Human Pax2 Antibody Summary
Asp229-Pro363
Accession # Q02962
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Mouse Pax2 by Western Blot. Western blot shows lysates of mouse fetal kidney. PVDF Membrane was probed with 1 µg/mL of Goat Anti-Human Pax2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3364) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF017). A specific band was detected for Pax2 at approximately 47 kDa (as indicated). This experiment was conducted under reducing conditions and using Western Blot Buffer Group 1.
 View Larger
View Larger
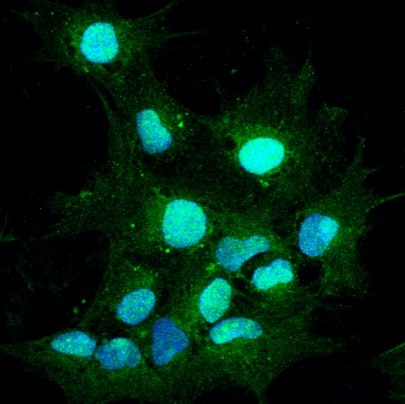
Pax2 in BG01V Human Embyonic Stem Cells. Pax2 was detected in immersion fixed BG01V human embryonic stem cells differentiated into the early otic lineage using Goat Anti-Human Pax2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3364) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; NL001) and counterstained with DAPI (blue). Specific staining was localized to nuclei. View our protocol for Fluorescent ICC Staining of Stem Cells on Coverslips.
 View Larger
View Larger
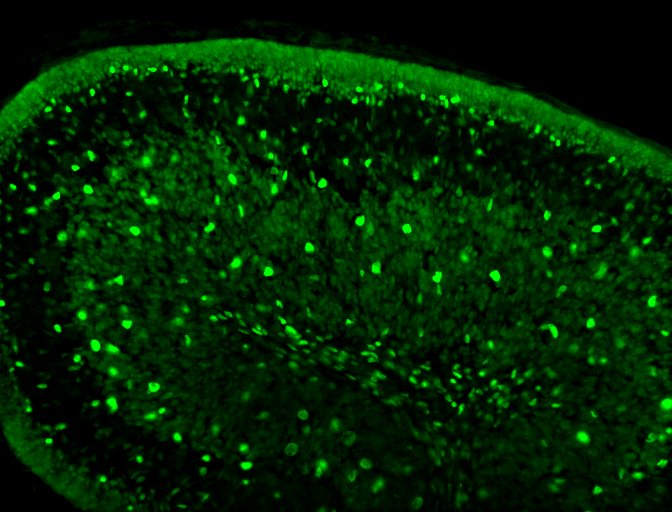
Pax2 in Human Kidney. Pax2 was detected in immersion fixed paraffin-embedded sections of human kidney using Goat Anti-Human Pax2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3364) at 1 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (VC004). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cell nuclei in convoluted tubules. Staining was performed using our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
Detection of Rat Human Pax2 Antibody by Immunohistochemistry Ablation of AAV-PdynP+ spinal dorsal horn (SDH) neurons does not affect behavioral responses evoked by the optical stimulation of A beta fibers, nociceptive mechanical force, and heat in rats. (A) Enhanced green fluorescent protein (EGFP) expression (green) in the L4 SDH at 4 weeks after intra-SDH microinjection of AAV-PdynP-DTR-EGFP (indicated by an upper schematic illustration). Colocalization of EGFP and PAX2 (red) in the SDH (indicated by arrowheads). Scale bar, 100 μm. The percentage of co-expressing neurons was quantified (n = 3 rats tested, 94 total EGFP+ cells quantified, and 339 total PAX2+ cells quantified). (B) Diphtheria toxin receptors (DTR) immunofluorescence in the SDH after administration of diphtheria toxin (DTX: 50 μg/kg, i.p., two injection 72 h apart) or PBS. Scale bar, 200 μm. (C) Immunohistochemical analysis and quantification of PAX2+ neurons in the superficial laminae of the ipsilateral and contralateral SDH at 4 weeks after DTX injection (n = 6 rats tested, PAX2+ neurons: 992 [ipsi] and 1574 [contra] cells quantified). Scale bar, 50 μm. ****P < 0.0001, Unpaired t-test. (D) Paw withdrawal behaviors by light (score), von Frey filaments (threshold), and heat (latency) at 4 weeks after DTX or PBS injection (n = 7–9 rats). Data shown as mean ± SEM. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35813063), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Rat Human Pax2 Antibody by Immunohistochemistry Ablation of AAV-PdynP+ spinal dorsal horn (SDH) neurons does not affect behavioral responses evoked by the optical stimulation of A beta fibers, nociceptive mechanical force, and heat in rats. (A) Enhanced green fluorescent protein (EGFP) expression (green) in the L4 SDH at 4 weeks after intra-SDH microinjection of AAV-PdynP-DTR-EGFP (indicated by an upper schematic illustration). Colocalization of EGFP and PAX2 (red) in the SDH (indicated by arrowheads). Scale bar, 100 μm. The percentage of co-expressing neurons was quantified (n = 3 rats tested, 94 total EGFP+ cells quantified, and 339 total PAX2+ cells quantified). (B) Diphtheria toxin receptors (DTR) immunofluorescence in the SDH after administration of diphtheria toxin (DTX: 50 μg/kg, i.p., two injection 72 h apart) or PBS. Scale bar, 200 μm. (C) Immunohistochemical analysis and quantification of PAX2+ neurons in the superficial laminae of the ipsilateral and contralateral SDH at 4 weeks after DTX injection (n = 6 rats tested, PAX2+ neurons: 992 [ipsi] and 1574 [contra] cells quantified). Scale bar, 50 μm. ****P < 0.0001, Unpaired t-test. (D) Paw withdrawal behaviors by light (score), von Frey filaments (threshold), and heat (latency) at 4 weeks after DTX or PBS injection (n = 7–9 rats). Data shown as mean ± SEM. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35813063), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Rat Human Pax2 Antibody by Immunohistochemistry AAV-PdynP injected into rat spinal dorsal horn (SDH) captures a subset of inhibitory neurons. (A) tdTomato (tdT) expression in the fourth lumbar (L4) SDH at 4 weeks after microinjection of AAV-PdynP-tdT. Schematic illustration indicates intra-SDH microinjection of the AAV vector. Scale bar, 200 μm. (B) Immunolabeling of tdT+ cells (red) with lamina-selective markers (green, NK1R; blue, IB4) in the L4 SDH. Scale bar, 200 μm. Quantification of the distribution of tdT+ cells (n = 3 rats tested, 272 total tdT+ cells quantified). (C) Immunofluorescence of prodynorphin (PDYN; green) in tdT+ SDH neurons (red). Scale bar, 200 μm (above), 20 μm (below). Arrowheads indicate tdT+ PDYN+ neurons. The percentage of tdT+ PDYN+ neurons per total tdT+ neurons was quantified (n = 4 rats tested, 1,281 total tdT+ cells quantified). (D) No colocalization of tdT (red) with NK1R (green). Scale bar, 50 μm. The percentage of co-expressing neurons was quantified (n = 3 rats tested, 187 total tdT+ cells quantified). (E) Firing patterns of tdT+ SDH neurons, and the percentage of neurons displaying each pattern. APs were evoked by current injection (80 pA). (F) Colocalization of tdT (red) with PAX2 (blue) and VGLUT2 (green) in the superficial laminae. Arrowheads and arrows indicate tdT+PAX2+ and tdT+VGLUT2+ neurons, respectively. Scale bar, 50 μm. The percentage of co-expressing neurons was quantified (n = 4 rats tested, 456 total tdT+ cells quantified). Data shown as mean ± SEM. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/35813063), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Pax2
Pax2 is a 40-45 kDa protein belonging to the small but developmentally important family of transcription regulators. Human Pax2 is a 416 amino acid (aa) residue protein with an N-terminal 128 aa DNA-binding paired box domain, a centrally-located octapeptide motif and a C-terminal truncated homeodomain. Based on the presence of the structural domains, Pax2 belongs to subgroup 2 in the Pax family. Pax2 is important for stem cell survival and lineage commitment during development. Pax2 is also expressed in various carcinomas where it seems to mediate anti-apoptotic functions. At least five splice isoforms of human Pax2 have been described within the region used as immunogen (shared by all isoforms). Human and mouse Pax2 share 98% aa sequence identity.
Product Datasheets
Citations for Human Pax2 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
28
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Developmental Expression of the Cell Cycle Regulator p16INK4a in Retinal Glial Cells: A Novel Marker for Immature Ocular Astrocytes?
Authors: Cristina Martinez-Fernandez Martinez-Fernandez de la Camara, Tina Storm, Ahmed Salman, Thomas Burgoyne, Martin Qvist Rasmussen, Harry O. Orlans et al.
Journal of Histochemistry & Cytochemistry
-
Selective Involvement of a Subset of Spinal Dorsal Horn Neurons Operated by a Prodynorphin Promoter in A beta Fiber-Mediated Neuropathic Allodynia-Like Behavioral Responses in Rats
Authors: Tadayuki Ishibashi, Yu Yoshikawa, Daichi Sueto, Ryoichi Tashima, Hidetoshi Tozaki-Saitoh, Keisuke Koga et al.
Frontiers in Molecular Neuroscience
-
Tsc1 Haploinsufficiency Leads to Pax2 Dysregulation in the Developing Murine Cerebellum
Authors: Ines Serra, Ana Stravs, Catarina Osório, Maria Roa Oyaga, Martijn Schonewille, Christian Tudorache et al.
Frontiers in Molecular Neuroscience
-
The single-cell and spatial transcriptional landscape of human gastrulation and early brain development
Authors: Zeng B, Liu Z, Lu Y et al.
Cell stem cell
-
Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells
Authors: Garreta E, Prado P, Tarantino C et al.
Nature Materials
-
MicroRNAs mediate precise control of spinal interneuron populations to exert delicate sensory-to-motor outputs
Authors: Chang SH, Su YC, Chang M, Chen JA.
eLife
-
Voltage-gated calcium channel subunit alpha 2δ-1 in spinal dorsal horn neurons contributes to aberrant excitatory synaptic transmission and mechanical hypersensitivity after peripheral nerve injury
Authors: Keisuke Koga, Kenta Kobayashi, Makoto Tsuda, Kazufumi Kubota, Yutaka Kitano, Hidemasa Furue
Frontiers in Molecular Neuroscience
-
Conserved and Divergent Molecular and Anatomic Features of Human and Mouse Nephron Patterning
Authors: Nils O. Lindström, Tracy Tran, Jinjin Guo, Elisabeth Rutledge, Riana K. Parvez, Matthew E. Thornton et al.
Journal of the American Society of Nephrology
-
Integrating collecting systems in kidney organoids through fusion of distal nephron to ureteric bud
Authors: Shi, M;Crouse, B;Sundaram, N;Shakked, NP;Ester, L;Zhang, W;Janakiram, V;Kopan, R;Helmrath, MA;Bonventre, JV;McCracken, KW;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Organoid
Applications: Immunocytochemistry -
Stepwise developmental mimicry generates proximal-biased kidney organoids
Authors: Schnell, J;Miao, Z;Achieng, M;Fausto, CC;Wang, V;Kuyper, F;Thornton, ME;Grubbs, B;Kim, J;Lindström, NO;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Organoid
Applications: Immunohistochemistry -
c-Maf-positive spinal cord neurons are critical elements of a dorsal horn circuit for mechanical hypersensitivity in neuropathy
Authors: N Frezel, M Ranucci, E Foster, H Wende, P Pelczar, R Mendes, RP Ganley, K Werynska, S d'Aquin, C Beccarini, C Birchmeier, HU Zeilhofer, H Wildner
Cell Reports, 2023-03-21;42(4):112295.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Identification of Spinal Inhibitory Interneurons Required for Attenuating Effect of Duloxetine on Neuropathic Allodynia-like Signs in Rats
Authors: T Ishibashi, D Sueto, Y Yoshikawa, K Koga, K Yamaura, M Tsuda
Cells, 2022-12-14;11(24):.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Switch of serotonergic descending inhibition into facilitation by a spinal chloride imbalance in neuropathic pain
Authors: F Aby, LE Lorenzo, Z Grivet, R Bouali-Ben, H Martin, S Valerio, S Whitestone, D Isabel, W Idi, O Bouchatta, P De Deurwae, AG Godin, C Herry, X Fioramonti, M Landry, Y De Koninck, P Fossat
Science Advances, 2022-07-27;8(30):eabo0689.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A gastruloid model of the interaction between embryonic and extra-embryonic cell types
Authors: NM Bérenger-C, M Mircea, E Adegeest, PR van den Be, M Feliksik, M Hochane, T Idema, SJ Tans, S Semrau
Journal of tissue engineering, 2022-06-11;13(0):2041731422110.
Species: Mouse
Sample Types: Organoids
Applications: IHC -
Stage-specific regulation of DNA methylation by TET enzymes during human cardiac differentiation
Authors: Y Lan, KM Banks, H Pan, N Verma, GR Dixon, T Zhou, B Ding, O Elemento, S Chen, D Huangfu, T Evans
Cell Reports, 2021-12-07;37(10):110095.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, ICC, Western Blot -
Incidence of malignant transformation in the oviductal fimbria in laying hens, a preclinical model of spontaneous ovarian cancer
Authors: EA Paris, JM Bahr, P Bitterman, S Basu, JS Abramowicz, A Barua
PLoS ONE, 2021-07-27;16(7):e0255007.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
MicroRNAs mediate precise control of spinal interneuron populations to exert delicate sensory-to-motor outputs
Authors: Chang SH; Su YC; Chang M; Chen JA.
eLife
-
Neuron-specific spinal cord translatomes reveal a neuropeptide code for mouse dorsal horn excitatory neurons
Authors: RR Das Gupta, L Scheurer, P Pelczar, H Wildner, HU Zeilhofer
Scientific Reports, 2021-03-04;11(1):5232.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Phox2a Defines a Developmental Origin of the Anterolateral System in Mice and Humans
Authors: RB Roome, FB Bourojeni, B Mona, S Rastegar-P, R Blain, A Dumouchel, C Salesse, WS Thompson, M Brookbank, Y Gitton, L Tessarollo, M Goulding, JE Johnson, M Kmita, A Chédotal, A Kania
Cell Rep, 2020-11-24;33(8):108425.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Spinal Inhibitory Ptf1a-Derived Neurons Prevent Self-Generated Itch
Authors: A Escalante, R Klein
Cell Rep, 2020-11-24;33(8):108422.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Targeting spinal neuropeptide Y1 receptor-expressing interneurons to alleviate chronic pain and itch
Authors: TS Nelson, BK Taylor
Prog. Neurobiol., 2020-08-07;0(0):101894.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Bcl-2 Expression in Pericytes and Astrocytes Impacts Vascular Development and Homeostasis
Authors: IS Zaitoun, CM Wintheiser, N Jamali, S Wang, A Suscha, SR Darjatmoko, K Schleck, BA Hanna, V Lindner, N Sheibani, CM Sorenson
Sci Rep, 2019-07-04;9(1):9700.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Facilitation of neuropathic pain by the NPY Y1 receptor-expressing subpopulation of excitatory interneurons in the dorsal horn
Authors: TS Nelson, W Fu, RR Donahue, GF Corder, T Hökfelt, RG Wiley, BK Taylor
Sci Rep, 2019-05-10;9(1):7248.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC-Fr -
Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells
Authors: Garreta E, Prado P, Tarantino C et al.
Nature Materials
-
Histone deacetylase expression patterns in developing murine optic nerve.
Authors: Tiwari, Sarika, Dharmarajan, Subraman, Shivanna, Mahesh, Otteson, Deborah, Belecky-Adams, Teri L
BMC Dev Biol, 2014-07-09;14(0):30.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Genetic manipulation of ureteric bud tip progenitors in the mammalian kidney through an Adamts18 enhancer driven tet-on inducible system
Authors: Rutledge EA, LindstrOm NO, Michos O, McMahon AP
Dev. Biol.
-
In vivo Regeneration of Ganglion Cells for Vision Restoration in Mammalian Retinas
Authors: Xiao D, Jin K, Qiu S Et al.
Frontiers in cell and developmental biology
-
Inhibitory Kcnip2 neurons of the spinal dorsal horn control behavioral sensitivity to environmental cold
Authors: Gioele W. Albisetti, Robert P. Ganley, Francesca Pietrafesa, Karolina Werynska, Marília Magalhaes de Sousa, Rebecca Sipione et al.
Neuron
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human Pax2 Antibody
Average Rating: 4 (Based on 2 Reviews)
Have you used Human Pax2 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Green is PAX2. Blue is DAPI.