Human VEGF-C Quantikine ELISA Kit Summary
Product Summary
Precision
Cell Culture Supernates
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 572 | 1622 | 3243 | 520 | 1527 | 3069 |
| Standard Deviation | 39.3 | 54.4 | 136 | 50.1 | 102 | 200 |
| CV% | 6.9 | 3.4 | 4.2 | 9.6 | 6.7 | 6.5 |
Serum, EDTA Plasma, Platelet-poor Plasma, Heparin Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 512 | 1543 | 3195 | 514 | 1540 | 3066 |
| Standard Deviation | 33.8 | 54.2 | 136 | 43.6 | 110 | 198 |
| CV% | 6.6 | 3.5 | 4.3 | 8.5 | 7.2 | 6.4 |
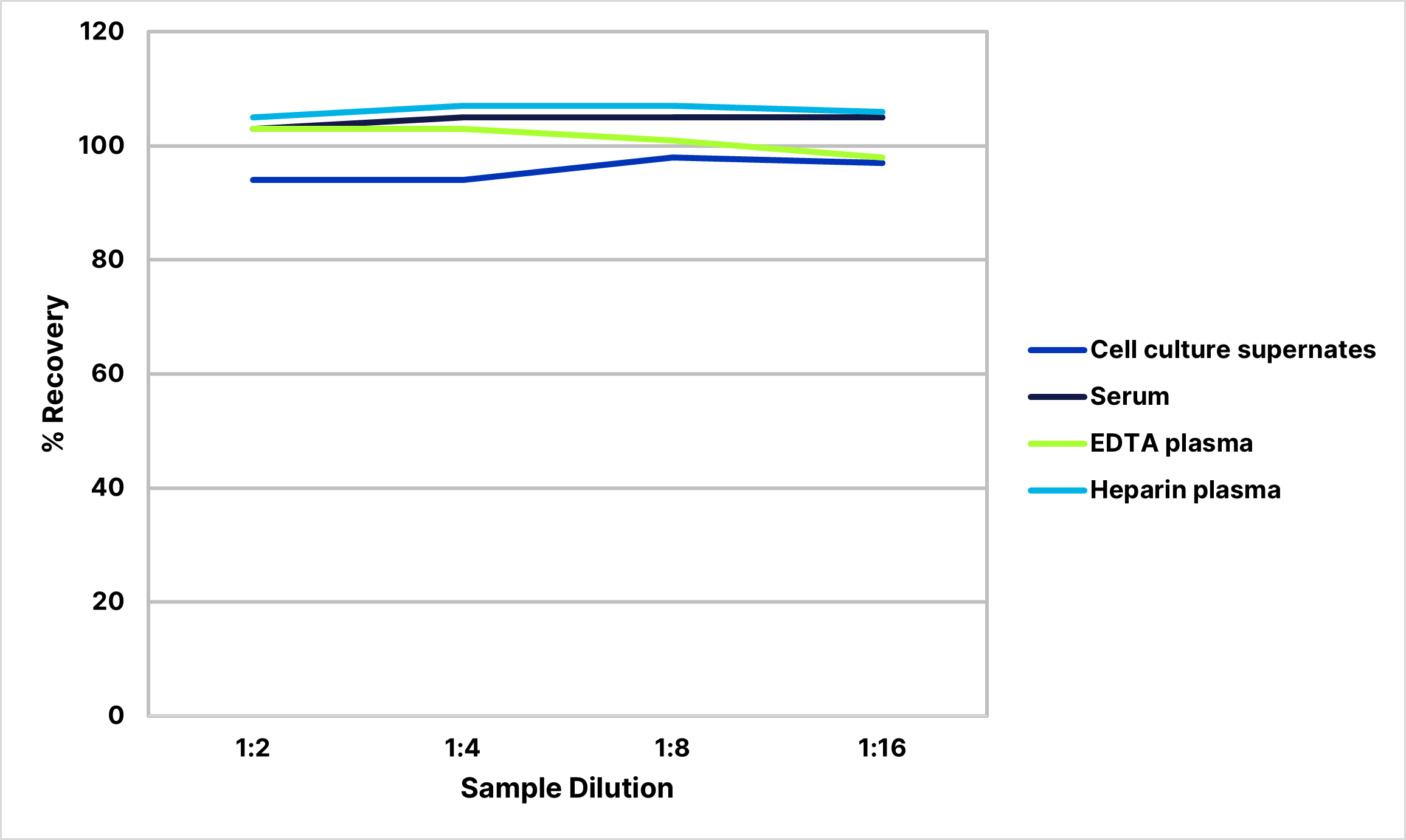
Recovery
The recovery of VEGF-C spiked to levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Supernates (n=4) | 107 | 93-115 |
| EDTA Plasma (n=4) | 101 | 96-110 |
| Heparin Plasma (n=4) | 97 | 88-103 |
| Serum (n=4) | 99 | 89-106 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: VEGF-C
Vascular endothelial growth factor C (VEGF-C) is a homodimeric ligand of VEGF R3/Flt-4 and is synthesized with N- and C-terminal propeptides. Fully processed VEGF-C containing only the VEGF homology domain can additionally bind and activate VEGF R2/KDR/Flk-1. VEGF-C interactions with VEGF R3 are critical for lymphangiogenesis. Both the ligand and receptor are usually co-expressed at sites of lymphatic vessel sprouting, in the embryo, and in various pathological conditions. Over-expression of VEGF-C in tumor cells induces tumoral lymphatic hyperplasia, neoangiogenesis, and vessel sprouting, resulting in enhanced lymph flow and metastasis to regional lymph nodes.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 µL of Assay Diluent to each well.
- Add 50 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours on a horizontal orbital microplate shaker.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes.
- Add 200 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours on the shaker.
- Aspirate and wash 4 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes on the benchtop. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Human VEGF-C Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
55
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Anti-Inflammatory and Anti-(Lymph)angiogenic Properties of an ABCB5+ Limbal Mesenchymal Stem Cell Population
Authors: Meshko, B;Volatier, TLA;Mann, J;Kluth, MA;Ganss, C;Frank, MH;Frank, NY;Ksander, BR;Cursiefen, C;Notara, M;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Development of potent isoflavone-based formyl peptide receptor 1 (FPR1) antagonists and their effects in gastric cancer cell models
Authors: Francavilla, F;Sarcina, F;Schepetkin, IA;Kirpotina, LN;Contino, M;Schirizzi, A;De Leonardis, G;Khlebnikov, AI;D'Alessandro, R;Quinn, MT;Lacivita, E;Leopoldo, M;
European journal of medicinal chemistry
Species: Human
Sample Types: Cell Cultue Supernates
-
Sodium accumulation in the skin is associated with higher density of skin lymphatic vessels in patients with arterial hypertension
Authors: Chachaj, A;Stanimirova, I;Chabowski, M;Gomu?kiewicz, A;Hodurek, P;Glatzel-Pluci?ska, N;Olbromski, M;Piotrowska, A;Kuzan, A;Grzegrzó?ka, J;Ratajczak-Wielgomas, K;Nowak, A;Szahidewicz-Krupska, E;Wi?niewski, J;Bromke, MA;Podhorska-Oko?ów, M;Gamian, A;Janczak, D;Dzi?giel, P;Szuba, A;
Advances in medical sciences
Species: Human
Sample Types: Plasma, Serum
-
High Plasma Angiopoietin-2 Levels Predict the Need to Initiate Dialysis within Two Years in Patients with Chronic Kidney Disease
Authors: Szymczak, A;Kusztal, M;Go??biowski, T;Letachowicz, K;Go?dzik, A;Ko?cielska-Kasprzak, K;Tukiendorf, A;Krajewska, M;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Relationship of Vascular Endothelial Growth Factor C, a Lymphangiogenesis Modulator, with Edema Formation, Congestion and Outcomes in Acute Heart Failure
Authors: G Iwanek, B Ponikowska, A Zdanowicz, M Fudim, M Hurkacz, R Zymli?ski, P Ponikowski, J Biegus
Journal of cardiac failure, 2023-04-29;0(0):.
Species: Human
Sample Types: Serum
-
Dicarbonyl-modified lipoproteins contribute to proteinuric kidney injury
Authors: J Zhong, HC Yang, EL Shelton, T Matsusaka, AJ Clark, V Yermalitsk, Z Mashhadi, LS May-Zhang, MF Linton, AB Fogo, A Kirabo, SS Davies, V Kon
JCI Insight, 2022-11-08;7(21):.
Species: Human
Sample Types: Cell Lysates
-
Hydrogen Sulfide Attenuates Lymphedema Via the Induction of Lymphangiogenesis Through a PI3K/Akt-Dependent Mechanism
Authors: J Suzuki, Y Shimizu, T Hayashi, Y Che, Z Pu, K Tsuzuki, S Narita, R Shibata, I Ishii, JW Calvert, T Murohara
Journal of the American Heart Association, 2022-10-26;11(21):e026889.
Species: Human
Sample Types: Cell Lysates
-
Implementing subtype-specific pre-clinical models of breast cancer to study pre-treatment aspirin effects
Authors: IS Miller, S Khan, LP Shiels, S Das, AC O' Farrell, K Connor, A Lafferty, B Moran, C Isella, P Loadman, E Conroy, S Cohrs, R Schibli, RS Kerbel, WM Gallagher, E Marangoni, K Bennett, DP O' Connor, RM Dwyer, AT Byrne
Cancer Medicine, 2022-04-17;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
CCT5 induces epithelial-mesenchymal transition to promote gastric cancer lymph node metastasis by activating the Wnt/beta-catenin signalling pathway
Authors: Y Li, C Liu, X Zhang, X Huang, S Liang, F Xing, H Tian
British Journal of Cancer, 2022-02-22;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Platelet-derived growth factor BB is reduced in endometrial endothelial cells of women with abnormal uterine bleeding-endometrial disorder
Authors: S Biswas Shi, Q Lu, D Sun, H Hou, JN Bulmer, BA Innes, DK Hapangama, GE Lash
Oncogene, 2022-02-13;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Retinal VEGF-A Overexpression Is Not Sufficient to Induce Lymphangiogenesis Regardless of VEGF-C Upregulation and Lyve1+ Macrophage Infiltration
Authors: I Wada, S Nakao, M Yamaguchi, Y Kaizu, M Arima, S Sawa, KH Sonoda
Investigative Ophthalmology & Visual Science, 2021-10-04;62(13):17.
Species: Human
Sample Types: Vitreous Humor
-
Neutralization of the induced VEGF-A potentiates the therapeutic effect of an anti-VEGFR2 antibody on gastric cancer in vivo
Authors: T Mashima, T Wakatsuki, N Kawata, MK Jang, A Nagamori, H Yoshida, K Nakamura, T Migita, H Seimiya, K Yamaguchi
Scientific Reports, 2021-07-23;11(1):15125.
Species: Mouse
Sample Types: Plasma
-
Comparison of lung ultrasound and other volumetric methods in peritoneal dialysis patients
Authors: M Sevinc, NB Hasbal, T Basturk, PN Ozcafer, BB Kocas, K Kilickesme, A Ozel, T Sakaci, E Ahbap, A Unsal, Y Koc
Medicine, 2021-01-22;100(3):e23856.
Species: Human
Sample Types: Serum
-
Lymphangiogenic therapy prevents cardiac dysfunction by ameliorating inflammation and hypertension
Authors: LouJin Song, Xian Chen, Terri A Swanson, Brianna LaViolette, Jincheng Pang, Teresa Cunio et al.
eLife
Species: Mouse
Sample Types: Plasma
-
Extracellular vesicle-associated VEGF-C promotes lymphangiogenesis and immune cells infiltration in endometriosis
Authors: WN Li, KY Hsiao, CA Wang, N Chang, PL Hsu, CH Sun, SR Wu, MH Wu, SJ Tsai
Proc Natl Acad Sci U S A, 2020-10-01;0(0):.
Species: Human
Sample Types: Serum
-
GRK2-Dependent HuR Phosphorylation Regulates HIF1&alpha Activation under Hypoxia or Adrenergic Stress
Authors: C Reglero, V Lafarga, V Rivas, Á Albitre, P Ramos, SR Berciano, O Tapia, ML Martínez-C, F Mayor, P Penela
Cancers (Basel), 2020-05-13;12(5):.
Species: Human
Sample Types: Cell Lysates
-
Distinct Characteristics of VEGF-D and VEGF-C to Predict Mortality in Patients With Suspected or Known Coronary Artery Disease
Authors: H Wada, M Suzuki, M Matsuda, Y Ajiro, T Shinozaki, S Sakagami, K Yonezawa, M Shimizu, J Funada, T Takenaka, Y Morita, T Nakamura, K Fujimoto, H Matsubara, T Kato, T Unoki, D Takagi, K Wada, M Wada, M Iguchi, N Masunaga, M Ishii, H Yamakage, T Kusakabe, A Yasoda, A Shimatsu, K Kotani, N Satoh-Asah, M Abe, M Akao, K Hasegawa
J Am Heart Assoc, 2020-04-22;9(9):e015761.
Species: Human
Sample Types: Serum
-
Phase Ib/II Clinical Trial of Pembrolizumab With Bevacizumab for Metastatic Renal Cell Carcinoma: BTCRC-GU14-003
Authors: AZ Dudek, LC Liu, S Gupta, TF Logan, EA Singer, M Joshi, YN Zakharia, JM Lang, JK Schwarz, A Al-Janadi, AS Alva
J. Clin. Oncol., 2020-02-25;38(11):1138-1145.
Species: Human
Sample Types: Serum
-
KLK3/PSA and cathepsin D activate VEGF-C and VEGF-D
Authors: SK Jha, K Rauniyar, E Chronowska, K Mattonet, EW Maina, H Koistinen, UH Stenman, K Alitalo, M Jeltsch
Elife, 2019-05-17;8(0):.
Species: Human
Sample Types: Seminal Plasma
-
Distribution and Activity of Lenvatinib in Brain Tumor Models of Human Anaplastic Thyroid Cancer Cells in Severe Combined Immune Deficient Mice
Authors: R Wang, T Yamada, S Arai, K Fukuda, H Taniguchi, A Tanimoto, A Nishiyama, S Takeuchi, K Yamashita, K Ohtsubo, J Matsui, N Onoda, E Hirata, S Taira, S Yano
Mol. Cancer Ther., 2019-03-29;18(5):947-956.
Species: Human
Sample Types: Cell Culture Supernates
-
Antibody-mediated delivery of VEGF-C potently reduces chronic skin inflammation
Authors: S Schwager, S Renner, T Hemmerle, S Karaman, ST Proulx, R Fetz, AM Golding-Oc, P Probst, C Halin, D Neri, M Detmar
JCI Insight, 2018-12-06;3(23):.
Species: Mouse
Sample Types: Serum
-
Docetaxel facilitates lymphatic-tumor crosstalk to promote lymphangiogenesis and cancer progression
Authors: AR Harris, MJ Perez, JM Munson
BMC Cancer, 2018-07-06;18(1):718.
Species: Mouse
Sample Types: Tissue Homogenates
-
RIP1 regulates TNF-?-mediated lymphangiogenesis and lymphatic metastasis in gallbladder cancer by modulating the NF-?B-VEGF-C pathway
Authors: CZ Li, XJ Jiang, B Lin, HJ Hong, SY Zhu, L Jiang, XQ Wang, NH Tang, FF She, YL Chen
Onco Targets Ther, 2018-05-16;11(0):2875-2890.
Species: Human
Sample Types: Cell Culture Supernates
-
cIAP2 promotes gallbladder cancer invasion and lymphangiogenesis by activating the NF-?B pathway
Authors: X Jiang, C Li, B Lin, H Hong, L Jiang, S Zhu, X Wang, N Tang, X Li, F She, Y Chen
Cancer Sci., 2017-05-31;108(6):1144-1156.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of fatty acid synthase inhibitors on lymphatic vessels: an in vitro and in vivo study in a melanoma model
Authors: Débora C Bastos
Lab. Invest, 2016-12-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Vascular endothelial growth factor A is associated with the subsequent development of moderate or severe cardiac allograft vasculopathy in pediatric heart transplant recipients
Authors: David M Briscoe
J. Heart Lung Transplant., 2016-10-03;0(0):.
Species: Human
Sample Types: Plasma
-
Human neural stem cell-induced endothelial morphogenesis requires autocrine/paracrine and juxtacrine signaling
Sci Rep, 2016-07-04;6(0):29029.
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of tumour-reactive lymphatic endothelial cells capable of inducing progression of gastric cancer.
Authors: Tokumoto M, Tanaka H, Tauchi Y, Kasashima H, Kurata K, Yashiro M, Sakurai K, Toyokawa T, Kubo N, Amano R, Kimura K, Muguruma K, Maeda K, Ohira M, Hirakawa K
Br J Cancer, 2015-09-10;113(7):1046-54.
Species: Human
Sample Types: Cell Culture Supernates
-
The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis.
Authors: Tutunea-Fatan, Elena, Majumder, Mousumi, Xin, Xiping, Lala, Peeyush
Mol Cancer, 2015-02-10;14(1):35.
Species: Human
Sample Types: Cell Culture Supernates
-
Telmisartan, a possible PPAR-delta agonist, reduces TNF-alpha-stimulated VEGF-C production by inhibiting the p38MAPK/HSP27 pathway in human proximal renal tubular cells.
Authors: Kimura H, Mikami D, Kamiyama K, Sugimoto H, Kasuno K, Takahashi N, Yoshida H, Iwano M
Biochem Biophys Res Commun, 2014-10-22;454(2):320-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum angiopoietin-2 and soluble VEGFR-2 levels predict malignancy of ovarian neoplasm and poor prognosis in epithelial ovarian cancer.
Authors: Sallinen H, Heikura T, Koponen J, Kosma V, Heinonen S, Yla-Herttuala S, Anttila M
BMC Cancer, 2014-09-23;14(0):696.
Species: Human
Sample Types: Serum
-
Filarial excretory-secretory products induce human monocytes to produce lymphangiogenic mediators.
Authors: Weinkopff T, Mackenzie C, Eversole R, Lammie P
PLoS Negl Trop Dis, 2014-07-10;8(7):e2893.
Species: Human
Sample Types: Cell Culture Supernates
-
KML001 inhibits cell proliferation and invasion in pancreatic cancer cells through suppression of NF-kappaB and VEGF-C.
Authors: Yang M, Kim H, Lee K, Yang S, Lee J, Lee K, Rhee J
Anticancer Res, 2014-07-01;34(7):3469-74.
Species: Human
Sample Types: Cell Culture Supernates
-
Vascular endothelial growth factor c promotes ovarian carcinoma progression through paracrine and autocrine mechanisms.
Authors: Decio A, Taraboletti G, Patton V, Alzani R, Perego P, Fruscio R, Jurgensmeier J, Giavazzi R, Belotti D
Am J Pathol, 2014-02-06;184(4):1050-61.
Species: Human
Sample Types: Ascites Fluid
-
Long-term survival and biomarker correlates of tasquinimod efficacy in a multicenter randomized study of men with minimally symptomatic metastatic castration-resistant prostate cancer.
Authors: Armstrong A, Haggman M, Stadler W, Gingrich J, Assikis V, Polikoff J, Damber J, Belkoff L, Nordle O, Forsberg G, Carducci M, Pili R
Clin Cancer Res, 2013-11-19;19(24):6891-901.
Species: Human
Sample Types: Plasma
-
Role of serum concentration of VEGFR1 and TIMP2 on clinical outcome in primary cervical cancer: results of a companion protocol of the randomized, NOGGO-AGO phase III adjuvant trial of simultaneous cisplatin-based radiochemotherapy vs. carboplatin and paclitaxel containing sequential radiotherapy.
Authors: Braicu E, Fotopoulou C, Chekerov R, Richter R, Blohmer J, Kummel S, Stamatian F, Yalcinkaya I, Mentze M, Lichtenegger W, Sehouli J
Cytokine, 2013-02-14;61(3):755-8.
Species: Human
Sample Types: Serum
-
Proteinuria triggers renal lymphangiogenesis prior to the development of interstitial fibrosis.
Authors: Yazdani S, Poosti F, Kramer A, Mirkovic K, Kwakernaak A, Hovingh M, Slagman M, Sjollema K, de Borst M, Navis G, van Goor H, van den Born J
PLoS ONE, 2012-11-26;7(11):e50209.
Species: Human
Sample Types: Cell Culture Supernates
-
Lysophosphatidic Acid Enhances Vascular Endothelial Growth Factor-C Expression in Human Prostate Cancer PC-3 Cells.
Authors: Lin CE, Chen SU, Lin CC
PLoS ONE, 2012-07-20;7(7):e41096.
Species: Human
Sample Types: Cell Culture Supernates
-
Co-expression of alpha9beta1 integrin and VEGF-D confers lymphatic metastatic ability to a human breast cancer cell line MDA-MB-468LN.
Authors: Majumder M, Tutunea-Fatan E, Xin X, Rodriguez-Torres M, Torres-Garcia J, Wiebe R, Timoshenko AV, Bhattacharjee RN, Chambers AF, Lala PK
PLoS ONE, 2012-04-24;7(4):e35094.
Species: Human
Sample Types: Cell Culture Supernates
-
Prognostic significance of angiogenic factors in uterine cervical cancer.
Authors: Landt S, Wehling M, Heidecke H, Jeschke S, Korlach S, Stoblen F, Schmid P, Blohmer JU, Lichtenegger W, Sehouli J, Kummel S
Anticancer Res., 2011-08-01;31(8):2589-95.
Species: Human
Sample Types: Serum
-
Mechanism-related circulating proteins as biomarkers for clinical outcome in patients with unresectable hepatocellular carcinoma receiving sunitinib.
Authors: Harmon CS, DePrimo SE, Raymond E, Cheng AL, Boucher E, Douillard JY, Lim HY, Kim JS, Lechuga MJ, Lanzalone S, Lin X, Faivre S
J Transl Med, 2011-07-25;9(0):120.
Species: Human
Sample Types: Plasma
-
Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast.
Authors: Scheel C, Eaton E, Li S, Chaffer C, Reinhardt F, Kah K, Bell G, Guo W, Rubin J, Richardson A, Weinberg R
Cell, 2011-06-10;145(6):926-40.
Species: Human
Sample Types: Cell Culture Supernates
-
Phase I and pharmacological study of the broad-spectrum tyrosine kinase inhibitor JNJ-26483327 in patients with advanced solid tumours.
Authors: Konings IR, de Jonge MJ, Burger H
Br. J. Cancer, 2010-09-07;103(7):987-92.
Species: Human
Sample Types: Serum
-
Human pregnancy specific beta-1-glycoprotein 1 (PSG1) has a potential role in placental vascular morphogenesis.
Authors: Ha CT, Wu JA, Irmak S, Lisboa FA, Dizon AM, Warren JW, Ergun S, Dveksler GS
Biol. Reprod., 2010-03-24;83(1):27-35.
Species: Human
Sample Types: Cell Culture Supernates
-
VEGF-C regulates lymphangiogenesis and capillary stability by regulation of PDGF-B.
Authors: Onimaru M, Yonemitsu Y, Fujii T, Tanii M, Nakano T, Nakagawa K, Kohno R, Hasegawa M, Nishikawa S, Sueishi K
Am. J. Physiol. Heart Circ. Physiol., 2009-09-04;297(5):H1685-96.
Species: Human
Sample Types: Serum
-
Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study.
Authors: Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J, Bhargava P, Meyerhardt J, Clark JW, Kwak EL, Hezel AF, Miksad R, Abrams TA, Enzinger PC, Fuchs CS, Ryan DP, Jain RK
J. Clin. Oncol., 2009-05-26;27(18):3027-35.
Species: Human
Sample Types: Plasma
-
Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism.
Authors: Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, Van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J
Nat. Med., 2009-05-03;15(5):545-52.
Species: Human
Sample Types: Serum
-
Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma.
Authors: Rini BI, Michaelson MD, Rosenberg JE, Bukowski RM, Sosman JA, Stadler WM, Hutson TE, Margolin K, Harmon CS, DePrimo SE, Kim ST, Chen I, George DJ
J. Clin. Oncol., 2008-08-01;26(22):3743-8.
Species: Human
Sample Types: Plasma
-
Isolation and characterization of CD146+ multipotent mesenchymal stromal cells.
Authors: Sorrentino A, Ferracin M, Castelli G, Biffoni M, Tomaselli G, Baiocchi M, Fatica A, Negrini M, Peschle C, Valtieri M
Exp. Hematol., 2008-05-27;36(8):1035-46.
Species: Human
Sample Types: Cell Culture Supernates
-
Cutting edge: rapid accumulation of epidermal CCL27 in skin-draining lymph nodes following topical application of a contact sensitizer recruits CCR10-expressing T cells.
Authors: Huang V, Lonsdorf AS, Fang L, Kakinuma T, Lee VC, Cha E, Zhang H, Nagao K, Zaleska M, Olszewski WL, Hwang ST
J. Immunol., 2008-05-15;180(10):6462-6.
Species: Human
Sample Types: Serum
-
Hypoxia-induced epithelial VEGF-C/VEGFR-3 upregulation in carcinoma cell lines.
Authors: Simiantonaki N, Jayasinghe C, Michel-Schmidt R, Peters K, Hermanns MI, Kirkpatrick CJ
Int. J. Oncol., 2008-03-01;32(3):585-92.
Species: Human
Sample Types: Cell Culture Supernates
-
Marrow angiogenesis-associated factors as prognostic biomarkers in patients with acute myelogenous leukaemia.
Authors: Lee CY, Tien HF, Hu CY, Chou WC, Lin LI
Br. J. Cancer, 2007-09-11;97(7):877-82.
Species: Human
Sample Types: Plasma
-
Expression of Tie-2 and other receptors for endothelial growth factors in acute myeloid leukemias is associated with monocytic features of leukemic blasts.
Authors: Riccioni R, Diverio D, Mariani G, Buffolino S, Riti V, Saulle E, Petrucci E, Cedrone M, Lo-Coco F, Foa R, Peschle C, Testa U
Stem Cells, 2007-04-19;25(8):1862-71.
Species: Human
Sample Types: Cell Culture Supernates
-
Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth.
Authors: Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L, Alitalo K
Cancer Res., 2007-01-15;67(2):593-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor.
Authors: Lin J, Lalani AS, Harding TC
Cancer Res., 2005-08-01;65(15):6901-9.
Species: Human
Sample Types: Tissue Homogenates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human VEGF-C Quantikine ELISA Kit
Average Rating: 4 (Based on 3 Reviews)
Have you used Human VEGF-C Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Works for mouse samples