Human Pappalysin-1/PAPP-A Antibody Summary
Glu82-Asp1214
Accession # Q13219
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human Pappalysin‑1/ PAPP‑A by Western Blot. Western blot shows lysate of human pregnant sera. PVDF membrane was probed with 0.1 µg/mL of Goat Anti-Human Pappalysin-1/PAPP-A Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2487) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for Pappalysin-1/PAPP-A at approximately 200 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
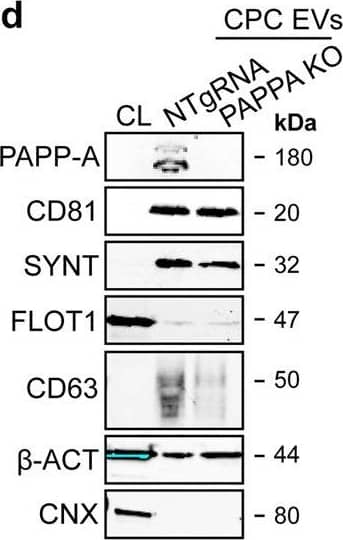
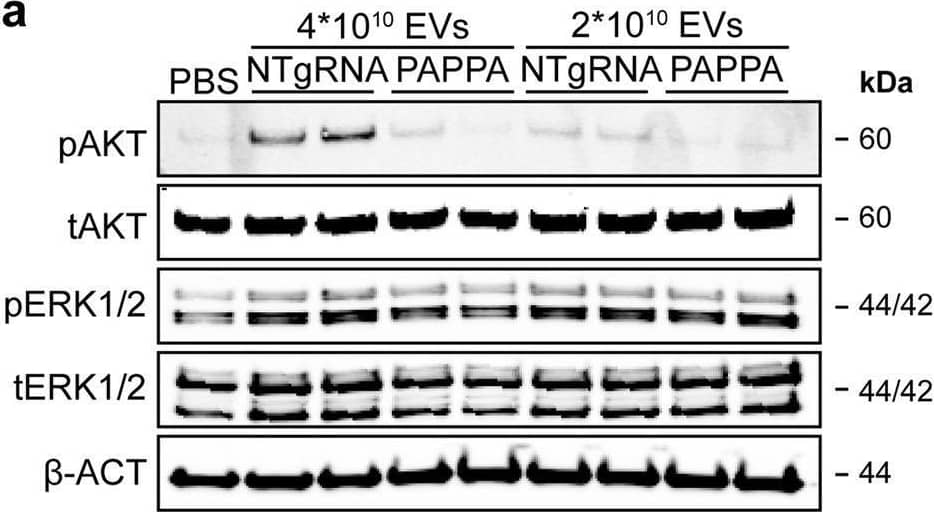
Detection of Human Pappalysin-1/PAPP-A by Western Blot PAPPA KO-EVs were generated using CRISPR/Cas9.a Schematic depicting hypothesized mechanism of (intra)cellular signalling activated by EV-associated PAPP-A, based on identified proteins and significantly altered phosphosites measured in HMEC-1 upon veh-EV stimulation by (phopho)proteomic analysis. Detected proteins in HMEC-1 are displayed in grey, while significantly changing phosphosites present in cluster C1 (see Fig. 3d) are displayed in brown. b Representative western blot analysis of phosphorylated AKT (pAKT), total AKT (tAKT), phosphorylated ERK1/2 (pERK1/2) and total ERK1/2 (tERK1/2) in HMEC-1 treated with 6 × 1010 or 2 × 1010 CPC-EVs, or with 200 ng/mL free IGF-1 after pre-incubation with different doses of picropodophyllin (PPP). beta -actin ( beta -ACT) was included as housekeeping protein (I = phosphorylated protein blot, II = total protein blot). Biological replicates of (b) are displayed in Supplementary Fig. 9g, h. c Sanger sequencing results confirming 1 bp insertion in exon 3 of PAPPA at the CRISPR/Cas9 target site of the PAPPA KO-CPC clone, compared with the NTgRNA polyclonal CPC line. d Western blot analysis showing the absence of PAPP-A in PAPPA KO-EVs, compared with NTgRNA-EVs; the presence of CD81, CD63, Syntenin-1 (SYNT), Flotillin (FLOT1), beta -ACT, and absence of Calnexin (CNX) in both EV populations. FLOT1, beta -ACT and CNX were present in CPC lysate (CL). e Representative NTA plot showing the size distribution and particle concentration of PAPPA KO- and NTgRNA-CPC-EVs. f Protein content per 1 × 1010 PAPPA KO- and NTgRNA-EVs of two representative experiments. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37528162), licensed under a CC-BY license. Not internally tested by R&D Systems.
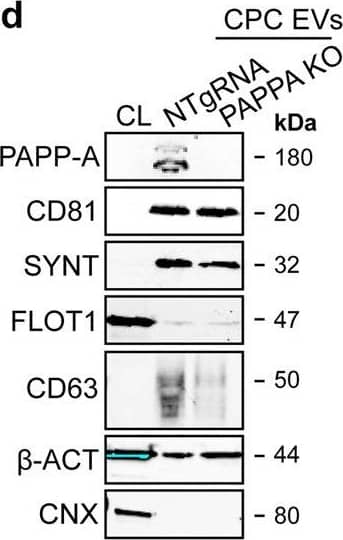 View Larger
View Larger
Detection of Human Pappalysin-1/PAPP-A by Western Blot PAPPA KO-EVs were generated using CRISPR/Cas9.a Schematic depicting hypothesized mechanism of (intra)cellular signalling activated by EV-associated PAPP-A, based on identified proteins and significantly altered phosphosites measured in HMEC-1 upon veh-EV stimulation by (phopho)proteomic analysis. Detected proteins in HMEC-1 are displayed in grey, while significantly changing phosphosites present in cluster C1 (see Fig. 3d) are displayed in brown. b Representative western blot analysis of phosphorylated AKT (pAKT), total AKT (tAKT), phosphorylated ERK1/2 (pERK1/2) and total ERK1/2 (tERK1/2) in HMEC-1 treated with 6 × 1010 or 2 × 1010 CPC-EVs, or with 200 ng/mL free IGF-1 after pre-incubation with different doses of picropodophyllin (PPP). beta -actin ( beta -ACT) was included as housekeeping protein (I = phosphorylated protein blot, II = total protein blot). Biological replicates of (b) are displayed in Supplementary Fig. 9g, h. c Sanger sequencing results confirming 1 bp insertion in exon 3 of PAPPA at the CRISPR/Cas9 target site of the PAPPA KO-CPC clone, compared with the NTgRNA polyclonal CPC line. d Western blot analysis showing the absence of PAPP-A in PAPPA KO-EVs, compared with NTgRNA-EVs; the presence of CD81, CD63, Syntenin-1 (SYNT), Flotillin (FLOT1), beta -ACT, and absence of Calnexin (CNX) in both EV populations. FLOT1, beta -ACT and CNX were present in CPC lysate (CL). e Representative NTA plot showing the size distribution and particle concentration of PAPPA KO- and NTgRNA-CPC-EVs. f Protein content per 1 × 1010 PAPPA KO- and NTgRNA-EVs of two representative experiments. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37528162), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
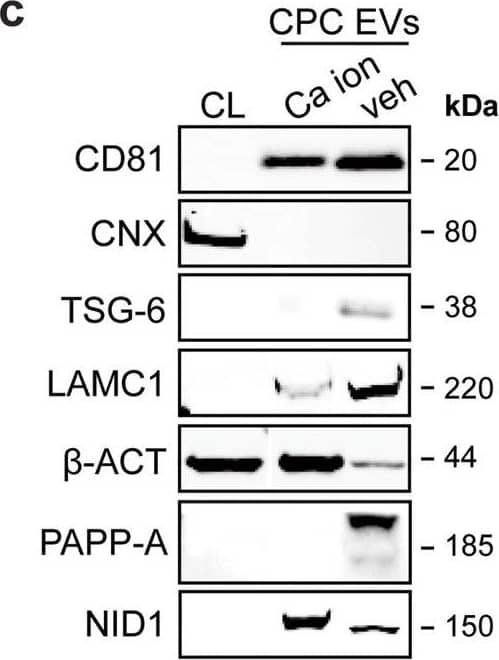
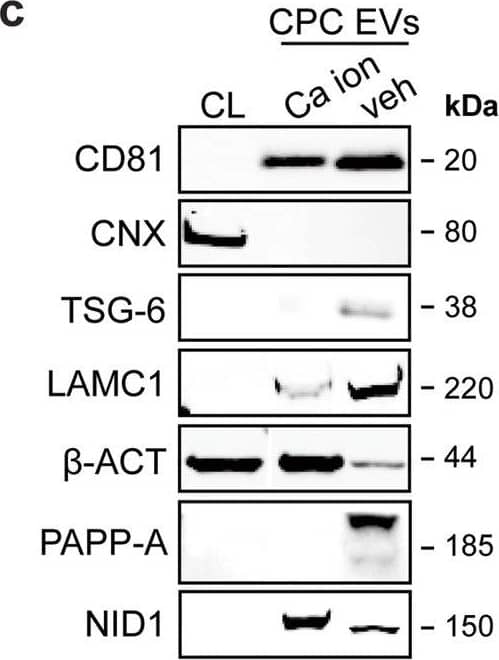
Detection of Human Pappalysin-1/PAPP-A by Western Blot LC–MS/MS identified enriched proteins in veh-EVs compared with Ca ion- and SKOV-3-EVs.a Heat map of protein abundance (log2) of proteins identified in each biological replicate (veh-, Ca ion- and SKOV-3-EVs), as identified by LC–MS/MS. b Volcano plots showing average fold changes for protein abundance (log2) of proteins identified in veh-EVs compared to (i) Ca ion-EVs and (ii) SKOV-3-EVs. P-values were calculated using student’s T-test, and significantly changing proteins (p-value ≤ 0.05 and fold change >2) in veh-EVs are highlighted in red, while significantly changing proteins in Ca ion- and SKOV-3-EVs are highlighted in blue. c Western blot analysis confirming the enrichment of MS-identified proteins NID1, TSG-6, LAMC1, PAPP-A, CD81 and beta -actin ( beta -ACT) in veh-EVs compared with Ca ion-EVs. CNX was solely present in CPC lysate (CL). Complete blots of beta -ACT, PAPP-A and NID1 are displayed in Supplementary Fig. 10. d Venn diagram showing number of proteins with >2-fold significant enrichment (p ≤ 0.05) in veh-EVs compared to Ca ion- and SKOV-3-EVs, and overlap between those two populations. e Gene ontology analysis using PANTHER of enriched biological processes for the 105 overlapping proteins, depicting number of identified proteins in each group, ranked on smallest corrected p-value (−log10(FDR)). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37528162), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
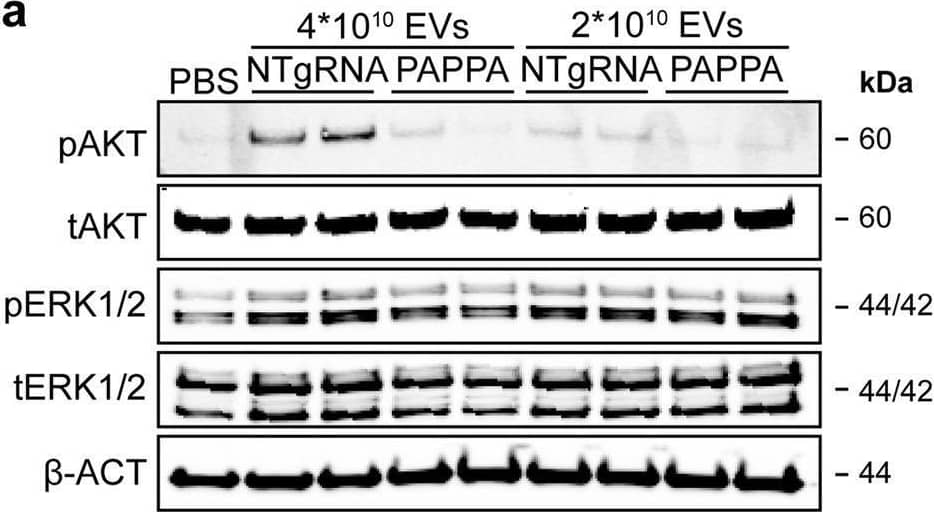
Detection of Pappalysin-1/PAPP-A by Western Blot PAPPA KO-EVs showed reduced activation of intracellular signalling and activation of HMEC-1 migration and sprout formation.a–d Representative western blot analyses of pAKT, tAKT, pERK1/2 and tERK1/2 in HMEC-1 treated with PAPPA KO- and NTgRNA-EVs normalized on two doses of a, b total particle numbers or c, d total protein content. beta -ACT was included as housekeeping protein. b, d Quantification of pAKT, tAKT, pERK1/2 and tERK1/2 expression levels using densitometry expressed as pAKT/AKT and pERK/ERK ratios (n = 3). Biological replicates of (a, c) are also displayed in Supplementary Fig. 9i–k. e Wound healing assay showing effects of 1 µg and 2 × 1010 NTgRNA- and PAPPA KO-EVs on HMEC-1 migration, analysed both as % wound closure and absolute migration distance (n = 3, technical replicates. Data are representative of three biologically independent experiments). f, g Sprouting assay showing NTgRNA- and PAPPA KO-EV-induced HMEC-1 sprout formation on beads, analysed both as (g) mean length per sprout and total sprout length per bead (n = 3, technical replicates. Data are representative of two biologically independent experiments). Data are presented as mean ± SD. *p < 0.033, **p < 0.0021, ***p < 0.0002. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37528162), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Pappalysin-1/PAPP-A by Western Blot LC–MS/MS identified enriched proteins in veh-EVs compared with Ca ion- and SKOV-3-EVs.a Heat map of protein abundance (log2) of proteins identified in each biological replicate (veh-, Ca ion- and SKOV-3-EVs), as identified by LC–MS/MS. b Volcano plots showing average fold changes for protein abundance (log2) of proteins identified in veh-EVs compared to (i) Ca ion-EVs and (ii) SKOV-3-EVs. P-values were calculated using student’s T-test, and significantly changing proteins (p-value ≤ 0.05 and fold change >2) in veh-EVs are highlighted in red, while significantly changing proteins in Ca ion- and SKOV-3-EVs are highlighted in blue. c Western blot analysis confirming the enrichment of MS-identified proteins NID1, TSG-6, LAMC1, PAPP-A, CD81 and beta -actin ( beta -ACT) in veh-EVs compared with Ca ion-EVs. CNX was solely present in CPC lysate (CL). Complete blots of beta -ACT, PAPP-A and NID1 are displayed in Supplementary Fig. 10. d Venn diagram showing number of proteins with >2-fold significant enrichment (p ≤ 0.05) in veh-EVs compared to Ca ion- and SKOV-3-EVs, and overlap between those two populations. e Gene ontology analysis using PANTHER of enriched biological processes for the 105 overlapping proteins, depicting number of identified proteins in each group, ranked on smallest corrected p-value (−log10(FDR)). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37528162), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Pappalysin-1/PAPP-A by Western Blot PAPPA KO-EVs showed reduced activation of intracellular signalling and activation of HMEC-1 migration and sprout formation.a–d Representative western blot analyses of pAKT, tAKT, pERK1/2 and tERK1/2 in HMEC-1 treated with PAPPA KO- and NTgRNA-EVs normalized on two doses of a, b total particle numbers or c, d total protein content. beta -ACT was included as housekeeping protein. b, d Quantification of pAKT, tAKT, pERK1/2 and tERK1/2 expression levels using densitometry expressed as pAKT/AKT and pERK/ERK ratios (n = 3). Biological replicates of (a, c) are also displayed in Supplementary Fig. 9i–k. e Wound healing assay showing effects of 1 µg and 2 × 1010 NTgRNA- and PAPPA KO-EVs on HMEC-1 migration, analysed both as % wound closure and absolute migration distance (n = 3, technical replicates. Data are representative of three biologically independent experiments). f, g Sprouting assay showing NTgRNA- and PAPPA KO-EV-induced HMEC-1 sprout formation on beads, analysed both as (g) mean length per sprout and total sprout length per bead (n = 3, technical replicates. Data are representative of two biologically independent experiments). Data are presented as mean ± SD. *p < 0.033, **p < 0.0021, ***p < 0.0002. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37528162), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Pappalysin-1/PAPP-A
Pappalysins belong to a fifth family of metzincins that consists of ADAMs/ADAMTSs, MMPs, astacins and serrylysins (1). PAPP-A is an important pregnancy protein and increases in plasma by a factor of about 150 during pregnancy as compared to the nonpregnant state. PAPP-A is also a major marker of Down syndrome in the first trimester of pregnancy because maternal serum levels of PAPP-A are significantly reduced when a fetus affected by Down syndrome is present (2). PAPP-A cleaves Insulin-like Growth Factor-Binding Protein-4 and -5 (IGFBP-4 and -5) at a single site, resulting in the release of bioactive IGF (3). Lack of IGFBP-4 cleavage in embryonic fibroblasts derived from PAPP-A knockout mice indicates that PAPP-A functions as a physiological IGFBP-4 protease (4). Three Lin12-Notch repeats (LNR) in the PAPP-A protein bind Ca2+ and are required for the cleavage of IGFBP-4, not IGFBP-5, by PAPP-A (5). The C-terminal LNR (residues 1476 to 1503) is not present in rhPAPP-A (residues 82 to 1214), which starts at the N-terminus of the mature chain and ends before the five Sushi (SCR) modules. As an active protease, rhPAPP-A cleaves IGFBP-5, which can be inhibited by 1,10-phenanthroline.
- Boldt, H.B. et al. (2001) Biochem. J. 358:359.
- Fialova L. and I.M. Malbohan (2002) Bratisl. Lek. Listy 103:194.
- Laursen, L.S. et al. (2001) FEBS Lett. 504:36.
- Conover, C.A. et al. (2004) Development 131:1187.
- Boldt, H.B. et al. (2004) J. Biol. Chem. 279:38525.
Product Datasheets
Citations for Human Pappalysin-1/PAPP-A Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
5
Citations: Showing 1 - 5
Filter your results:
Filter by:
-
Impaired Plakophilin-2 in obesity breaks cell cycle dynamics to breed adipocyte senescence
Authors: Lluch, A;Latorre, J;Serena-Maione, A;Espadas, I;Caballano-Infantes, E;Moreno-Navarrete, JM;Oliveras-Cañellas, N;Ricart, W;Malagón, MM;Martin-Montalvo, A;Birchmeier, W;Szymanski, W;Graumann, J;Gómez-Serrano, M;Sommariva, E;Fernández-Real, JM;Ortega, FJ;
Nature communications
-
Cardiac progenitor cell-derived extracellular vesicles promote angiogenesis through both associated- and co-isolated proteins
Authors: Roefs MT, Bauzá-Martinez J, van de Wakker SI et al.
Communications biology
-
Altered Biomarkers in Trophoblast Cells Obtained Noninvasively Prior to Clinical Manifestation of Perinatal Disease
Sci Rep, 2016-09-23;6(0):32382.
Species: Human
Sample Types: Whole Cells
Applications: IHC -
Expression of pregnancy-associated plasma protein A2 during pregnancy in human and mouse.
Authors: Wang J, Qiu Q, Haider M, Bell M, Gruslin A, Christians JK
J. Endocrinol., 2009-05-27;202(3):337-45.
Species: Human
Sample Types: Serum
Applications: Western Blot -
Increased levels of pregnancy-associated plasma protein-A2 in the serum of pre-eclamptic patients.
Authors: Nishizawa H, Pryor-Koishi K, Suzuki M
Mol. Hum. Reprod., 2008-09-18;14(10):595-602.
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human Pappalysin-1/PAPP-A Antibody
Average Rating: 5 (Based on 1 Review)
Have you used Human Pappalysin-1/PAPP-A Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:

