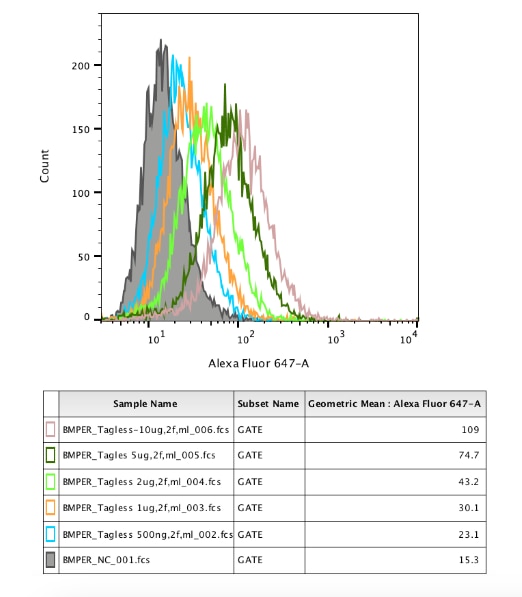
Mouse Crossveinless-2/CV-2 Antibody Summary
Ala39-Arg685
Accession # AAH66153
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Crossveinless‑2/CV‑2 in Mouse Embryo. Crossveinless-2/CV-2 was detected in immersion fixed frozen sections of mouse embryo (E13.5) using Mouse Crossveinless-2/CV-2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2299) at 10 µg/mL overnight at 4 °C. Tissue was stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counterstained with DAPI (blue). Specific staining was localized to the epithelium surrounding the nasal cavity. View our protocol for Fluorescent IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
Detection of Mouse Crossveinless-2/CV-2/BMPER by Immunocytochemistry/Immunofluorescence Negative correlation between distributions of BMPER and pSMAD1/5/8 along with BMP4-induced expression suggests BMPER regulates BMP signaling through negative feedback. (A–C) Localization of pSMAD1/5/8 and BMPER protein in the AGM region from E9.5 (A), E10.5 (B), and E11.5 (C) embryos. Magenta, BMPER; cyan, pSMAD1/5/8; green, CD31; blue, DAPI. Bars, 100 µm. Boxes highlight regions of complementarity between BMPER and pSMAD1/5/8 shown at higher magnification in D and Fig. S5 A. UGR, one of two indicated. (D) Higher magnification of E10.5 ventro-lateral region highlighted in B. Magenta, BMPER; cyan, pSMAD1/5/8; green, CD31; blue, DAPI. Bar, 50 µm. Dotted line indicates the boundary around regions with high BMPER protein. (E) Higher magnification view of intra-aortic cluster highlighted in box “E” of B. Magenta, BMPER; cyan, pSMAD1/5/8; green, CD31; blue, DAPI. Arrowheads indicate intra-aortic cluster. Asterisks indicate subendothelial cells with strong nuclear pSMAD1/5/8 signal. Bar, 50 µm. (F) Quantification of pSMAD1/5/8 staining intensity (mean gray values) over an 80-µm band around the dorsal aorta on transverse embryo sections from E9.5, E10.5, and E11.5 (shown in Fig. S5 C). Quantification was on sections from at least two embryos (littermates). Significance measured by Student’s t test: **, P = 0.0015. (G) Expression of Bmper and Bmp4 in AGMs dissected from E9.5, E10.5, and E11.5 stage embryos normalized to Tbp. Each point represents one embryo (littermates). Significance measured by Student’s t test: ****, Bmper E9.5 versus E11.5 P = 8 × 105. (H) Expression of Bmper in E11.5 AGM explants after 24-h culture with BMP4 at displayed dose, without cytokines or serum normalized to Tbp. Experiments were performed twice. Error bars represent SD from the mean. Significance measured by Student’s t test: *, P = 0.035. (I) Expression of Bmper in OP9-BMP4 after reaggregation and culture with doxycycline to induce Bmp4 overexpression. Expression was normalized to Tbp. For each condition, reaggregates were cultured in two separate wells in parallel. Error bars represent the SD from the mean (n = 2). Significance measured by Student’s t test: *, P = 0.026. Image collected and cropped by CiteAb from the following publication (https://rupress.org/jem/article/214/12/3731/42286/A-molecular-roadmap-of-the-AGM-region-reveals), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Crossveinless-2/CV-2/BMPER by Immunocytochemistry/Immunofluorescence Perivascular cells and subaortic mesenchyme are the main source of BMPER within the AGM region. (A) Distribution of BMPER protein in a transverse section of E10.5 AGM measured by immunostaining. Green, CD31; magenta, BMPER; cyan, RUNX1; blue, DAPI. gut, hindgut; nc, notochord. Bar, 50 µm. (B) Bmper mRNA in transverse section of the E10.5 AGM region by in situ hybridization. Bar, 50 µm. (C) Quantification of the mean immunostaining signal intensity (mean gray values) of DAPI (blue) and BMPER (magenta) along a box (not depicted) drawn over the dorsal-ventral axis of the AGM region from A. Distance is from the notochord (dorsal), position 0, to the intersection with the gut and the AGM region (ventral), position 500. (D) Higher magnification of the highlighted region from A showing the aortic endothelium and perivascular population. Green, CD31; magenta, BMPER; cyan, RUNX1; blue, DAPI. Bar, 50 µm. (E) Higher magnification of the region highlighted in B showing Bmper mRNA around the lining of the aorta. Bar, 50 µm. (F) Expression level of Bmper relative to Tbp in each sorted population: Lin−VC−CD45+, representing hematopoietic cells; Lin−VC+CD45−, endothelial cells; Lin−VC−CD45−CD146+, putative perivascular cells; and Lin−VC−CD45−CD146−, remaining stroma. Expression was normalized to the Lin−VC−CD45−CD146+ population. Each population as percentage of Lin− cells indicated below. Sorting was performed twice, first from one pool of embryos from four to five litters and second from two pools of embryos from four to five litters. Error bars represent SD from the mean (n = 3). Significance calculated by t test: **, P = 0.0016; ***, perivascular versus stroma, P = 0.0006; perivascular versus hematopoietic, P = 0.0002. (G) The distribution of nonendothelial, CD146-positive cells and endothelial CD146-positive cells in transverse section of the E10.5 AGM region. Green, CD31; red, CD146; blue, DAPI. Bar, 50 µm. (H) Higher magnification view of the region highlighted in G showing CD31- and CD146-positive endothelial layer and the CD146-positive CD31-negative perivascular layer around the dorsal aorta. Green, CD31; red, CD146; blue, DAPI. Bar, 50 µm. (I) Expression level of Bmper relative to Tbp in each sorted population: Lin−VC+CD45−CD43−CD41lo proHSC, Lin−VC+CD43+CD41+ type I preHSC, and Lin−VC+CD45+ type II preHSC and Lin−VC−CD45−CD146+ putative perivascular cells. Each population as percentage of Lin− cells indicated below. Sorting was performed twice, from pools of two and six litters, respectively. Expression was normalized to the Lin−VC−CD45−CD146+ population. Error bars represent SD from the mean (n = 2). Significance calculated by t test: *, perivascular versus preHSCI, P = 0.04; perivascular versus preHSCII, P = 0.03. (J) Higher magnification view of highlighted region from A showing the localization of BMPER protein in the hematopoietic clusters of the E10.5 dorsal aorta in sections measured by immunostaining. Magenta, BMPER; green, CD31; cyan, RUNX1; blue, DAPI. Bar, 50 µm. (K and L) Higher magnification view of highlighted region from B (K) and from Fig. S3 C (L) showing Bmper mRNA in some but not all cells of the intra-aortic cluster by in situ hybridization. Bars, 50 µm. Image collected and cropped by CiteAb from the following publication (https://rupress.org/jem/article/214/12/3731/42286/A-molecular-roadmap-of-the-AGM-region-reveals), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Crossveinless-2/CV-2/BMPER by Immunocytochemistry/Immunofluorescence Perivascular cells and subaortic mesenchyme are the main source of BMPER within the AGM region. (A) Distribution of BMPER protein in a transverse section of E10.5 AGM measured by immunostaining. Green, CD31; magenta, BMPER; cyan, RUNX1; blue, DAPI. gut, hindgut; nc, notochord. Bar, 50 µm. (B) Bmper mRNA in transverse section of the E10.5 AGM region by in situ hybridization. Bar, 50 µm. (C) Quantification of the mean immunostaining signal intensity (mean gray values) of DAPI (blue) and BMPER (magenta) along a box (not depicted) drawn over the dorsal-ventral axis of the AGM region from A. Distance is from the notochord (dorsal), position 0, to the intersection with the gut and the AGM region (ventral), position 500. (D) Higher magnification of the highlighted region from A showing the aortic endothelium and perivascular population. Green, CD31; magenta, BMPER; cyan, RUNX1; blue, DAPI. Bar, 50 µm. (E) Higher magnification of the region highlighted in B showing Bmper mRNA around the lining of the aorta. Bar, 50 µm. (F) Expression level of Bmper relative to Tbp in each sorted population: Lin−VC−CD45+, representing hematopoietic cells; Lin−VC+CD45−, endothelial cells; Lin−VC−CD45−CD146+, putative perivascular cells; and Lin−VC−CD45−CD146−, remaining stroma. Expression was normalized to the Lin−VC−CD45−CD146+ population. Each population as percentage of Lin− cells indicated below. Sorting was performed twice, first from one pool of embryos from four to five litters and second from two pools of embryos from four to five litters. Error bars represent SD from the mean (n = 3). Significance calculated by t test: **, P = 0.0016; ***, perivascular versus stroma, P = 0.0006; perivascular versus hematopoietic, P = 0.0002. (G) The distribution of nonendothelial, CD146-positive cells and endothelial CD146-positive cells in transverse section of the E10.5 AGM region. Green, CD31; red, CD146; blue, DAPI. Bar, 50 µm. (H) Higher magnification view of the region highlighted in G showing CD31- and CD146-positive endothelial layer and the CD146-positive CD31-negative perivascular layer around the dorsal aorta. Green, CD31; red, CD146; blue, DAPI. Bar, 50 µm. (I) Expression level of Bmper relative to Tbp in each sorted population: Lin−VC+CD45−CD43−CD41lo proHSC, Lin−VC+CD43+CD41+ type I preHSC, and Lin−VC+CD45+ type II preHSC and Lin−VC−CD45−CD146+ putative perivascular cells. Each population as percentage of Lin− cells indicated below. Sorting was performed twice, from pools of two and six litters, respectively. Expression was normalized to the Lin−VC−CD45−CD146+ population. Error bars represent SD from the mean (n = 2). Significance calculated by t test: *, perivascular versus preHSCI, P = 0.04; perivascular versus preHSCII, P = 0.03. (J) Higher magnification view of highlighted region from A showing the localization of BMPER protein in the hematopoietic clusters of the E10.5 dorsal aorta in sections measured by immunostaining. Magenta, BMPER; green, CD31; cyan, RUNX1; blue, DAPI. Bar, 50 µm. (K and L) Higher magnification view of highlighted region from B (K) and from Fig. S3 C (L) showing Bmper mRNA in some but not all cells of the intra-aortic cluster by in situ hybridization. Bars, 50 µm. Image collected and cropped by CiteAb from the following publication (https://rupress.org/jem/article/214/12/3731/42286/A-molecular-roadmap-of-the-AGM-region-reveals), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Crossveinless-2/CV-2/BMPER by Immunocytochemistry/Immunofluorescence Negative correlation between distributions of BMPER and pSMAD1/5/8 along with BMP4-induced expression suggests BMPER regulates BMP signaling through negative feedback. (A–C) Localization of pSMAD1/5/8 and BMPER protein in the AGM region from E9.5 (A), E10.5 (B), and E11.5 (C) embryos. Magenta, BMPER; cyan, pSMAD1/5/8; green, CD31; blue, DAPI. Bars, 100 µm. Boxes highlight regions of complementarity between BMPER and pSMAD1/5/8 shown at higher magnification in D and Fig. S5 A. UGR, one of two indicated. (D) Higher magnification of E10.5 ventro-lateral region highlighted in B. Magenta, BMPER; cyan, pSMAD1/5/8; green, CD31; blue, DAPI. Bar, 50 µm. Dotted line indicates the boundary around regions with high BMPER protein. (E) Higher magnification view of intra-aortic cluster highlighted in box “E” of B. Magenta, BMPER; cyan, pSMAD1/5/8; green, CD31; blue, DAPI. Arrowheads indicate intra-aortic cluster. Asterisks indicate subendothelial cells with strong nuclear pSMAD1/5/8 signal. Bar, 50 µm. (F) Quantification of pSMAD1/5/8 staining intensity (mean gray values) over an 80-µm band around the dorsal aorta on transverse embryo sections from E9.5, E10.5, and E11.5 (shown in Fig. S5 C). Quantification was on sections from at least two embryos (littermates). Significance measured by Student’s t test: **, P = 0.0015. (G) Expression of Bmper and Bmp4 in AGMs dissected from E9.5, E10.5, and E11.5 stage embryos normalized to Tbp. Each point represents one embryo (littermates). Significance measured by Student’s t test: ****, Bmper E9.5 versus E11.5 P = 8 × 105. (H) Expression of Bmper in E11.5 AGM explants after 24-h culture with BMP4 at displayed dose, without cytokines or serum normalized to Tbp. Experiments were performed twice. Error bars represent SD from the mean. Significance measured by Student’s t test: *, P = 0.035. (I) Expression of Bmper in OP9-BMP4 after reaggregation and culture with doxycycline to induce Bmp4 overexpression. Expression was normalized to Tbp. For each condition, reaggregates were cultured in two separate wells in parallel. Error bars represent the SD from the mean (n = 2). Significance measured by Student’s t test: *, P = 0.026. Image collected and cropped by CiteAb from the following publication (https://rupress.org/jem/article/214/12/3731/42286/A-molecular-roadmap-of-the-AGM-region-reveals), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Crossveinless-2/CV-2/BMPER by Immunocytochemistry/Immunofluorescence Perivascular cells and subaortic mesenchyme are the main source of BMPER within the AGM region. (A) Distribution of BMPER protein in a transverse section of E10.5 AGM measured by immunostaining. Green, CD31; magenta, BMPER; cyan, RUNX1; blue, DAPI. gut, hindgut; nc, notochord. Bar, 50 µm. (B) Bmper mRNA in transverse section of the E10.5 AGM region by in situ hybridization. Bar, 50 µm. (C) Quantification of the mean immunostaining signal intensity (mean gray values) of DAPI (blue) and BMPER (magenta) along a box (not depicted) drawn over the dorsal-ventral axis of the AGM region from A. Distance is from the notochord (dorsal), position 0, to the intersection with the gut and the AGM region (ventral), position 500. (D) Higher magnification of the highlighted region from A showing the aortic endothelium and perivascular population. Green, CD31; magenta, BMPER; cyan, RUNX1; blue, DAPI. Bar, 50 µm. (E) Higher magnification of the region highlighted in B showing Bmper mRNA around the lining of the aorta. Bar, 50 µm. (F) Expression level of Bmper relative to Tbp in each sorted population: Lin−VC−CD45+, representing hematopoietic cells; Lin−VC+CD45−, endothelial cells; Lin−VC−CD45−CD146+, putative perivascular cells; and Lin−VC−CD45−CD146−, remaining stroma. Expression was normalized to the Lin−VC−CD45−CD146+ population. Each population as percentage of Lin− cells indicated below. Sorting was performed twice, first from one pool of embryos from four to five litters and second from two pools of embryos from four to five litters. Error bars represent SD from the mean (n = 3). Significance calculated by t test: **, P = 0.0016; ***, perivascular versus stroma, P = 0.0006; perivascular versus hematopoietic, P = 0.0002. (G) The distribution of nonendothelial, CD146-positive cells and endothelial CD146-positive cells in transverse section of the E10.5 AGM region. Green, CD31; red, CD146; blue, DAPI. Bar, 50 µm. (H) Higher magnification view of the region highlighted in G showing CD31- and CD146-positive endothelial layer and the CD146-positive CD31-negative perivascular layer around the dorsal aorta. Green, CD31; red, CD146; blue, DAPI. Bar, 50 µm. (I) Expression level of Bmper relative to Tbp in each sorted population: Lin−VC+CD45−CD43−CD41lo proHSC, Lin−VC+CD43+CD41+ type I preHSC, and Lin−VC+CD45+ type II preHSC and Lin−VC−CD45−CD146+ putative perivascular cells. Each population as percentage of Lin− cells indicated below. Sorting was performed twice, from pools of two and six litters, respectively. Expression was normalized to the Lin−VC−CD45−CD146+ population. Error bars represent SD from the mean (n = 2). Significance calculated by t test: *, perivascular versus preHSCI, P = 0.04; perivascular versus preHSCII, P = 0.03. (J) Higher magnification view of highlighted region from A showing the localization of BMPER protein in the hematopoietic clusters of the E10.5 dorsal aorta in sections measured by immunostaining. Magenta, BMPER; green, CD31; cyan, RUNX1; blue, DAPI. Bar, 50 µm. (K and L) Higher magnification view of highlighted region from B (K) and from Fig. S3 C (L) showing Bmper mRNA in some but not all cells of the intra-aortic cluster by in situ hybridization. Bars, 50 µm. Image collected and cropped by CiteAb from the following publication (https://rupress.org/jem/article/214/12/3731/42286/A-molecular-roadmap-of-the-AGM-region-reveals), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Crossveinless-2/CV-2
Crossveinless-2 (CV-2), also known as bone morphogenetic protein-binding endothelial cell precursor-derived regulator (BMPER), is a secreted chordin-like protein that modulates the BMP signaling pathway (1‑3). Mouse CV-2 is synthesized as a 685 amino acid (aa) residue precursor protein with a putative 39 aa signal peptide, five tandem chordin-like cysteine-rich (CR) domains, a partial von Willebrand factor type D domain (vWD), and a carboxyl trypsin inhibitor-like cysteine-rich domain (TIL) (1, 2, 4). Secreted CV-2 is reported to be proteolytically cleaved to generate two fragments that are disulfide-linked (1, 2). The GDPH sequence is conserved in CV-2 from other species. It is also found in multiple proteins that undergo a similar type of cleavage (5). Mouse CV-2 message is detected in many tissues, with the highest expression detected in the heart, lungs, and skin (2). It is also expressed in flk-1+ endothelial cell precursors and in primary chondrocytes (2). During embryonic development, CV-2 is expressed in the dorsal midline, regions of the telencephalon, migrating cells of the branchial neural crest and endothelial cells in the yolk sac (2). Mouse CV-2 shares 92% and 34% aa sequence identity with the human and Drosophila homologs, respectively (1, 4). Results from biochemical experiments using recombinant CV-2 show that CV-2 directly interacts with BMP-2, -4, and -6 to antagonize BMP signaling, which can regulate a wide range of differentiation processes (1, 2). In contrast, genetic data from Drosophila suggest that CV-2 potentiates BMP-signaling (6). It is possible that like TSG, CV-2 can positively and negatively modulate BMP signal transduction depending on the cell context (7).
- Binnerts, M.E. et al. (2004) Biochem Biophys Res Commun. 315:272.
- Moser, M. et al. (2003) Mol Cell Biol. 23:5664.
- Garcia-Abreu, J. et al. (2002) Gene 287:39.
- Coffinier, C. et al. (2002) Mech Dev. 119:S179.
- Lidell, M.E. et al. (2003) J. Biol. Chem. 278:13944.
- Conley, C.A. et al. (2000) Development 127:3947.
- Kamimura, M. et al. (2004) Developmental Dynamics 230:434.
Product Datasheets
Citations for Mouse Crossveinless-2/CV-2 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
7
Citations: Showing 1 - 7
Filter your results:
Filter by:
-
A molecular roadmap of the AGM region reveals BMPER as a novel regulator of HSC maturation
Authors: Alison C. McGarvey, Stanislav Rybtsov, Céline Souilhol, Sara Tamagno, Ritva Rice, David Hills et al.
Journal of Experimental Medicine
Species: Mouse
Sample Types: Organoid
Applications: Immunohistochemistry -
BMPER Promotes Epithelial-Mesenchymal Transition in the Developing Cardiac Cushions
Authors: Laura Dyer, Pamela Lockyer, Yaxu Wu, Arnab Saha, Chelsea Cyr, Martin Moser et al.
PLOS ONE
-
BMPER-induced BMP signaling promotes coronary artery remodeling.
Authors: Dyer L, Wu Y, Moser M, Patterson C
Dev Biol, 2013-12-27;386(2):385-94.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Crossveinless-2 is required for the relocalization of Chordin protein within the vertebral field in mouse embryos.
Authors: Zakin L, Chang EY, Plouhinec JL, De Robertis EM
Dev. Biol., 2010-08-31;347(1):204-15.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Development of the vertebral morphogenetic field in the mouse: interactions between Crossveinless-2 and Twisted Gastrulation.
Authors: Zakin L, Metzinger CA, Chang EY, Coffinier C, De Robertis EM
Dev. Biol., 2008-08-29;323(1):6-18.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning.
Authors: Ambrosio AL, Taelman VF, Lee HX, Metzinger CA, Coffinier C, De Robertis EM
Dev. Cell, 2008-08-01;15(2):248-60.
Species: Mouse
Sample Types: Recombinant Protein
Applications: Immunoprecipitation, Western Blot -
BMPER is a conserved regulator of hematopoietic and vascular development in zebrafish.
Authors: Moser M, Yu Q, Bode C, Xiong JW, Patterson C
J. Mol. Cell. Cardiol., 2007-05-18;43(3):243-53.
Species: Zebrafish
Sample Types: Cell Culture Supernates
Applications: Western Blot
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse Crossveinless-2/CV-2 Antibody
Average Rating: 4.5 (Based on 2 Reviews)
Have you used Mouse Crossveinless-2/CV-2 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: