Human Serum Albumin Antibody Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human Albumin by Western Blot. Western blot shows lysate of human liver tissue. PVDF membrane was probed with 1 µg/mL of Mouse Anti-Human Serum Albumin Monoclonal Antibody (Catalog # MAB1455) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF018). A specific band was detected for Albumin at approximately 65-70 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Albumin in Hepatocytes Derived from Human Embryonic Stem Cells. Albumin was detected in immersion fixed BG01V human embryonic stem cells differentiated to hepatocytes using Mouse Anti-Human Serum Albumin Monoclonal Antibody (Catalog # MAB1455) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (red; Catalog # NL007) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. Cells were co-stained using Sheep Anti-Human CEBP alpha (Catalog # AF7094) and NorthernLights™ 493-conjugated Anti-Sheep IgG Secondary Antibody (green, Catalog # NL012). View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
Albumin in Human Liver. Albumin was detected in immersion fixed paraffin-embedded sections of human liver using Mouse Anti-Human Serum Albumin Monoclonal Antibody (Catalog # MAB1455) at 0.1 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Mouse IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC001). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (Catalog # CTS013). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cytoplasm and plasma membrane. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
Detection of Albumin in HepG2 Human Cell Line by Flow Cytometry. HepG2 human hepatocellular carcinoma cell line was stained with Mouse Anti-Human Serum Albumin Monoclonal Antibody (Catalog # MAB1455, filled histogram) or isotype control antibody (Catalog # MAB003, open histogram), followed by Allophycocyanin-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # F0101B). To facilitate intracellular staining, cells were fixed with paraformaldehyde and permeabilized with saponin.
 View Larger
View Larger
Detection of Human Albumin by Simple WesternTM. Simple Western lane view shows lysates of human serum, loaded at 1:25000. A specific band was detected for Albumin at approximately 64 kDa (as indicated) using 1 µg/mL of Mouse Anti-Human Serum Albumin Monoclonal Antibody (Catalog # MAB1455). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
Detection of Human Albumin by Simple WesternTM. Simple Western lane view shows lysates of human liver tissue, loaded at 0.2 mg/mL. A specific band was detected for Albumin at approximately 64 kDa (as indicated) using 10 µg/mL of Mouse Anti-Human Serum Albumin Monoclonal Antibody (Catalog # MAB1455). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
Serum Albumin in Human Liver Using Dual RNAscope®ISH and IHC. Serum Albumin mRNA was detected in formalin-fixed paraffin-embedded tissue sections of human liver probed with ACD RNAScope®Probe (Catalog # 600941) and stained using ACD RNAscope®2.5 HD Detection Reagents-Red (top image, Catalog # 32260). Adjacent tissue section was processed for immunohistochemistry using R&D Systems Mouse Anti-Human Serum Albumin Monoclonal Antibody (Catalog # MAB1455) at 0.05 ug/mL for 1 hour at room temperature followed by incubation with the Anti-Mouse IgG VisUCyte HRP Polymer Antibody (R&D Systems, Catalog # VC001) and DAB chromogen (lower image, yellow-brown). Tissues were counterstained with hematoxylin (blue).
 View Larger
View Larger
Detection of Mouse Albumin by Immunohistochemistry Heterochronic blood exchange effects on muscle regeneration and performance.One day after blood exchange mice were injured by intramuscular injections of CTX into TA. Five days after injury, TA muscles were isolated, cryo-sectioned and analysed. (a) TA muscles from young mice receiving young blood (YY), young mice receiving old blood (YO), old mice receiving young blood (OY) and old mice receiving old blood (OO) were analysed by haematoxylin and eosin (H&E) staining and immunofluorescence with anti-eMyHC antibody. Representative images show an injury site and nascent de-novo formed eMyHC+ myofibers which are smaller in size with central nuclei than uninjured myofibers. Scale bar, 50 μm for H&E panel and 25 μm for immunofluorescence panel. (b,c) Regeneration indices ±s.e.m. were quantified from H&E images (b) and eMyHC images (c) by counting the number of nascent de-novo formed myofibers and dividing by the total number of nuclei present at the injury/regeneration site. By H&E: *P<0.05 N=4 per group. Significant students t test differences exist between YO and OY (P=0.045), YY and OY (P=0.043), YY and OO (P=0.0004), YO and OO (P=0.0042) and between OY and OO (P=0.015). By eMyHC: *P<0.05, N=4 per group; OY and OO P=0.041, YY and OO P=0.00009, and YO to OO P=0.001. (d) Fibrotic/inflammatory indexes were quantified as total injury area minus regenerated myofiber area, per injury site, using the H&E images15. T-test **P<0.005, n=3–4 per group. Muscle from old to old isochronic exchange had diminished regenerative capacity and more fibrosis, as compared with muscle from young to young isochronic exchange. Heterochronic blood exchange significantly improved regeneration of old muscle after experimental injury and reduced fibrosis, but no significant decline in young muscle regeneration was seen. (e) A four-limb hanging test was conducted with isochronically and heterochronically transfused mice that were not injured, before and at 6 days after the blood exchange. Maximal hanging time was multiplied by body weight (hang index). T-test n=4–8, P=0.01 YY post transfusion compared with O training, and YO, OY, OO post-transfusion performance. Y to O training and YO, OY and OO were NS=not statistically different. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27874859), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Albumin by Immunohistochemistry Heterochronic blood exchange effects on hepatogenesis and liver fibrosis and adiposity.(a) Livers from YY, YO, OY and OO mice with and without experimental muscle injury as above were cryo-sectioned at 10 μm and immuno-stained for Ki67 (red), hepatocyte marker albumin (green) and Hoechst (blue). Representative images show YY livers with and without injury. Scale bar, 50 μm. (B&C. Quantification of hepatocyte proliferation was by counting the average number of Ki67+,abumin+,Hoechst+ cells per 10 μm section from multiple sections of each blood exchange cohort. (b) Old hepatocyte showed increased proliferation and young hepatocytes showed less proliferation with heterochronic blood as compared with isochronic blood exchanges in animals with injured muscle (t test P=0.00028). (c) This trend continues without muscle injury, but the total numbers of proliferating hepatocytes decline by twofold, (P=0.02411). *P<0.05; **P<0.005; n=3–5. (d) As previously published4, there were fibrotic clusters exclusively in the old livers of small Ki67+ve, albumin negative Ki67+ cells. Scale bar, 50 μm, × 40 magnification. (e,f) Fibrotic index was calculated as the average number of albumin negative proliferative cell clusters per four 10 μm sections. The fibrotic index diminished in old mice exchanged with young blood with muscle injury (e) (t test P=0.048 N=4, *P<0.05) or without (f) (t test P=0.00776. N=3; **P<0.005). (g) Liver adiposity was assayed by Oil Red in 10 μm cryosections. Shown are representative images acquired at × 20 magnification. (h). Liver adiposity (red) was quantified by Image J, dramatically increased with age and was attenuated by young blood in old mice (t test N=3, P=0.022), while adiposity remained unchanged in young mice that were transfused with the old blood (see Supplementary Figure 4). Shown are means±s.e.m. for all histograms. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27874859), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Albumin by Immunohistochemistry Heterochronic blood exchange effects on hepatogenesis and liver fibrosis and adiposity.(a) Livers from YY, YO, OY and OO mice with and without experimental muscle injury as above were cryo-sectioned at 10 μm and immuno-stained for Ki67 (red), hepatocyte marker albumin (green) and Hoechst (blue). Representative images show YY livers with and without injury. Scale bar, 50 μm. (B&C. Quantification of hepatocyte proliferation was by counting the average number of Ki67+,abumin+,Hoechst+ cells per 10 μm section from multiple sections of each blood exchange cohort. (b) Old hepatocyte showed increased proliferation and young hepatocytes showed less proliferation with heterochronic blood as compared with isochronic blood exchanges in animals with injured muscle (t test P=0.00028). (c) This trend continues without muscle injury, but the total numbers of proliferating hepatocytes decline by twofold, (P=0.02411). *P<0.05; **P<0.005; n=3–5. (d) As previously published4, there were fibrotic clusters exclusively in the old livers of small Ki67+ve, albumin negative Ki67+ cells. Scale bar, 50 μm, × 40 magnification. (e,f) Fibrotic index was calculated as the average number of albumin negative proliferative cell clusters per four 10 μm sections. The fibrotic index diminished in old mice exchanged with young blood with muscle injury (e) (t test P=0.048 N=4, *P<0.05) or without (f) (t test P=0.00776. N=3; **P<0.005). (g) Liver adiposity was assayed by Oil Red in 10 μm cryosections. Shown are representative images acquired at × 20 magnification. (h). Liver adiposity (red) was quantified by Image J, dramatically increased with age and was attenuated by young blood in old mice (t test N=3, P=0.022), while adiposity remained unchanged in young mice that were transfused with the old blood (see Supplementary Figure 4). Shown are means±s.e.m. for all histograms. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27874859), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Albumin by Immunocytochemistry/Immunofluorescence Differentiation of hESCs into HLCs.a The relative hepatocyte (ALB, AAT, CK18, and ASGPR1), cholangiocyte (SOX9), and hepatoblast (AFP) gene expression levels of day 19 differentiated cells with different treatments (groups A and B) were determined by qPCR. HGF (hepatocyte growth factor, 20 ng/mL); OSM (oncostatin M, 20 ng/mL); Dex (dexamethasone, 10 µM); SB431542 (TGF beta inhibitor, 2 µM); and RO4929097 (Notch inhibitor, 1 µM). b Immunofluorescence analysis of ALB, AAT, ASGPR1, and CK18 expression in group B-induced differentiated cells on day 19. c The expression levels of ALB and ASGPR1 in group B-induced differentiated cells were determined by flow cytometry on day 19. Isotype control antibodies were used as controls. d The relative hepatocyte (ALB, AAT, CK18, ASGPR1, and AFP) gene expression levels of differentiated HLCs (group B) compared with those of hESCs and primary human hepatocytes (PHHs) were determined by qPCR. e Albumin secretion of HLCs (black line) on days 12, 14, 16, 18, and 20 and PHHs (dotted line) on day 2 were determined by ELISA. *p < 0.05, **p < 0.01; data are represented as the mean ± SD. Scale bar, 50 µm Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31601782), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
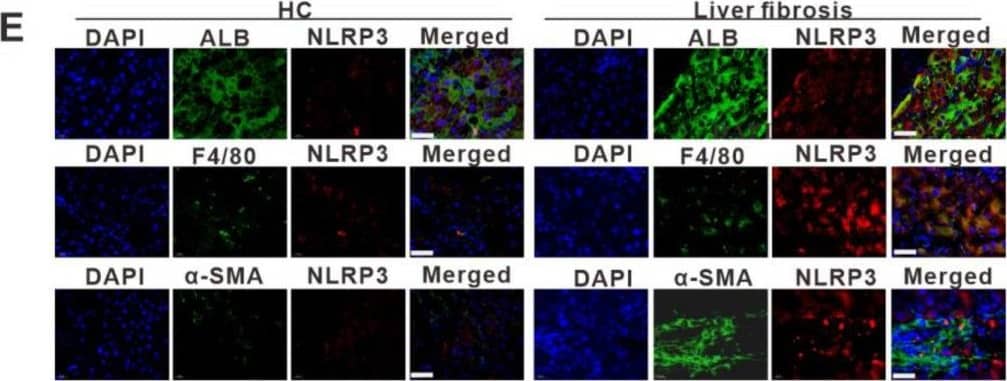
Detection of Human Albumin by Immunohistochemistry NLRP3 inflammasome-dependent pyroptosis occurs in liver fibrosis. (A) IHC staining for GSDMD, IL-1 beta, and IL-18 in liver sections from liver fibrosis patients and HCs. Scale bar: 40 µm. (B–D) ELISA analyses of serum levels of GSDMD (B), IL-1 beta (C), and IL-18 (D) in liver fibrosis patients (n = 89) and HCs (n = 60). (E) Representative immunofluorescence images of NLRP3 (red) and albumin (hepatocyte marker) (top), F4/80 (KC marker) (middle) or alpha -SMA (HSC marker) (bottom) (green) from the human fibrotic liver tissues. Scale bar: 40 µm. (F) Schematic diagram of the study. Liver fibrosis was induced by CCl4 injection for 8 weeks. (G) Representative mouse liver histology of H&E, Sirius Red staining, and IHC staining for alpha -SMA, GSDMD, and IL-1 beta. Black scale bar: 100 µm; Red scale bar: 50 µm. (H–J) ELISA analyses for serum levels of GSDMD (H), IL-1 beta (I), and IL-18 (J) in CCl4 group mouse (n = 5) and vehicle group mouse (n = 5). (K) Representative immunofluorescence images of NLRP3 (red) and albumin (hepatocyte marker) (top), F4/80 (KC marker) (middle) or alpha -SMA (HSC marker) (bottom) (green) from the 8-week CCl4-treated mouse liver. The vehicle group mouse liver was used as a control. Scale bar: 40 µm. (L) The qRT-PCR analysis for mRNA levels of IL-1 beta in THP-1 macrophages treated with LPS to induce pyroptosis. (M) ELISA analysis for IL-1 beta expression in supernatants from THP-1. (N) Western blot analysis of COL1A1, alpha -SMA, and TGF-beta expression in LX-2 cells which were exposed to CM from LPS-treated THP-1 macrophages. The protein expression was quantified by densitometry and normalized to beta -actin and are shown as fold changes relative to the control group (right panel). ** p < 0.01, *** p < 0.001. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36429008), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
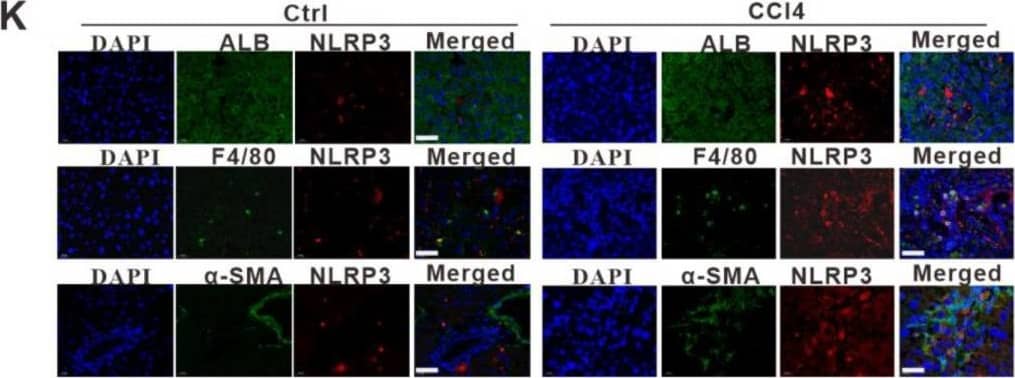
Detection of Mouse Albumin by Immunohistochemistry NLRP3 inflammasome-dependent pyroptosis occurs in liver fibrosis. (A) IHC staining for GSDMD, IL-1 beta, and IL-18 in liver sections from liver fibrosis patients and HCs. Scale bar: 40 µm. (B–D) ELISA analyses of serum levels of GSDMD (B), IL-1 beta (C), and IL-18 (D) in liver fibrosis patients (n = 89) and HCs (n = 60). (E) Representative immunofluorescence images of NLRP3 (red) and albumin (hepatocyte marker) (top), F4/80 (KC marker) (middle) or alpha -SMA (HSC marker) (bottom) (green) from the human fibrotic liver tissues. Scale bar: 40 µm. (F) Schematic diagram of the study. Liver fibrosis was induced by CCl4 injection for 8 weeks. (G) Representative mouse liver histology of H&E, Sirius Red staining, and IHC staining for alpha -SMA, GSDMD, and IL-1 beta. Black scale bar: 100 µm; Red scale bar: 50 µm. (H–J) ELISA analyses for serum levels of GSDMD (H), IL-1 beta (I), and IL-18 (J) in CCl4 group mouse (n = 5) and vehicle group mouse (n = 5). (K) Representative immunofluorescence images of NLRP3 (red) and albumin (hepatocyte marker) (top), F4/80 (KC marker) (middle) or alpha -SMA (HSC marker) (bottom) (green) from the 8-week CCl4-treated mouse liver. The vehicle group mouse liver was used as a control. Scale bar: 40 µm. (L) The qRT-PCR analysis for mRNA levels of IL-1 beta in THP-1 macrophages treated with LPS to induce pyroptosis. (M) ELISA analysis for IL-1 beta expression in supernatants from THP-1. (N) Western blot analysis of COL1A1, alpha -SMA, and TGF-beta expression in LX-2 cells which were exposed to CM from LPS-treated THP-1 macrophages. The protein expression was quantified by densitometry and normalized to beta -actin and are shown as fold changes relative to the control group (right panel). ** p < 0.01, *** p < 0.001. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/36429008), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
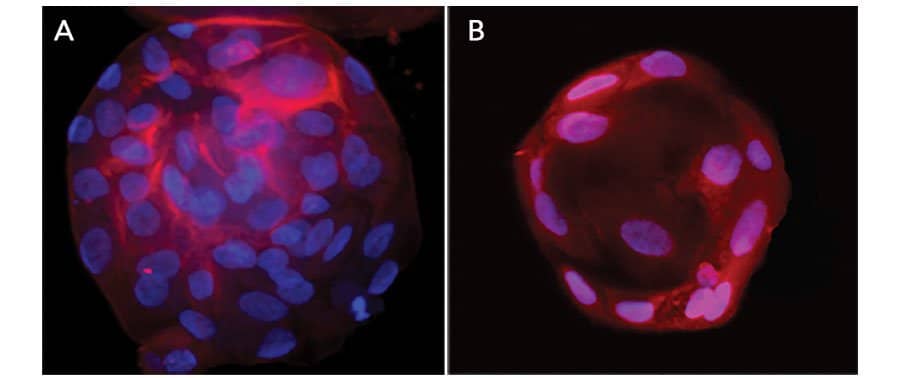
Immunofluorescent Staining of Adult Stem Cell-derived Liver Organoids. Adult stem cell-derived liver organoids were generated following the steps detailed in the human liver organoid culture protocol. Differentiated human liver organoids were stained using a (A) Mouse Anti-Human Serum Albumin Monoclonal Antibody (Catalog # MAB1455; red) and a (B) Goat Anti-Human HNF-3beta Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2400; red) and counterstained with DAPI (Catalog # 5748; blue).
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Albumin
Albumins are a family of globular proteins, the most common of which are serum albumins. Albumins are commonly found in blood plasma, and are unique from other blood proteins in that they are not glycosylated. Albumin is a 65-70 kDa protein with serum albumin being the main protein of human blood plasma. It binds water, cations (such as Ca2+, Na+ and K+), fatty acids, hormones, bilirubin, thyroxine (T4) and pharmaceuticals (including barbiturates) - its main function is to regulate the colloidal osmotic pressure of blood. Albumin comprises three homologous domains that assemble to form a heart-shaped molecule. Each domain is a product of two subdomains that possess common structural motifs. The principal regions of ligand binding to human serum albumin are located in hydrophobic cavities in subdomains IIA and IIIA, which exhibit similar chemistry. Structurally, the serum albumins are similar, each domain containing five or six internal disulfide bonds.
Product Datasheets
Citations for Human Serum Albumin Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
57
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Genetic Correction and Hepatic Differentiation of Hemophilia B-specific Human Induced Pluripotent Stem Cells
Authors: 何琼, Qiong He, 王惠荟, 程涛, 袁卫平, 马钰波 et al.
Chinese Medical Sciences Journal
-
Rejuvenation of brain, liver and muscle by simultaneous pharmacological modulation of two signaling determinants, that change in opposite directions with age
Authors: Melod Mehdipour, Jessy Etienne, Chia-Chien Chen, Ranveer Gathwala, Maryam Rehman, Cameron Kato et al.
Aging (Albany NY)
-
Rapid and Efficient Generation of Transgene-Free iPSC from a Small Volume of Cryopreserved Blood
Authors: Hongyan Zhou, Hector Martinez, Bruce Sun, Aiqun Li, Matthew Zimmer, Nicholas Katsanis et al.
Stem Cell Reviews and Reports
-
Extracellular Vesicle Capture by AnTibody of CHoice and Enzymatic Release (EV‐CATCHER): A customizable purification assay designed for small‐RNA biomarker identification and evaluation of circulating small‐EVs
Authors: Megan I. Mitchell, Iddo Z. Ben‐Dov, Christina Liu, Kenny Ye, Kar Chow, Yael Kramer et al.
Journal of Extracellular Vesicles
-
SIX1/EYA1 are novel liver damage biomarkers in chronic hepatitis B and other liver diseases
Authors: Baoyan Xu, Qiao Yang, Yingzi Tang, Zhaoxia Tan, Haiyan Fu, Jing Peng et al.
Annals of Translational Medicine
-
Recellularization of Rat Liver Scaffolds by Human Liver Stem Cells
Authors: Victor Navarro-Tableros, Maria Beatriz Herrera Sanchez, Federico Figliolini, Renato Romagnoli, Ciro Tetta, Giovanni Camussi
Tissue Engineering Part A
-
Generation of iPS Cells from Human Hair Follice Dermal Papilla Cells
Authors: I. A. Muchkaeva, E. B. Dashinimaev, A. S. Artyuhov, E. P. Myagkova, E. A. Vorotelyak, Y. Y. Yegorov et al.
Acta Naturae
-
Comprehensive proteomic profiling of plasma and serum phosphatidylserine-positive extracellular vesicles reveals tissue-specific proteins
Authors: Satoshi Muraoka, Masayo Hirano, Junko Isoyama, Satoshi Nagayama, Takeshi Tomonaga, Jun Adachi
iScience
-
Signals from dying hepatocytes trigger growth of liver progenitors
Authors: Youngmi Jung, Rafal P. Witek, Wing-Kin Syn, Steve S. Choi, Alessia Omenetti, Richard Premont et al.
Gut
-
Combinatorial microenvironmental regulation of liver progenitor differentiation by Notch ligands, TGF beta, and extracellular matrix
Authors: Kerim B. Kaylan, Viktoriya Ermilova, Ravi Chandra Yada, Gregory H. Underhill
Scientific Reports
-
Modeling drug-induced liver injury and screening for anti-hepatofibrotic compounds using human PSC-derived organoids
Authors: Xiaoshan Wu, Dacheng Jiang, Yi Yang, Shuang Li, Qiurong Ding
Cell Regeneration
-
Phenotypical, functional and transcriptomic comparison of two modified methods of hepatocyte differentiation from human induced pluripotent stem cells
Authors: Rong Li, Yang Zhao, Jeffrey J. Yourick, Robert L. Sprando, Xiugong Gao
Biomedical Reports
-
Acute liver steatosis translationally controls the epigenetic regulator MIER1 to promote liver regeneration in a study with male mice
Authors: Y Chen, L Chen, X Wu, Y Zhao, Y Wang, D Jiang, X Liu, T Zhou, S Li, Y Wei, Y Liu, C Hu, B Zhou, J Qin, H Ying, Q Ding
Nature Communications, 2023-03-18;14(1):1521.
-
Fetal hepatocytes protect the HSPC genome via fetuin-A
Authors: Guo, XL;Wang, YD;Liu, YJ;Chu, L;Zhu, H;Hu, Y;Wu, RY;Xie, HY;Yu, J;Li, SP;Xiong, ZY;Li, RY;Ke, F;Chen, L;Chen, GQ;Chen, L;Bai, F;Enver, T;Li, GH;Li, HF;Hong, DL;
Nature
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Oxygen Gradient Induced in Microfluidic Chips Can Be Used as a Model for Liver Zonation
Authors: S Ghafoory, C Stengl, S Kopany, M Mayadag, N Mechtel, B Murphy, S Schattschn, N Wilhelmi, S Wölfl
Cells, 2022-11-23;11(23):.
Species: Human
Sample Types: Whole Cells
Applications: ICC/IF -
CRISPR/Cas9-engineered human ES cells harboring heterozygous and homozygous c-KIT knockout
Authors: MAS de Toledo, X Fu, F Kluge, K Götz, S Schmitz, P Wanek, HM Schüler, K Pannen, N Chatain, S Koschmiede, TH Brümmendor, M Zenke
Stem Cell Research, 2022-03-01;60(0):102732.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Potential of miRNAs in urinary extracellular vesicles for management of active surveillance in prostate cancer patients
Authors: M Ramirez-Ga, V Berge, A Lin?, A Llorente
British Journal of Cancer, 2021-11-22;0(0):.
Species: Human
Sample Types: Extracellular Vesicles
Applications: Western Blot -
Fecal host biomarkers predicting severity of Clostridioides difficile infection
Authors: M Golizeh, K Winter, L Roussel, M Landekic, M Langelier, VG Loo, M Ndao, DC Vinh
JCI Insight, 2021-01-11;0(0):.
Species: Human
Sample Types: Protein
Applications: Western Blot -
The Microfluidic Environment Reveals a Hidden Role of Self-Organizing Extracellular Matrix in Hepatic Commitment and Organoid Formation of hiPSCs
Authors: F Michielin, GG Giobbe, C Luni, Q Hu, I Maroni, MR Orford, A Manfredi, L Di Filippo, AL David, D Cacchiarel, P De Coppi, S Eaton, N Elvassore
Cell Rep, 2020-12-01;33(9):108453.
Species: Human
Sample Types: Organoid, Whole Cells
Applications: ICC, IHC -
Repair of acute liver damage with immune evasive hESC derived hepato-blasts
Authors: J Liu, T Pan, Y Chen, Y Liu, F Yang, Q Chen, N Abbas, M Zhong, Q Zhang, Y Xu, YX Li
Stem Cell Res, 2020-09-26;49(0):102010.
Species: Human, Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Integration of Hydrogel Microparticles With Three-Dimensional Liver Progenitor Cell Spheroids
Authors: Stefan D. Gentile, Andreas P. Kourouklis, Hyeon Ryoo, Gregory H. Underhill
Frontiers in Bioengineering and Biotechnology
Species: Mouse
Sample Types: Spheroid
Applications: Immunocytochemistry -
Mechano-modulatory synthetic niches for liver organoid derivation
Authors: G Sorrentino, S Rezakhani, E Yildiz, S Nuciforo, MH Heim, MP Lutolf, K Schoonjans
Nat Commun, 2020-07-10;11(1):3416.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Plasmodium vivax liver stage assay platforms using Indian clinical isolates
Authors: PA Subramani, N Vartak-Sha, S Sreekumar, P Mathur, B Nayer, S Dakhore, SK Basavanna, DM Kalappa, RV Krishnamur, B Mukhi, P Mishra, N Yoshida, SK Ghosh, R Shandil, S Narayanan, B Campo, K Hasegawa, AR Anvikar, N Valecha, V Sundaramur
Malar. J., 2020-06-22;19(1):214.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline-albumin
Authors: M Mehdipour, C Skinner, N Wong, M Lieb, C Liu, J Etienne, C Kato, D Kiprov, MJ Conboy, IM Conboy
Aging (Albany NY), 2020-05-30;12(10):8790-8819.
Species: Human, Mouse
Sample Types: Serum, Whole Tissue
Applications: IHC, Western Blot -
Fabrication of Perfusable Vascular Channels and Capillaries in 3D Liver-like Tissue
Authors: N Mori, Y Akagi, Y Imai, Y Takayama, YS Kida
Sci Rep, 2020-04-14;10(1):5646.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Human liver stem cells express UGT1A1 and improve phenotype of immunocompromised Crigler Najjar syndrome type I mice
Authors: ES Famulari, V Navarro-Ta, MB Herrera Sa, G Bortolussi, M Gai, L Conti, L Silengo, E Tolosano, C Tetta, AF Muro, G Camussi, S Fagoonee, F Altruda
Sci Rep, 2020-01-21;10(1):887.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Psoralea corylifolia L. Seed Extract Attenuates Methylglyoxal-Induced Insulin Resistance by Inhibition of Advanced Glycation End Product Formation
Authors: Cao-Sang Truong, Eunhui Seo, Hee-Sook Jun
Oxidative Medicine and Cellular Longevity
Species: Mouse
Sample Types: Cell Lysates, Tissue Homogenates
Applications: Western Blot -
In vitro metabolic zonation through oxygen gradient on a chip
Authors: F Tonon, GG Giobbe, A Zambon, C Luni, O Gagliano, A Floreani, G Grassi, N Elvassore
Sci Rep, 2019-09-19;9(1):13557.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Neutrophil-derived miR-223 as local biomarker of bacterial peritonitis
Authors: AC Brook, RH Jenkins, A Clayton, A Kift-Morga, AC Raby, AP Shephard, B Mariotti, SM Cuff, F Bazzoni, T Bowen, DJ Fraser, M Eberl
Sci Rep, 2019-07-12;9(1):10136.
Species: Human
Sample Types: Peritoneal Dialysate
Applications: ELISA Detection -
The role of insulin in transdifferentiated hepatocyte proliferation and function in serum-free medium
Authors: C Gu, P Li, W Liu, Y Zhou, WS Tan
J. Cell. Mol. Med., 2019-04-04;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Function and Immunogenicity of Gene-corrected iPSC-derived Hepatocyte-Like Cells in Restoring Low Density Lipoprotein Uptake in Homozygous Familial Hypercholesterolemia
Authors: H Okada, C Nakanishi, S Yoshida, M Shimojima, J Yokawa, M Mori, H Tada, T Yoshimuta, K Hayashi, T Yamano, R Hanayama, M Yamagishi, MA Kawashiri
Sci Rep, 2019-03-18;9(1):4695.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
4 in 1: Antibody-free protocol for isolating the main hepatic cells from healthy and cirrhotic single rat livers
Authors: A Fernández-, M Ortega-Rib, S Guixé-Munt, J Gracia-San
J. Cell. Mol. Med., 2018-11-12;0(0):.
Species: Rat
Sample Types: Whole Cells
Applications: ICC -
Noncoding RNA Transcripts during Differentiation of Induced Pluripotent Stem Cells into Hepatocytes
Authors: A Skrzypczyk, S Kehr, I Krystel, SH Bernhart, S Giri, A Bader, PF Stadler
Stem Cells Int, 2018-08-19;2018(0):5692840.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Extracellular vesicles from human liver stem cells restore argininosuccinate synthase deficiency
Authors: MB Herrera Sa, S Previdi, S Bruno, V Fonsato, MC Deregibus, S Kholia, S Petrillo, E Tolosano, R Critelli, M Spada, R Romagnoli, M Salizzoni, C Tetta, G Camussi
Stem Cell Res Ther, 2017-07-27;8(1):176.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Nucleic acid-based polymers effective against hepatitis B Virus infection in patients don't harbor immunostimulatory properties in primary isolated liver cells
Authors: CI Real, M Werner, A Paul, G Gerken, JF Schlaak, A Vaillant, R Broering
Sci Rep, 2017-03-08;7(0):43838.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
A single heterochronic blood exchange reveals rapid inhibition of multiple tissues by old blood
Nat Commun, 2016-11-22;7(0):13363.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Effect of Secreted Molecules of Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells on Acute Hepatic Failure Model
Stem Cells Dev, 2016-10-27;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC-Fr -
Differences in the Epigenetic Regulation of Cytochrome P450 Genes between Human Embryonic Stem Cell-Derived Hepatocytes and Primary Hepatocytes.
Authors: Park H, Choi Y, Kim J, Chun H, Im I, Yoon S, Han Y, Song C, Kim H
PLoS ONE, 2015-07-15;10(7):e0132992.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Fatty acid-binding proteins (FABPs) are intracellular carriers for Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD).
Authors: Elmes M, Kaczocha M, Berger W, Leung K, Ralph B, Wang L, Sweeney J, Miyauchi J, Tsirka S, Ojima I, Deutsch D
J Biol Chem, 2015-02-09;290(14):8711-21.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Identification of transcription factors for lineage-specific ESC differentiation.
Authors: Yamamizu K, Piao Y, Sharov A, Zsiros V, Yu H, Nakazawa K, Schlessinger D, Ko M
Stem Cell Reports, 2013-11-27;1(6):545-59.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Production of hepatocyte-like cells from human pluripotent stem cells.
Authors: Hannan, Nicholas, Segeritz, Charis-P, Touboul, Thomas, Vallier, Ludovic
Nat Protoc, 2013-01-01;8(2):430-7.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Induction of neuronal death by microglial AGE-albumin: implications for Alzheimer's disease.
Authors: Byun K, Bayarsaikhan E, Kim D, Kim CY, Mook-Jung I, Paek SH, Kim SU, Yamamoto T, Won MH, Song BJ, Park YM, Lee B
PLoS ONE, 2012-05-25;7(5):e37917.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Netrin-4 enhances angiogenesis and neurologic outcome after cerebral ischemia.
Authors: Hoang S, Liauw J, Choi M
J. Cereb. Blood Flow Metab., 2008-11-05;29(2):385-97.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Fate-mapping evidence that hepatic stellate cells are epithelial progenitors in adult mouse livers.
Authors: Yang L, Jung Y, Omenetti A, Witek RP, Choi S, Vandongen HM, Huang J, Alpini GD, Diehl AM
Stem Cells, 2008-05-29;26(8):2104-13.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition.
Authors: Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R
J. Biol. Chem., 2007-06-11;282(32):23337-47.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Stem cells derived from human fetal membranes display multilineage differentiation potential.
Authors: Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U
Biol. Reprod., 2007-05-09;77(3):577-88.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Isolation and characterization of a stem cell population from adult human liver.
Authors: Herrera MB, Bruno S, Buttiglieri S, Tetta C, Gatti S, Deregibus MC, Bussolati B, Camussi G
Stem Cells, 2006-08-31;24(12):2840-50.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro.
Authors: Baharvand H, Hashemi SM, Kazemi Ashtiani S, Farrokhi A
Int. J. Dev. Biol., 2006-01-01;50(7):645-52.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Longitudinal in vivo bioimaging of hepatocyte transcription factor activity following cholestatic liver injury in mice
Authors: JM Delhove, SM Buckley, DP Perocheau, R Karda, P Arbuthnot, NC Henderson, SN Waddington, TR McKay
Sci Rep, 2017-02-03;7(0):41874.
-
Rigorous characterization of urinary extracellular vesicles (uEVs) in the low centrifugation pellet - a neglected source for uEVs
Authors: Musante L, Bontha Sv, La Salvia S et Al.
Sci Rep
-
Psoralea corylifolia L. Seed Extract Attenuates Methylglyoxal-Induced Insulin Resistance by Inhibition of Advanced Glycation End Product Formation
Authors: Cao-Sang Truong, Eunhui Seo, Hee-Sook Jun
Oxidative Medicine and Cellular Longevity
-
Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC
Authors: Marcus Grohmann, Florian Wiede, Garron T. Dodd, Esteban N. Gurzov, Geraldine J. Ooi, Tariq Butt et al.
Cell
-
SOX9 accelerates ESC differentiation to three germ layer lineages by repressing SOX2 expression through P21 (WAF1/CIP1)
Authors: Kohei Yamamizu, David Schlessinger, Minoru S. H. Ko
Development
-
A human cell atlas of fetal gene expression
Authors: Cao J, O'Day DR, Pliner HA et al.
Science
-
Non-Invasive Approach for Evaluation of Pulmonary Hypertension Using Extracellular Vesicle-Associated Small Non-Coding RNA
Authors: C Lipps, P Northe, R Figueiredo, M Rohde, A Brahmer, EM Krämer-Alb, C Liebetrau, CB Wiedenroth, E Mayer, SD Kriechbaum, O Dörr, H Nef, CW Hamm, T Keller, C Troidl
Biomolecules, 2019-10-29;9(11):.
-
Stroma‐derived extracellular vesicle mRNA signatures inform histological nature of prostate cancer
Authors: Alex P. Shephard, Peter Giles, Mariama Mbengue, Amr Alraies, Lisa K. Spary, Howard Kynaston et al.
Journal of Extracellular Vesicles
-
Rapid actions of anti‐Müllerian hormone in regulating synaptic transmission and long‐term synaptic plasticity in the hippocampus
Authors: Kang Wang, Fuhua Xu, Shawn P. Campbell, Kyle D. Hart, Tyler Durham, James Maylie et al.
The FASEB Journal
FAQs
-
What is the light chain of Human Serum Albumin Antibody, Catalog #s MAB1455 and IC1455, Clone #188835?
Catalog #s MAB1455 and IC1455 have a kappa light chain.
Reviews for Human Serum Albumin Antibody
Average Rating: 5 (Based on 2 Reviews)
Have you used Human Serum Albumin Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:


