout human embryonic stem cell line (WAe009-A-73) by the CRISPR/Cas9 editing system
Authors: S Zhang, T Guo, Y Song, M Jiang, Y Jiang, T Dong, S Zhang, H Wang, X Hou
Stem Cell Research, 2021-12-20;59(0):102631.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Establishment of a KCNQ1 homozygous knockout human embryonic stem cell line by episomal vector-based CRISPR/Cas9 system
Authors: X Liu, S Zhang, Y Chang, F Wu, R Bai
Stem Cell Research, 2021-07-22;55(0):102467.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Generating dHAND homozygous knockout human embryonic stem cell line (WAe009-A-59) by episomal vector-based CRISPR/Cas9 system
Authors: W Wang, R Bai, X Song
Stem Cell Research, 2021-07-20;55(0):102471.
Species: Human
Sample Types: Whole Cell
Applications: Flow Cytometry, ICC -
Generation of a TPM1 homozygous knockout embryonic stem cell line by CRISPR/Cas9 editing
Authors: S Ma, Q Xu, R Bai, T Dong, Z Peng, X Liu
Stem Cell Research, 2021-07-19;55(0):102470.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Generation of a TLR2 homozygous knockout human embryonic stem cell line WAe001-A-64 using CRISPR/Cas9 editing
Authors: A Getachew, Z Yang, X Huang, F Wu, YY Liu, C Yan, F Yang, Y Li
Stem Cell Research, 2021-05-21;54(0):102401.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Generation of a TBX5 homozygous knockout embryonic stem cell line (WAe009-A-45) by CRISPR/Cas9 genome editing
Authors: T Zhao, R Bai, F Wu, WJ Lu, J Zhang
Stem Cell Research, 2021-01-06;51(0):102156.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Differentiation of induced pluripotent stem cells into definitive endoderm on Activin A-functionalized gradient surfaces
Authors: L Andreasson, H Evenbratt, R Mobini, S Simonsson
J Biotechnol, 2020-11-02;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay, ICC -
Generation of a Junctophilin-2 homozygous knockout human embryonic stem cell line (WAe009-A-36) by an episomal vector-based CRISPR/Cas9 system
Authors: F Wu, X Li, R Bai, Y Li, J Gao, F Lan
Stem Cell Res, 2020-08-03;48(0):101930.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Generation of an integration-free induced pluripotent stem cell (iPSC) line (ZZUNEUi005-A) from a Wilson's disease patient harboring a homozygous Pro992Leu mutation in ATP7B gene
Authors: L Wei, J Zhang, D Chen, L Feng, C Wu, R Wang, X Li
Stem Cell Res, 2020-07-15;47(0):101909.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Generation of an integration-free induced pluripotent stem cell (iPSC) line (ZZUNEUi002-A) from a patient with spinocerebellar ataxia type 3
Authors: L Wei, J Zhang, D Chen, L Feng, C Wu, R Wang, X Li
Stem Cell Res, 2020-06-29;47(0):101898.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
The self-renewal dental pulp stem cell microtissues challenged by a toxic dental monomer
Authors: G Kaufman, NM Kiburi, D Skrtic
Biosci. Rep., 2020-06-26;40(6):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Generation of an integration-free induced pluripotent stem cell (iPSC) line (ZZUNEUi001-A) from a healthy male individual
Authors: J Zhang, L Wei, R Wang, X Li, Z Liu
Stem Cell Res, 2020-04-24;45(0):101809.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Generation of an integration-free induced pluripotent stem cell (iPSC) line (ZZUNEUi004-A) from a Wilson's disease patient harboring a homozygous Pro992Leu mutation in ATP7B gene
Authors: J Zhang, L Wei, D Chen, L Feng, C Wu, R Wang, X Li, H Liu
Stem Cell Res, 2020-02-20;44(0):101741.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Effect of ectopic high expression of transcription factor OCT4 on the "stemness" characteristics of human bone marrow-derived mesenchymal stromal cells
Authors: X Guo, Y Tang, P Zhang, S Li, Y Chen, B Qian, H Shen, N Zhao
Stem Cell Res Ther, 2019-06-03;10(1):160.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Generation of a GDE heterozygous mutation human embryonic stem cell line WAe001-A-14 by CRISPR/Cas9 editing
Authors: G Xu, D Guo, F Wu, N Abbas, K Lai, F Yuan, K You, Y Liu, Y Zhuang, Y Wu, Y Xu, Y Chen, F Yang, T Pan, YX Li
Stem Cell Res, 2017-12-13;27(0):38-41.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Generation of a SMO homozygous knockout human embryonic stem cell line WAe001-A-16 by CRISPR/Cas9 editing
Authors: F Wu, G Gao, T Pan, Z Yang, G Xu, N Abbas, Y Liu, Y Chen, S Tan, K You, X Ke, Y Zhuang, X Lin, F Yang, YX Li
Stem Cell Res, 2017-12-05;27(0):5-9.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Generation of an ASGR1 homozygous mutant human embryonic stem cell line WAe001-A-6 using CRISPR/Cas9
Authors: Y Xu, Y Wu, D Guo, G Gao, K Lai, F Yang, K Wang, H Wu, L Lai, J Li, K Xu, YX Li
Stem Cell Res, 2017-05-19;22(0):29-32.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
HIF1? regulates single differentiated glioma cell dedifferentiation to stem-like cell phenotypes with high tumorigenic potential under hypoxia
Authors: P Wang, C Lan, S Xiong, X Zhao, Y Shan, R Hu, W Wan, S Yu, B Liao, G Li, J Wang, D Zou, B Chen, H Feng, N Wu
Oncotarget, 2017-04-25;8(17):28074-28092.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Spatial development of gingival fibroblasts and dental pulp cells: Effect of extracellular matrix
Authors: G Kaufman, D Skrtic
Tissue Cell, 2017-04-10;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Molecular and cellular features of murine craniofacial and trunk neural crest cells as stem cell-like cells.
Authors: Hagiwara, Kunie, Obayashi, Takeshi, Sakayori, Nobuyuki, Yamanishi, Emiko, Hayashi, Ryuhei, Osumi, Noriko, Nakazawa, Toru, Nishida, Kohji
PLoS ONE, 2014-01-20;9(1):e84072.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Suppression of OCT4B enhances sensitivity of lung adenocarcinoma A549 cells to cisplatin via increased apoptosis.
Authors: Cortes-Dericks, Lourdes, Yazd, Ehsan Fa, Mowla, Seyed J, Schmid, Ralph A, Karoubi, Golnaz
Anticancer Res, 2013-12-01;33(12):5365-73.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
The effect of human and mouse fibroblast feeder cells on cardiac differentiation of human pluripotent stem cells.
Authors: Pekkanen-Mattila M, Ojala M, Kerkela E, Rajala K, Skottman H, Aalto-Setala K
Stem Cells Int, 2012-01-19;2012(0):875059.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Generation of HIV-1 resistant and functional macrophages from hematopoietic stem cell-derived induced pluripotent stem cells.
Authors: Kambal A, Mitchell G, Cary W, Gruenloh W, Jung Y, Kalomoiris S, Nacey C, McGee J, Lindsey M, Fury B, Bauer G, Nolta JA, Anderson JS
Mol. Ther., 2010-11-30;19(3):584-93.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
A defined glycosaminoglycan-binding substratum for human pluripotent stem cells.
Authors: Klim JR, Li L, Wrighton PJ
Nat. Methods, 2010-11-14;7(12):989-94.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Innate immune suppression enables frequent transfection with RNA encoding reprogramming proteins.
Authors: Angel M, Yanik MF
PLoS ONE, 2010-07-23;5(7):e11756.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Multipotent mesenchymal stem cells from human placenta: critical parameters for isolation and maintenance of stemness after isolation.
Authors: Semenov O, Koestenbauer S, Riegel M, Zech N, Zimmermann R, Zisch A, Malek A
Am J Obstet Gynecol, 2009-12-24;202(2):193.e1-193.e1.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Histone deacetylase inhibition elicits an evolutionarily conserved self-renewal program in embryonic stem cells.
Authors: Ware CB, Wang L, Mecham BH, Shen L, Nelson AM, Bar M, Lamba DA, Dauphin DS, Buckingham B, Askari B, Lim R, Tewari M, Gartler SM, Issa JP, Pavlidis P, Duan Z, Blau CA
Cell Stem Cell, 2009-04-03;4(4):359-69.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Octamer 4 small interfering RNA results in cancer stem cell-like cell apoptosis.
Authors: Hu T, Liu S, Breiter DR, Wang F, Tang Y, Sun S
Cancer Res., 2008-08-15;68(16):6533-40.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Enhancing the reliability and throughput of neurosphere culture on hydrogel microwell arrays.
Authors: Cordey M, Limacher M, Kobel S, Taylor V, Lutolf MP
Stem Cells, 2008-07-31;26(10):2586-94.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Nucleofection mediates high-efficiency stable gene knockdown and transgene expression in human embryonic stem cells.
Authors: Hohenstein KA, Pyle AD, Chern JY, Lock LF, Donovan PJ
Stem Cells, 2008-03-06;26(6):1436-43.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Differential requirements for hematopoietic commitment between human and rhesus embryonic stem cells.
Authors: Rajesh</LastName><ForeNam D</Initial, Rajesh D, Chinnasamy N, Mitalipov SM, Wolf DP, Slukvin I, Thomson JA, Shaaban AF
Stem Cells, 2007-02-01;25(2):490-9.
Species: Human, Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Flow Cytometry -
A Novel Combination Cancer Therapy with Iron Chelator Targeting Cancer Stem Cells via Suppressing Stemness
Authors: Katsura Y, Ohara T, Noma K et al.
Cancers (Basel)
-
Podocytes derived from human induced pluripotent stem cells: characterization, comparison, and modeling of diabetic kidney disease
Authors: Julie Bejoy, Justin M. Farry, Jennifer L. Peek, Mariana C. Cabatu, Felisha M. Williams, Richard C. Welch et al.
Stem Cell Research & Therapy
-
Accelerated protocol for the differentiation of podocytes from human pluripotent stem cells
Authors: Julie Bejoy, Eddie Spencer Qian, Lauren Elizabeth Woodard
STAR Protocols
-
Iron depletion is a novel therapeutic strategy to target cancer stem cells
Authors: Takayuki Ninomiya, Toshiaki Ohara, Kazuhiro Noma, Yuki Katsura, Ryoichi Katsube, Hajime Kashima et al.
Oncotarget
-
Ribosome Incorporation into Somatic Cells Promotes Lineage Transdifferentiation towards Multipotency
Authors: N Ito, K Katoh, H Kushige, Y Saito, T Umemoto, Y Matsuzaki, H Kiyonari, D Kobayashi, M Soga, T Era, N Araki, Y Furuta, T Suda, Y Kida, K Ohta
Sci Rep, 2018-01-26;8(1):1634.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse Oct-3/4 Antibody
Average Rating: 5 (Based on 3 Reviews)
Have you used Human/Mouse Oct-3/4 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: