Mouse Ly-6G/Ly-6C (Gr-1) Antibody Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Ly-6G/Ly-6C (Gr-1) in Mouse Spleno-cytes. Ly-6G/Ly-6C (Gr-1) was detected in immersion fixed mouse splenocytes using Mouse Ly-6G/Ly-6C (Gr-1) Monoclonal Anti-body (Catalog # MAB1037) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Rat IgG Secondary Antibody (red; Catalog # NL013) and counter-stained with DAPI (blue). View our protocol for Fluorescent ICC Staining of Non-adherent Cells.
 View Larger
View Larger
Ly-6G/Ly-6C (Gr-1) in Mouse Spleen. Ly-6G/Ly-6C (Gr-1) was detected in perfusion fixed frozen sections of mouse spleen using Rat Anti-Mouse Ly-6G/Ly-6C (Gr-1)Monoclonal Antibody (Catalog # MAB1037) at 3 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Rat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS017) and counterstained with hematoxylin (blue). Specific staining was localized to cytoplasm in splenocytes. View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
Detection of Mouse Mouse Ly-6G/Ly-6C (Gr-1) Antibody by Immunohistochemistry Lymphocyte/neutrophil ratio increases upon NHE1 inhibition in tumor sections of KPfC mice.(A) H&E (left) and PAS-stained KPfC mouse tissue sections after vehicle and gemcitabine + cariporide (GEM+CARI) therapy. Cells of innate immunity, such as neutrophils (arrows), utilize glycogen and are thus PAS+ (purple), in contrast to, for example, lymphocytes. Scale bar: 50 μm. (B) Representative IHC images stained for Ly6G+ neutrophils (magenta, arrows on left image), CD3+ lymphocytes (yellow, arrows on the right image), and nuclei with DAPI (cyan). Scale bar: 50 μm. (C) CD3/Ly6G ratio was assessed by dividing the number of CD3+ cells by the number of Ly6G+ cells in every tumor node. Data points depict the mean CD3/Ly6G ratio derived from each tumor node in individual mice; NVehicle = 10, NGEM = 9, NCARI = 10, NGEM+CARI = 11 mice. (D) To obtain the CD3/Ly6G ratio per tumor node, the number of CD3+ cells was divided by the respective number of Ly6G+ cells in each tumor node. Data points depict individual tumor nodes; nVehicle = 386, nGEM = 276, nCARI = 301, nGEM+CARI = 398. Data and statistical comparison in D and E are represented as median ± 95% CI using Kruskal-Wallis statistical test with Dunn’s post hoc test. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/37643024), licensed under a CC-BY license. Not internally tested by R&D Systems.
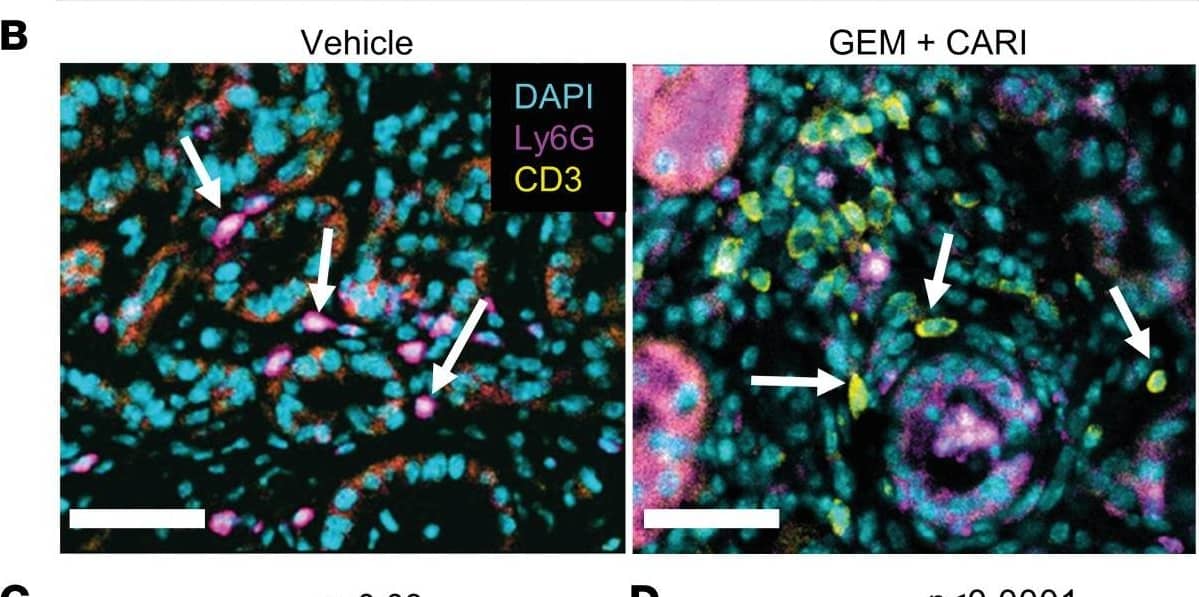 View Larger
View Larger
Detection of Mouse Gr-1/Ly-6G by Immunohistochemistry Lymphocyte/neutrophil ratio increases upon NHE1 inhibition in tumor sections of KPfC mice.(A) H&E (left) and PAS-stained KPfC mouse tissue sections after vehicle and gemcitabine + cariporide (GEM+CARI) therapy. Cells of innate immunity, such as neutrophils (arrows), utilize glycogen and are thus PAS+ (purple), in contrast to, for example, lymphocytes. Scale bar: 50 μm. (B) Representative IHC images stained for Ly6G+ neutrophils (magenta, arrows on left image), CD3+ lymphocytes (yellow, arrows on the right image), and nuclei with DAPI (cyan). Scale bar: 50 μm. (C) CD3/Ly6G ratio was assessed by dividing the number of CD3+ cells by the number of Ly6G+ cells in every tumor node. Data points depict the mean CD3/Ly6G ratio derived from each tumor node in individual mice; NVehicle = 10, NGEM = 9, NCARI = 10, NGEM+CARI = 11 mice. (D) To obtain the CD3/Ly6G ratio per tumor node, the number of CD3+ cells was divided by the respective number of Ly6G+ cells in each tumor node. Data points depict individual tumor nodes; nVehicle = 386, nGEM = 276, nCARI = 301, nGEM+CARI = 398. Data and statistical comparison in D and E are represented as median ± 95% CI using Kruskal-Wallis statistical test with Dunn’s post hoc test. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37643024), licensed under a CC-BY license. Not internally tested by R&D Systems.
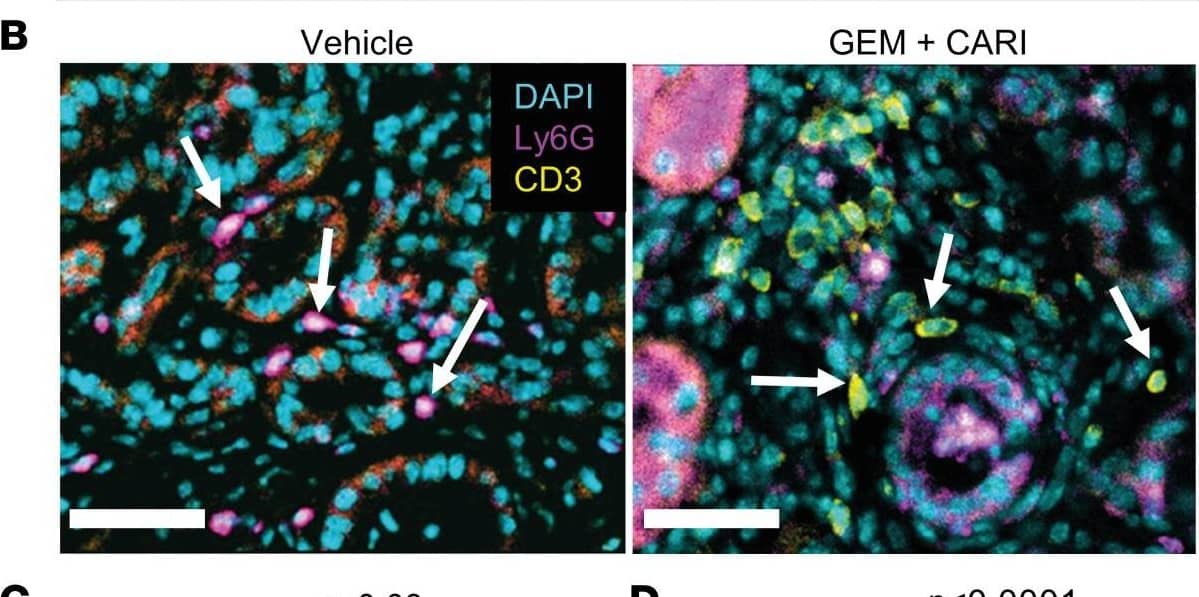 View Larger
View Larger
Detection of Mouse Ly-6G/Ly-6C (Gr-1) by Immunohistochemistry Lymphocyte/neutrophil ratio increases upon NHE1 inhibition in tumor sections of KPfC mice.(A) H&E (left) and PAS-stained KPfC mouse tissue sections after vehicle and gemcitabine + cariporide (GEM+CARI) therapy. Cells of innate immunity, such as neutrophils (arrows), utilize glycogen and are thus PAS+ (purple), in contrast to, for example, lymphocytes. Scale bar: 50 μm. (B) Representative IHC images stained for Ly6G+ neutrophils (magenta, arrows on left image), CD3+ lymphocytes (yellow, arrows on the right image), and nuclei with DAPI (cyan). Scale bar: 50 μm. (C) CD3/Ly6G ratio was assessed by dividing the number of CD3+ cells by the number of Ly6G+ cells in every tumor node. Data points depict the mean CD3/Ly6G ratio derived from each tumor node in individual mice; NVehicle = 10, NGEM = 9, NCARI = 10, NGEM+CARI = 11 mice. (D) To obtain the CD3/Ly6G ratio per tumor node, the number of CD3+ cells was divided by the respective number of Ly6G+ cells in each tumor node. Data points depict individual tumor nodes; nVehicle = 386, nGEM = 276, nCARI = 301, nGEM+CARI = 398. Data and statistical comparison in D and E are represented as median ± 95% CI using Kruskal-Wallis statistical test with Dunn’s post hoc test. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/37643024), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Ly-6G/Ly-6C (Gr-1)
The myeloid differentiation antigen Gr-1 is a member of the Ly-6 family, Ly-6G (1). The RB6‑8C5 antibody also reacts weakly with Ly-6C-transfected EL4 cells (2). In the periphery, this antibody specifically recognized granulocytes (3, 4).
- Spangrude, G.J. et al. (1988) Science 241:58.
- Fleming, T.J. et al. (1993) J. Immunol. 151:2399.
- Lewinsohn, D.M. et al.(1987) J. Immunol. 147:22.
- Lagasse, E. and I.L. Weissman (1996) J. Immunol. Methods 197:139.
Product Datasheets
Citations for Mouse Ly-6G/Ly-6C (Gr-1) Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
63
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Novel peptide-based vaccine targeting heat shock protein 90 induces effective antitumor immunity in a HER2+ breast cancer murine model
Authors: Jinho Kang, Hye-Jin Lee, Jimin Lee, Jinhwa Hong, Yeul Hong Kim, Mary L Disis et al.
Journal for ImmunoTherapy of Cancer
-
miR‐34b‐5p inhibition attenuates lung inflammation and apoptosis in an LPS‐induced acute lung injury mouse model by targeting progranulin
Authors: Wang Xie, Qingchun Lu, Kailing Wang, Jingjing Lu, Xia Gu, Dongyi Zhu et al.
Journal of Cellular Physiology
-
Novel TGF-beta inhibitors ameliorate oral squamous cell carcinoma progression and improve the anti-tumor immune response of anti-PD-L1 immunotherapy
Authors: Nils Ludwig, Łukasz Wieteska, Cynthia S. Hinck, Saigopalakrishna S. Yerneni, Juliana H. Azambuja, Richard J. Bauer et al.
Molecular Cancer Therapeutics
-
Early Subretinal Allograft Rejection is Characterized by Innate Immune Activity
Authors: Kevin P. Kennelly, Toby M. Holmes, Deborah M. Wallace, Cliona O'farrelly, David J. Keegan
Cell Transplantation
-
Regenerative Metaplastic Clones in COPD Lung Drive Inflammation and Fibrosis
Authors: Wei Rao, Shan Wang, Marcin Duleba, Suchan Niroula, Kristina Goller, Jingzhong Xie et al.
Cell
-
Effect of the new silicon-based agent on the symptoms of interstitial pneumonitis
Authors: M Shimada, Y Koyama, Y Kobayashi, H Kobayashi, S Shimada
Scientific Reports, 2023-04-07;13(1):5707.
-
Cavin1; a Regulator of Lung Function and Macrophage Phenotype
Authors: Praveen Govender, Freddy Romero, Dilip Shah, Jesus Paez, Shi-Ying Ding, Libin Liu et al.
PLoS ONE
-
A Retroviral Replicating Vector Encoding Cytosine Deaminase and 5-FC Induces Immune Memory in Metastatic Colorectal Cancer Models
Authors: Kader Yagiz, Maria E. Rodriguez-Aguirre, Fernando Lopez Espinoza, Tiffany T. Montellano, Daniel Mendoza, Leah A. Mitchell et al.
Molecular Therapy - Oncolytics
-
Multiscale topology classifies cells in subcellular spatial transcriptomics
Authors: Benjamin, K;Bhandari, A;Kepple, JD;Qi, R;Shang, Z;Xing, Y;An, Y;Zhang, N;Hou, Y;Crockford, TL;McCallion, O;Issa, F;Hester, J;Tillmann, U;Harrington, HA;Bull, KR;
Nature
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Acid-base homeostasis orchestrated by NHE1 defines pancreatic stellate cell phenotype in pancreatic cancer
Authors: Peth?, Z;Najder, K;Beel, S;Fels, B;Neumann, I;Schimmelpfennig, S;Sargin, S;Wolters, M;Grantins, K;Wardelmann, E;Mitkovski, M;Oeckinghaus, A;Schwab, A;
JCI insight
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
GPR55 contributes to neutrophil recruitment and mechanical pain induction after spinal cord compression in mice
Authors: T Ono, T Yamashita, R Kano, M Inoue, S Okada, K Kano, S Koizumi, K Iwabuchi, Y Hirabayash, I Matsuo, Y Nakashima, H Kamiguchi, Y Kohro, M Tsuda
Brain, Behavior, and Immunity, 2023-03-08;110(0):276-287.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Fructose-1,6-bisphosphatase 1 dephosphorylates IkappaBalpha and suppresses colorectal tumorigenesis
Authors: W Zhu, H Chu, Y Zhang, T Luo, H Yu, H Zhu, Y Liu, H Gao, Y Zhao, Q Li, X Wang, G Li, W Yang
Cell Research, 2023-01-16;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
GPR55 contributes to neutrophil recruitment and mechanical pain induction after spinal cord compression in mice
Authors: T Ono, T Yamashita, R Kano, M Inoue, S Okada, K Kano, S Koizumi, K Iwabuchi, Y Hirabayash, I Matsuo, Y Nakashima, H Kamiguchi, Y Kohro, M Tsuda
Brain, Behavior, and Immunity, 2023;110(0):276-287.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Cellular infiltration, cytokines, and histopathology of skin lesions associated with different clinical forms and stages of naturally occurring lumpy skin disease in cattle
Authors: Y Badr, AE Noreldin, YHA Elewa, MS Ahmed, Y Inoshima, NM Baker, WN Aamer, OM Abas, M Nayel, MM Rahman, E Elgendy, AG Saleh, MS El-Neweshy
Comparative immunology, microbiology and infectious diseases, 2022-10-07;90(0):101894.
Species: Bovine
Sample Types: Whole Tissue
Applications: IHC -
Neuropathic pain caused by miswiring and abnormal end organ targeting
Authors: V Gangadhara, H Zheng, FJ Taberner, J Landry, TA Nees, J Pistolic, N Agarwal, D Männich, V Benes, M Helmstaedt, B Ommer, SG Lechner, T Kuner, R Kuner
Nature, 2022-05-25;606(7912):137-145.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Ephrin receptor A10 monoclonal antibodies and the derived chimeric antigen receptor T cells exert an antitumor response in mouse models of triple-negative breast cancer
Authors: JH Cha, LC Chan, YN Wang, YY Chu, CH Wang, HH Lee, W Xia, WC Shyu, SP Liu, J Yao, CW Chang, FR Cheng, J Liu, SO Lim, JL Hsu, WH Yang, GN Hortobagyi, C Lin, L Yang, D Yu, LB Jeng, MC Hung
The Journal of Biological Chemistry, 2022-03-10;0(0):101817.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
FcgammaRIIB potentiates differentiation of myeloid-derived suppressor cells to mediate tumor immunoescape
Authors: L Wu, Y Xu, H Zhao, Y Zhou, Y Chen, S Yang, J Lei, J Zhang, J Wang, Y Wu, Y Li
Theranostics, 2022-01-01;12(2):842-858.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Expansion of monocytic myeloid-derived suppressor cells ameliorated intestinal inflammatory response by radiation through SOCS3 expression
Authors: YY Choi, KM Seong, HJ Lee, SS Lee, A Kim
Cell Death & Disease, 2021-09-03;12(9):826.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Resveratrol Ameliorates Cardiac Remodeling in a Murine Model of Heart Failure With Preserved Ejection Fraction
Authors: L Zhang, J Chen, L Yan, Q He, H Xie, M Chen
Frontiers in Pharmacology, 2021-06-10;12(0):646240.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Characteristics of immune induction by transcutaneous vaccination using dissolving microneedle patches in mice
Authors: S Hirobe, R Susai, H Takeuchi, R Eguchi, S Ito, YS Quan, F Kamiyama, N Okada
International journal of pharmaceutics, 2021-03-31;601(0):120563.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Reprogramming immunosuppressive myeloid cells facilitates immunotherapy for colorectal cancer
Authors: W Lu, W Yu, J He, W Liu, J Yang, X Lin, Y Zhang, X Wang, W Jiang, J Luo, Q Zhang, H Yang, S Peng, Z Yi, S Ren, J Chen, S Siwko, R Nussinov, F Cheng, H Zhang, M Liu
Embo Molecular Medicine, 2020-12-07;0(0):e12798.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Microglia-organized scar-free spinal cord repair in neonatal mice
Authors: Y Li, X He, R Kawaguchi, Y Zhang, Q Wang, A Monavarfes, Z Yang, B Chen, Z Shi, H Meng, S Zhou, J Zhu, A Jacobi, V Swarup, PG Popovich, DH Geschwind, Z He
Nature, 2020-10-07;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Short-chain fatty acids bind to apoptosis-associated speck-like protein to activate inflammasome complex to prevent Salmonella infection
Authors: H Tsugawa, Y Kabe, A Kanai, Y Sugiura, S Hida, S Taniguchi, T Takahashi, H Matsui, Z Yasukawa, H Itou, K Takubo, H Suzuki, K Honda, H Handa, M Suematsu
PLoS Biol, 2020-09-29;18(9):e3000813.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Mesenchymal Stem Cells Alleviate Moderate-to-Severe Psoriasis by Reducing the Production of Type I Interferon (IFN-I) by Plasmacytoid Dendritic Cells (pDCs)
Authors: M Chen, J Peng, Q Xie, N Xiao, X Su, H Mei, Y Lu, J Zhou, Y Dai, S Wang, C Li, G Lin, L Cheng
Stem Cells Int, 2019-11-07;2019(0):6961052.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
A nano-liposome formulation of the PARP inhibitor Talazoparib enhances treatment efficacy and modulates immune cell populations in mammary tumors of BRCA-deficient mice
Authors: D Zhang, P Baldwin, AS Leal, S Carapelluc, S Sridhar, KT Liby
Theranostics, 2019-08-14;9(21):6224-6238.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Estrogen stimulates female cancer progression by inducing myeloid-derived suppressive cells: investigations on pregnant and non-pregnant experimental models
Authors: K Kozasa, S Mabuchi, Y Matsumoto, H Kuroda, E Yokoi, N Komura, M Kawano, R Takahashi, T Sasano, K Shimura, M Kodama, K Hashimoto, K Sawada, K Nagasaka, T Kimura
Oncotarget, 2019-03-08;10(20):1887-1902.
Species: Xenograft
Sample Types: Whole Tissue
Applications: IHC-P -
PU.1 controls fibroblast polarization and tissue fibrosis
Authors: T Wohlfahrt, S Rauber, S Uebe, M Luber, A Soare, A Ekici, S Weber, AE Matei, CW Chen, C Maier, E Karouzakis, HP Kiener, E Pachera, C Dees, C Beyer, C Daniel, K Gelse, AE Kremer, E Naschberge, M Stürzl, F Butter, M Sticherlin, S Finotto, A Kreuter, MH Kaplan, A Jüngel, S Gay, SL Nutt, DW Boykin, GMK Poon, O Distler, G Schett, JHW Distler, A Ramming
Nature, 2019-01-30;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Regulation of S100A8 Stability by RNF5 in Intestinal Epithelial Cells Determines Intestinal Inflammation and Severity of Colitis
Authors: Y Fujita, A Khateb, Y Li, R Tinoco, T Zhang, H Bar-Yoseph, MA Tam, Y Chowers, E Sabo, S Gerassy-Va, E Starosvets, B James, K Brown, SS Shen-Orr, LM Bradley, PA Tessier, ZA Ronai
Cell Rep, 2018-09-18;24(12):3296-3311.e6.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Group 2 Innate Lymphoid Cells Attenuate Inflammatory Arthritis and Protect from Bone Destruction in Mice
Authors: Y Omata, M Frech, T Primbs, S Lucas, D Andreev, C Scholtysek, K Sarter, M Kindermann, N Yeremenko, DL Baeten, N Andreas, T Kamradt, A Bozec, A Ramming, G Krönke, S Wirtz, G Schett, MM Zaiss
Cell Rep, 2018-07-03;24(1):169-180.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Vesicular nucleotide transporter mediates ATP release and migration in neutrophils
Authors: Y Harada, Y Kato, T Miyaji, H Omote, Y Moriyama, M Hiasa
J. Biol. Chem., 2018-01-23;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
SPLUNC1 knockout enhances LPS-induced lung injury by increasing recruitment of CD11b+Gr-1+ cells to the spleen of mice
Authors: Han Zhang, Xiaoling Li, Shan Liao, Heran Wang, Pan Chen, Guangchao Zhu et al.
Oncology Reports
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade
Authors: M Ouzounova, E Lee, R Piranliogl, A El Andalou, R Kolhe, MF Demirci, D Marasco, I Asm, A Chadli, KA Hassan, M Thangaraju, G Zhou, AS Arbab, JK Cowell, H Korkaya
Nat Commun, 2017-04-06;8(0):14979.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Malondialdehyde epitopes are sterile mediators of hepatic inflammation in hypercholesterolemic mice
Authors: Clara Jana-Lui Busch
Hepatology, 2017-02-03;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
[EXPRESS] Colchicine alleviates acute postoperative pain but delays wound repair in mice: roles of neutrophils and macrophages
Authors: A Sukeishi, K Isami, H Hiyama, S Imai, K Nagayasu, H Shirakawa, T Nakagawa, S Kaneko
Mol Pain, 2017-01-01;13(0):1744806917743.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
PTPN22 Is a Critical Regulator of Fc? Receptor-Mediated Neutrophil Activation
Authors: Sonja Vermeren
J. Immunol, 2016-11-02;197(12):4771-4779.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Diabetic Wounds Exhibit Decreased Ym1 and Arginase Expression with Increased Expression of IL-17 and IL-20
Authors: Laurie P Shornick
Adv Wound Care (New Rochelle), 2016-11-01;5(11):486-494.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Discovery of a Metastatic Immune Escape Mechanism Initiated by the Loss of Expression of the Tumour Biomarker Interleukin-33
Sci Rep, 2016-09-13;6(0):30555.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Exogenous Lipocalin 2 Ameliorates Acute Rejection in a Mouse Model of Renal Transplantation
Authors: F Aigner
Am. J. Transplant., 2015-11-23;16(3):808-20.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
C1q Deficiency Promotes Pulmonary Vascular Inflammation and Enhances the Susceptibility of the Lung Endothelium to Injury.
Authors: Shah D, Romero F, Zhu Y, Duong M, Sun J, Walsh K, Summer R
J Biol Chem, 2015-10-20;290(49):29642-51.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
The Src-Family Kinases Hck and Fgr Regulate Early Lipopolysaccharide-Induced Myeloid Cell Recruitment into the Lung and Their Ability To Secrete Chemokines.
Authors: Mazzi P, Caveggion E, Lapinet-Vera J, Lowell C, Berton G
J Immunol, 2015-07-31;195(5):2383-95.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Rhadinovirus host entry by co-operative infection.
Authors: Lawler C, Milho R, May J, Stevenson P
PLoS Pathog, 2015-03-19;11(3):e1004761.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Syndecan-1 in the mouse parietal peritoneum microcirculation in inflammation.
Authors: Kowalewska P, Patrick A, Fox-Robichaud A
PLoS ONE, 2014-09-03;9(9):e104537.
Species: Mouse
Sample Types: In Vivo
Applications: IHC-Fr -
CCR1 plays a critical role in modulating pain through hematopoietic and non-hematopoietic cells.
Authors: Lewis, Nuruddee, Muthukumarana, Akalushi, Fogal, Steven E, Corradini, Laura, Stefanopoulos, Dimitria, Adusumalli, Prathima, Pelletier, Josephin, Panzenbeck, Mark, Berg, Karen, Canfield, Melissa, Cook, Brian N, Razavi, Hossein, Kuzmich, Daniel, Anderson, Shawn, Allard, Devan, Harrison, Paul, Grimaldi, Christin, Souza, Donald, Harcken, Christia, Fryer, Ryan M, Modis, Louise K, Brown, Maryanne
PLoS ONE, 2014-08-29;9(8):e105883.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Tumor-derived interleukin-1 promotes lymphangiogenesis and lymph node metastasis through M2-type macrophages.
Authors: Watari K, Shibata T, Kawahara A, Sata K, Nabeshima H, Shinoda A, Abe H, Azuma K, Murakami Y, Izumi H, Takahashi T, Kage M, Kuwano M, Ono M
PLoS ONE, 2014-06-12;9(6):e99568.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Transcription factor binding site analysis identifies FOXO transcription factors as regulators of the cutaneous wound healing process.
Authors: Roupe K, Veerla S, Olson J, Stone E, Sorensen O, Hedrick S, Nizet V
PLoS ONE, 2014-02-19;9(2):e89274.
Species: Human, Mouse
Sample Types: Whole Tissue
Applications: IHC -
Decidual neutrophil infiltration is not required for preterm birth in a mouse model of infection-induced preterm labor.
Authors: Rinaldi S, Catalano R, Wade J, Rossi A, Norman J
J Immunol, 2014-02-05;192(5):2315-25.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy.
Authors: Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari B, Jha K, Barhanpurkar A, Wani M, Roy S, Mabalirajan U, Ghosh B, Agrawal A
EMBO J, 2014-01-15;33(9):994-1010.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Pseudomonas aeruginosa MucD protease mediates keratitis by inhibiting neutrophil recruitment and promoting bacterial survival.
Authors: Mochizuki Y, Suzuki T, Oka N, Zhang Y, Hayashi Y, Hayashi N, Gotoh N, Ohashi Y
Invest Ophthalmol Vis Sci, 2014-01-09;55(1):240-6.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Inhibition of PAI-1 induces neutrophil-driven neoangiogenesis and promotes tissue regeneration via production of angiocrine factors in mice.
Blood, 2012-05-09;119(26):6382-93.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
CRTH2 Is A Critical Regulator of Neutrophil Migration and Resistance to Polymicrobial Sepsis.
Authors: Ishii M, Asano K, Namkoong H, Tasaka S, Mizoguchi K, Asami T, Kamata H, Kimizuka Y, Fujiwara H, Funatsu Y, Kagawa S, Miyata J, Ishii K, Nakamura M, Hirai H, Nagata K, Kunkel SL, Hasegawa N, Betsuyaku T
J. Immunol., 2012-04-27;188(11):5655-64.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37.
Authors: Kai-Larsen Y, Luthje P, Chromek M, Peters V, Wang X, Holm A, Kadas L, Hedlund KO, Johansson J, Chapman MR, Jacobson SH, Romling U, Agerberth B, Brauner A
PLoS Pathog., 2010-07-22;6(0):e1001010.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
TLR4 activation is required for IL-17-induced multiple tissue inflammation and wasting in mice.
Authors: Tang H, Pang S, Wang M
J. Immunol., 2010-07-14;185(4):2563-9.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
The leukotriene B4 receptor, BLT1, is required for the induction of experimental autoimmune encephalomyelitis.
Authors: Kihara Y, Yokomizo T, Kunita A, Morishita Y, Fukayama M, Ishii S, Shimizu T
Biochem. Biophys. Res. Commun., 2010-03-11;394(3):673-8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression.
Authors: Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, Ochiai A
Int. J. Cancer, 2009-09-15;125(6):1276-84.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines.
Authors: Hayashida K, Parks WC, Park PW
Blood, 2009-07-28;114(14):3033-43.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
NOD1 expression in the eye and functional contribution to IL-1beta-dependent ocular inflammation in mice.
Authors: Rosenzweig HL, Galster KT, Planck SR, Rosenbaum JT
Invest. Ophthalmol. Vis. Sci., 2008-12-13;50(4):1746-53.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis.
Authors: Kim JV, Kang SS, Dustin ML, McGavern DB
Nature, 2008-11-16;457(7226):191-5.
Species: Mouse
Sample Types: In Vivo
Applications: Immunodepletion -
Immune or Genetic-Mediated Disruption of CASPR2 Causes Pain Hypersensitivity Due to Enhanced Primary Afferent Excitability
Authors: John M. Dawes, Greg A. Weir, Steven J. Middleton, Ryan Patel, Kim I. Chisholm, Philippa Pettingill et al.
Neuron
-
SPLUNC1 knockout enhances LPS-induced lung injury by increasing recruitment of CD11b+Gr-1+ cells to the spleen of mice
Authors: Han Zhang, Xiaoling Li, Shan Liao, Heran Wang, Pan Chen, Guangchao Zhu et al.
Oncology Reports
-
p300/CBP inhibitor A-485 alleviates acute liver injury by regulating macrophage activation and polarization
Authors: Jinjin Peng, Jiacheng Li, Jing Huang, Pan Xu, Heming Huang, Yanjun Liu et al.
Theranostics
-
Subversion of NK-cell and TNF? Immune Surveillance Drives Tumor Recurrence
Authors: T Kottke, L Evgin, KG Shim, D Rommelfang, N Boisgeraul, S Zaidi, RM Diaz, J Thompson, E Ilett, M Coffey, P Selby, H Pandha, K Harrington, A Melcher, R Vile
Cancer Immunol Res, 2017-10-15;5(11):1029-1045.
-
Neutrophilic inflammation during lung development disrupts elastin assembly and predisposes adult mice to COPD
Authors: John T. Benjamin, Erin J. Plosa, Jennifer M.S. Sucre, Riet van der Meer, Shivangi Dave, Sergey Gutor et al.
Journal of Clinical Investigation
-
Myeloid-Derived Suppressor Cells and gamma δT17 Cells Contribute to the Development of Gastric MALT Lymphoma in H. felis-Infected Mice
Authors: Yanan Zhao, Fei Lu, Jingjing Ye, Min Ji, Yihua Pang, Yan Wang et al.
Frontiers in Immunology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse Ly-6G/Ly-6C (Gr-1) Antibody
Average Rating: 4.6 (Based on 8 Reviews)
Have you used Mouse Ly-6G/Ly-6C (Gr-1) Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
A. Normal mouse colon; B. DSS induced mouse colitis colon.
The staining only work at 1:20 dilution instead of the recommended ~1:50 dilution.
We tried 4 difference sources and this is the best one.




