Mouse Nephrin Antibody Summary
Gln37-Thr1049
Accession # NP_062332
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
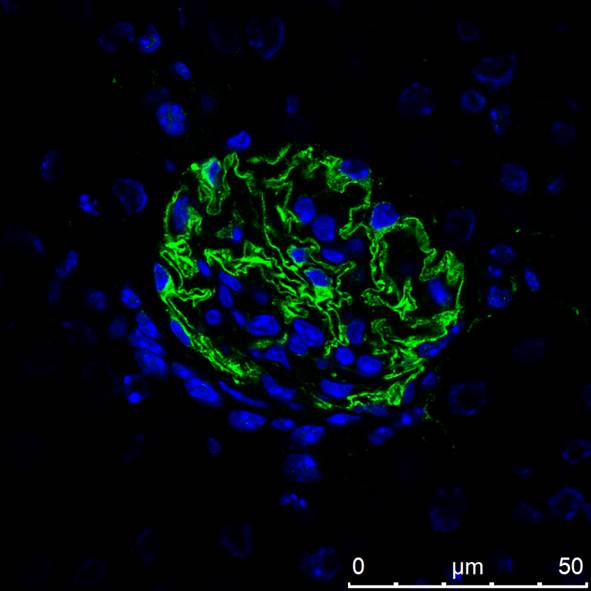
Nephrin in Mouse Kidney. Nephrin was detected in perfusion fixed frozen sections of mouse kidney using 10 µg/mL Goat Anti-Mouse Nephrin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3159) overnight at 4 °C. Tissue was stained with the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counterstained (green). View our protocol for Fluorescent IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
Nephrin in Mouse Kidney. Nephrin was detected in perfusion fixed frozen sections of mouse kidney using Goat Anti-Mouse Nephrin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3159) at 10 µg/mL overnight at 4 °C. Tissue was stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (yellow; Catalog # NL001) and counterstained with DAPI (blue). View our protocol for Fluorescent IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
Detection of Mouse Nephrin by Immunocytochemistry/Immunofluorescence Immunofluorescence analysis of PRCP and nephrin at 60x magnification eleven weeks after 2K1C.White arrows indicate PRCP staining. A) Localization of PRCP and nephrin with overlay in the control kidney. B) Localization of PRCP and nephrin with overlay in the unclipped kidney. C) Localization of PRCP and nephrin with overlay in the clipped kidney. D) Quantitative analysis of cortical, glomerular and tubular PRCP expression in control, unclipped and clipped kidneys. *p < 0.05 vs. Control. **p < 0.01 vs. Unclipped. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25706121), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Nephrin by Immunocytochemistry/Immunofluorescence Intraparenchymal delivery of lentiviral vectors preferentially infects epithelial cells of both kidneys and urinary bladder.(a) Confocal images of Strawberry expression in renal sections of ELS infected kidneys 60 days post injection. Sections were stained with the indicated antibodies and merged images with DAPI (blue) are presented. Preferential transduction was observed in proximal (AQP1) and distal (THP) renal tubular epithelial cells and renal fibroblasts (CD73) (white arrowheads). Strawberry was not localised to collecting ducts (AQP2) or podocytes (Nephrin) (white arrows). Scale bars, 50 μm. (b) Confocal images of Strawberry expression in the liver, spleen, lung, urinary bladder and left and right kidney of ELS infected animals 60 days post left intrarenal injection (top panels) and uninjected control animals (bottom panels). Sections were stained with an antibody against Strawberry (green) and merged images with DAPI (blue) are presented. Scale bars, 50 μm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26046460), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Nephrin by Immunocytochemistry/Immunofluorescence Podocyte‐specific knockout of KDM6A in mice protects against diabetes‐induced kidney injuryAQuantitative RT–PCR evaluation of KDM6A mRNA expression in kidney glomeruli isolated from wild‐type and KDM6A‐knockout (KO) mice. *P < 0.05, significant difference versus wild‐type controls (Wilcoxon two‐sample test; n = 8).BWestern blot analysis of KDM6A expression in glomeruli and podocytes isolated from wild‐type and KDM6A‐KO mice. Presented experiments were performed at least three times independently.CChanges in body weights and levels of blood glucose in wild‐type or KDM6A‐KO mice with or without STZ treatment. No significant differences in body weights or blood glucose levels between wild‐type and KDM6A‐KO mice with diabetes were observed during the 8‐week experimental period (Wilcoxon two‐sample test; n = 8).DLevels of urinary protein excretion, weights of kidney, and levels of HbA1c in wild‐type and KDM6A‐KO mice with or without STZ treatment (at 8 weeks after the onset of diabetes). The mean relative kidney weight (%) shown in the study is determined as the percent of kidneys out of total body weight, and the HbA1c level is defined as the ratio of HbA1c to the total hemoglobin (% HbA1c; DCCT unit). *P < 0.05 versus untreated wild‐type controls, #P < 0.05 versus STZ‐treated wild‐type mice (parametric ANOVA and a Bonferroni post hoc test; n = 8).EImmunofluorescence images of kidney sections stained with KDM6A and nephrin in wild‐type (WT‐NC), KDM6A‐KO (KO‐NC), STZ‐treated wild‐type (WT‐DM), or STZ‐treated KDM6A‐KO (KO‐DM) mice. Scale bars, 20 μm. *P < 0.05 versus untreated wild‐type controls, #P < 0.05 versus STZ‐treated wild‐type mice (parametric ANOVA and a Bonferroni post hoc test; n = 3).FImmunofluorescence images of kidney sections stained with KDM6A and H3K27me3 in wild‐type (WT‐NC), KDM6A‐KO (KO‐NC), STZ‐treated wild‐type (WT‐DM), or STZ‐treated KDM6A‐KO (KO‐DM) mice. Scale bars, 20 μm. *P < 0.05 versus untreated wild‐type controls, #P < 0.05 versus STZ‐treated wild‐type mice (parametric ANOVA and a Bonferroni post hoc test; n = 3).GImmunofluorescence staining of F‐actin and KDM6A in primary podocytes isolated from the above‐treated mice. Presented experiments were performed at least three times independently. Scale bars, 20 μm.HWestern blot analysis of nephrin, KDM6A, and WT‐1 expressed in primary podocytes isolated from the above‐treated mice. *P < 0.05 versus untreated wild‐type controls, #P < 0.05 versus STZ‐treated wild‐type mice (parametric ANOVA and a Bonferroni post hoc test; n = 3).IEffect of high glucose on the expression of podocyte‐related markers in primary cultured podocytes isolated from wild‐type or KDM6A‐KO mice. The primary podocytes isolated from wild‐type or KDM6A‐KO mice were cultured in normal or high glucose (30 mM) for 48 h. Protein lysates from the cultured podocytes were subjected to Western blot analysis with the indicated antibodies. *P < 0.05 versus wild‐type podocytes in normal glucose, #P < 0.05 versus wild‐type podocytes in high glucose (parametric ANOVA and a Bonferroni post hoc test; n = 3).Data information: Data are expressed as mean ± SEM. See the exact P‐values for comparison tests in Appendix Table S4. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30948420), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Nephrin by Immunocytochemistry/Immunofluorescence KLF10 is a downstream effector of KDM6A and is capable of directly binding to nephrin gene promoterAScreening of the potential KDM6A‐regulated transcriptional factors involved in repression of nephrin expression. After transduction with a control lentiviral vector or a lentiviral vector expressing KDM6A into primary podocytes for 48 h, intracellular RNAs were isolated and used for RNA sequencing (RNA‐Seq) analysis. The transcript expression patterns of 86 selected transcriptional factors from three independent RNA‐Seq experiments are presented in a heat map. Notably, among these 86 selected genes, KLF10 is the most up‐regulated gene.BValidation of increased KDM6A and KLF10 expression in primary podocytes that were infected with an empty lentiviral vector or KDM6A‐expressing lentiviral vector for 48 h. *P < 0.05 versus the empty vector control (Wilcoxon two‐sample test; n = 3).CIncreased expression of KDM6A and KLF10 in primary podocytes cultured in high glucose for 48 h. *P < 0.05 versus normal controls (Wilcoxon two‐sample test; n = 3).DEffect of KLF10 knockdown on high glucose‐mediated reduction of nephrin in primary podocytes. As noted, knockdown of KLF10 prevented down‐regulation of nephrin and up‐regulation of KDM6A in high glucose‐treated podocytes. *P < 0.05 versus normal controls, #P < 0.05 versus control siRNA with HG incubation (parametric ANOVA and a Bonferroni post hoc test; n = 3).EImmunofluorescence analysis of KLF10 and nephrin in renal sections of normal, diabetic, and GSK‐J4‐treated diabetic mice. Scale bars, 20 μm. *P < 0.05 versus the normal control group, #P < 0.05 versus the untreated diabetic group (parametric ANOVA and a Bonferroni post hoc test; n = 3).FImmunofluorescence images of KLF10 and nephrin in renal sections of wild‐type or KDM6A‐KO mice with or without STZ treatment. Scale bars, 20 μm. *P < 0.05 versus the untreated wild‐type group, #P < 0.05 versus the STZ‐treated wild‐type group (parametric ANOVA and a Bonferroni post hoc test; n = 3).GWestern blot analysis of KDM6A, KFL10, and nephrin expression in primary podocytes isolated from normal, diabetic, and GSK‐J4‐treated diabetic mice. *P < 0.05 versus normal controls, #P < 0.05 versus the untreated diabetic group (parametric ANOVA and a Bonferroni post hoc test; n = 3).HWestern blot analysis of KDM6A, KLF10, and nephrin expression in primary podocytes isolated from wild‐type or KDM6A‐KO mice with or without STZ treatment. *P < 0.05 versus the untreated wild‐type group, #P < 0.05 versus the STZ‐treated wild‐type group (parametric ANOVA and a Bonferroni post hoc test; n = 3).IChIP analysis of KLF10, acetyl‐histone H4 (H4‐Ac), Dnmt1 and Dnmt3 binding to nephrin gene promoter. ChIP assays were carried out using cross‐linked chromatin from primary podocytes that were cultured in normal or high glucose conditions. *P < 0.05, significant difference versus the normal control group (Wilcoxon two‐sample test; n = 3).JModulation of podocyte‐specific marker expression in primary podocytes by KLF10 overexpression. Ectopic overexpression of KLF10 in primary podocytes significantly repressed various podocyte‐specific markers, but conversely increased KDM6A expression. *P < 0.05 versus the empty vector control (Wilcoxon two‐sample test; n = 3).Data information: Data are expressed as mean ± SEM. See the exact P‐values for comparison tests in Appendix Table S5. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30948420), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Nephrin by Immunocytochemistry/Immunofluorescence Elevated levels of KDM6A and KLF10 expression, along with decreased levels of nephrin (or WT‐1) expression, are detected in kidney tissues and urinary exosomes of patients with diabetic nephropathyAImmunofluorescence analysis of kidney sections from diabetic nephropathy subjects and non‐diabetic controls stained with KDM6A, KLF10, nephrin, and WT‐1. Scale bars, 50 μm. *P < 0.05 by Wilcoxon two‐sample test (n = 6 for each group).BWestern blot analysis of kidney tissues from non‐diabetic controls and diabetic nephropathy subjects. Expression levels of KDM6A, KLF10, nephrin, and WT‐1 in kidney tissues were determined by immunoblotting using the indicated antibodies. Relative protein levels in kidney tissues were normalized to actin. *P < 0.05 by Wilcoxon two‐sample test (n = 6 for each group).CLevels of KDM6A and KLF10, nephrin mRNAs in urine exosomes of diabetic nephropathy patients and non‐diabetic controls. Relative mRNA levels in human urinary exosomes were normalized to 18S rRNA. Horizontal lines are medians. *P < 0.05, **P < 0.01, and ***P < 0.001 by Wilcoxon two‐sample test (n = 12 for each group).Data information: Data are expressed as mean ± SEM (A and B). See the exact P‐values for comparison tests in Appendix Table S7. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30948420), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Nephrin by Immunocytochemistry/Immunofluorescence KDM6A promotes podocyte and kidney dysfunction in diabetic miceARelative mRNA levels of KDM6A, nephrin and WT‐1, normalized to beta ‐actin, expressed in kidney glomeruli of normal and diabetic mice at 4, 8, and 12 weeks after diabetic induction. *Significant differences (P < 0.05) compared with normal controls (Wilcoxon two‐sample test; n = 8 each).BWestern blot analysis of nephrin, KDM6A, and H3K27me3 in kidney glomeruli of normal and diabetic mice at different time points. *P < 0.05 versus normal controls for the indicated time points (Wilcoxon two‐sample test; n = 3).CChanges in body weights and levels of blood glucose in normal, diabetic, and GSK‐J4‐treated diabetic mice. As noted, GSK‐J4 treatment did not significantly affect body weights or blood glucose levels in diabetic mice during the experimental period of 12 weeks (Wilcoxon two‐sample test; n = 8).DLevels of urinary protein excretion, weights of kidney, and levels of HbA1c in normal, diabetic, and GSK‐J4‐treated diabetic mice. Urinary total protein excretion, kidney weight, and levels of HbA1c were measured at 12 weeks after diabetic induction. The mean relative kidney weight (%) shown in the study is determined as the percent of kidneys out of total body weight, and the HbA1c level is defined as the ratio of HbA1c to the total hemoglobin (% HbA1c; DCCT unit). *P < 0.05 versus normal controls, #P < 0.05 versus untreated diabetic mice (parametric ANOVA and a Bonferroni post hoc test; n = 8).EWestern blot analysis of KDM6A, nephrin, WT‐1, and H3K27me3 expressed in kidney glomeruli of normal, diabetic, and GSK‐J4‐treated diabetic mice at 12 weeks after diabetic induction. *P < 0.05 versus normal controls, #P < 0.05 versus untreated diabetic mice (parametric ANOVA and a Bonferroni post hoc test; n = 3).FImmunofluorescence analysis of KDM6A, nephrin, and WT‐1 in kidney sections from normal, diabetic, and GSK‐J4‐treated diabetic mice. Green: nephrin or WT‐1; red: KDM6A; blue: DAPI. Scale bars, 20 μm. *P < 0.05 versus normal controls, #P < 0.05 versus untreated diabetic mice (parametric ANOVA and a Bonferroni post hoc test; n = 3).GImmunofluorescence staining of F‐actin and KDM6A in primary podocytes isolated from normal, diabetic, and GST‐J4‐treated diabetic mice. Green: F‐actin; red: KDM6A; blue: DAPI. Scale bars, 20 μm. Presented experiments were performed at least three times independently.Data information: Data are expressed as mean ± SEM. See the exact P‐values for comparison tests in Appendix Table S3. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30948420), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Nephrin
Nephrin is a 185 kDa type I transmembrane glycoprotein that belongs to the immunoglobulin superfamily (1). Mature mouse Nephrin consists of a 1042 amino acid (aa) extracellular domain (ECD) with eight Ig-like C2-set domains and one fibronectin type III domain, a 22 aa transmembrane segment, and a 156 aa cytoplasmic tail (2, 3). Within the ECD, mouse Nephrin shares 84% and 95% aa sequence identity with human and rat Nephrin, respectively. Usage of the alternate exon 1B results in a distinct N-terminal sequence that lacks a clearly defined signal peptide cleavage site (4). Nephrin is expressed primarily on podocytes in the renal glomerulus and to a lesser extent in the brain and pancreas (3, 5). The 1B isoform is not expressed in the kidney (4). Nephrin localizes to intercellular junctions between podocyte foot processes where it functions as a homophilic adhesion molecule (2, 6). Nephrin is required for formation and maintenance of the slit diaphragm between these processes (7). It associates with Neph1, podicin, P-cadherin, and multiple scaffolding proteins which couple it to the actin cytoskeleton (8-12). Nephrin expression is required for the anti-apoptotic effect of VEGF on podocytes as well as for the ability of podocytes to upregulate Glut1 and Glut4 glucose transporters in response to insulin (13, 14). Nephrin downregulation contributes to diabetic nephropathy, and Nephrin mutations underlie the lethal congenital nephritic syndrome NPHS1 (5, 15).
- Kawachi, H. et al. (2006) Nephrology 11:274.
- Holzman, L.B. et al. (1999) Kidney Int. 56:1481.
- Putaala, H. et al. (2000) J. Am. Soc. Nephrol. 11:991.
- Beltcheva, O. et al. (2003) J. Am. Soc. Nephrol. 14:352.
- Putaala, H. et al. (2001) Hum. Mol. Genet. 10:1.
- Khoshnoodi, J. et al. (2003) Am. J. Pathol. 163:2337.
- Ruotsalainen, V. et al. (2000) Am. J. Pathol. 157:1905.
- Barletta, G.M. et al. (2003) J. Biol. Chem. 278:19266.
- Huber, T.B. et al. (2001) J. Biol. Chem. 276:41543.
- Lehtonen, S. et al. (2004) Am. J. Pathol. 165:923.
- Lehtonen, S. et al. (2005) Proc. Natl. Acad. Sci. USA 102:9814.
- Verma, R. et al. (2006) J. Clin. Invest. 116:1346.
- Foster, R.R. et al. (2005) Am. J. Physiol. Renal Physiol. 288:F48.
- Coward, R.J. et al. (2007) Diabetes 56:1127.
- Cooper, M.E. et al. (2002) Semin. Nephrol. 22:393.
Product Datasheets
Citations for Mouse Nephrin Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
58
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Injury-induced actin cytoskeleton reorganization in podocytes revealed by super-resolution microscopy.
Authors: Suleiman HY, Roth R, Jain S et al.
JCI Insight
-
Lysophosphatidic Acid Receptor Antagonism Protects against Diabetic Nephropathy in a Type 2 Diabetic Model
Authors: Zhang MZ, Wang X, Yang H et al.
J Am Soc Nephrol.
-
MiR-320a induces diabetic nephropathy via inhibiting MafB
Authors: He M, Wang J, Yin Z et al.
Aging (Albany NY)
-
Intraglomerular Monocyte/Macrophage Infiltration and Macrophage-Myofibroblast Transition during Diabetic Nephropathy Is Regulated by the A2B Adenosine Receptor
Authors: Á Torres, K Muñoz, Y Nahuelpán, AP R Saez, P Mendoza, C Jara, C Cappelli, R Suarez, C Oyarzún, C Quezada, R San Martín
Cells, 2020-04-23;9(4):.
-
Mannan-Binding Lectin Is Associated with Inflammation and Kidney Damage in a Mouse Model of Type 2 Diabetes
Authors: Dørflinger, GH;Holt, CB;Thiel, S;Bech, JN;Østergaard, JA;Bjerre, M;
International journal of molecular sciences
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Genetic or pharmacologic blockade of mPGES-2 attenuates renal lipotoxicity and diabetic kidney disease by targeting Rev-Erb?/FABP5 signaling
Authors: Zhong, D;Chen, J;Qiao, R;Song, C;Hao, C;Zou, Y;Bai, M;Su, W;Yang, B;Sun, D;Jia, Z;Sun, Y;
Cell reports
Species: Mouse, Transgenic Mouse
Sample Types: Whole Cells
Applications: Immunohistochemistry -
Protective Role of the Podocyte IL-15 / STAT5 Pathway in Focal Segmental Glomerulosclerosis
Authors: Niasse, A;Louis, K;Lenoir, O;Schwarz, C;Xu, X;Couturier, A;Dobosziewicz, H;Corchia, A;Placier, S;Vandermeersch, S;Hennighausen, L;Frère, P;Galichon, P;Surin, B;Ouchelouche, S;Louedec, L;Migeon, T;Verpont, MC;Yousfi, N;Buob, D;Xu-Dubois, YC;François, H;Rondeau, E;Mesnard, L;Hadchouel, J;Luque, Y;
Kidney international reports
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
The crosstalk between glomerular endothelial cells and podocytes controls their responses to metabolic stimuli in diabetic nephropathy
Authors: Albrecht, M;Sticht, C;Wagner, T;Hettler, SA;De La Torre, C;Qiu, J;Gretz, N;Albrecht, T;Yard, B;Sleeman, JP;Garvalov, BK;
Scientific reports
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Periglomerular afferent innervation of the mouse renal cortex
Authors: Roman Tyshynsky, Sulagna Sensarma, Maureen Riedl, John Bukowy, Lawrence P. Schramm, Lucy Vulchanova et al.
Frontiers in Neuroscience
-
ZEB2 controls kidney stromal progenitor differentiation and inhibits abnormal myofibroblast expansion and kidney fibrosis
Authors: S Kumar, X Fan, H Milo Rasou, R Sharma, DJ Salant, W Lu
JCI Insight, 2023-01-10;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Microtubule associated protein 4 phosphorylation-induced epithelial-to-mesenchymal transition of podocyte leads to proteinuria in diabetic nephropathy
Authors: Lingfei Li, Yanhai Feng, Junhui Zhang, Qiong Zhang, Jun Ren, Cheng Sun et al.
Cell Communication and Signaling
-
A slit-diaphragm-associated protein network for dynamic control of renal filtration
Authors: MK Kocylowski, H Aypek, W Bildl, M Helmstädte, P Trachte, B Dumoulin, S Wittösch, L Kühne, U Aukschun, C Teetzen, O Kretz, B Gaal, A Kulik, C Antignac, G Mollet, A Köttgen, B Göcmen, J Schwenk, U Schulte, TB Huber, B Fakler, F Grahammer
Nature Communications, 2022-10-28;13(1):6446.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
An ex vivo culture model of kidney podocyte injury reveals mechanosensitive, synaptopodin-templating, sarcomere-like structures.
Authors: Shumeng J, Farid A, Yin-Yuan H et al.
Sci Adv.
-
Neutrophil Extracellular Traps Promote NLRP3 Inflammasome Activation and Glomerular Endothelial Dysfunction in Diabetic Kidney Disease
Authors: A Gupta, K Singh, S Fatima, S Ambreen, S Zimmermann, R Younis, S Krishnan, R Rana, I Gadi, C Schwab, R Biemann, K Shahzad, V Rani, S Ali, PR Mertens, S Kohli, B Isermann
Nutrients, 2022-07-20;14(14):.
Species: Mouse
Sample Types: Cell Lysates, Whole Tissue
Applications: IHC, Western Blot -
Role of Periostin and Nuclear Factor-kappaB Interplay in the Development of Diabetic Nephropathy
Authors: L Abbad, N Prakoura, A Michon, R Chalghoumi, S Reichelt-W, MC Banas, C Chatzianto
Cells, 2022-07-15;11(14):.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Metformin improves renal injury of MRL/lpr lupus-prone mice via the AMPK/STAT3 pathway
Authors: Chen XC, Wu D, Wu HL et al.
Lupus Science & Medicine
-
Insulin-activated store-operated Ca2+ entry via Orai1 induces podocyte actin remodeling and causes proteinuria
Authors: JH Kim, KH Hwang, BTN Dang, M Eom, ID Kong, Y Gwack, S Yu, HY Gee, L Birnbaumer, KS Park, SK Cha
Nature Communications, 2021-11-11;12(1):6537.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC -
Blockade of the natriuretic peptide clearance receptor attenuates proteinuria in a mouse model of focal segmental glomerulosclerosis
Authors: L Wang, Y Tang, AF Buckley, RF Spurney
Physiological Reports, 2021-11-01;9(21):e15095.
Species: Mouse, Transgenic Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Early type 1 diabetes aggravates renal ischemia/reperfusion-induced acute kidney injury
Authors: MC de Ponte, VG Cardoso, GL Gonçalves, JM Costa-Pess, M Oliveira-S
Scientific Reports, 2021-09-24;11(1):19028.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The important roles and molecular mechanisms of annexin A2 autoantibody in children with nephrotic syndrome
Authors: Qing Ye, Yingying Zhang, Jieqiu Zhuang, Ye Bi, Hong Xu, Qian Shen et al.
Annals of Translational Medicine
-
Superresolving the kidney—a practical comparison of fluorescence nanoscopy of the glomerular filtration barrier
Authors: Lucia C. S. Wunderlich, Florian Ströhl, Stefan Ströhl, Oliver Vanderpoorten, Luca Mascheroni, Clemens F. Kaminski
Analytical and Bioanalytical Chemistry
-
Plasminogen deficiency does not prevent sodium retention in a genetic mouse model of experimental nephrotic syndrome
Authors: Mengyun Xiao, Bernhard N. Bohnert, Hande Aypek, Oliver Kretz, Florian Grahammer, Ute Aukschun et al.
Acta Physiol (Oxf)
-
Proteolytic Cleavage of Podocin by Matriptase Exacerbates Podocyte Injury
Authors: S Ozawa, M Matsubayas, H Nanaura, M Yanagita, K Mori, K Asanuma, N Kajiwara, K Hayashi, H Ohashi, M Kasahara, H Yokoi, H Kataoka, E Mori, T Nakagawa
J. Biol. Chem., 2020-09-09;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Berberine protects against diabetic kidney disease via promoting PGC-1?-regulated mitochondrial energy homeostasis
Authors: Qin X, Jiang M, Zhao Y et al.
British Journal of Pharmacology
-
Co-immunostaining of ICAM-1, ICAM-2, and CD31 in Mouse Kidney Glomeruli
Authors: Sun-Sang J Sung
BIO-PROTOCOL
-
EDA2R mediates podocyte injury in high glucose milieu
Authors: Xiqian Lan, Vinod Kumar, Alok Jha, Rukhsana Aslam, Haichao Wang, Kehong Chen et al.
Biochimie
-
A highly potent lymphatic system-targeting nanoparticle cyclosporine prevents glomerulonephritis in mouse model of lupus
Authors: R Ganugula, M Arora, D Zou, SK Agarwal, C Mohan, MNVR Kumar
Sci Adv, 2020-06-12;6(24):eabb3900.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A highly potent lymphatic system-targeting nanoparticle cyclosporine prevents glomerulonephritis in mouse model of lupus
Authors: R Ganugula, M Arora, D Zou, SK Agarwal, C Mohan, MNVR Kumar
Sci Adv, 2020;6(24):eabb3900.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Effective reconstruction of functional organotypic kidney spheroid for in vitro nephrotoxicity studies
Authors: HM Kang, JH Lim, KH Noh, D Park, HS Cho, K Susztak, CR Jung
Sci Rep, 2019-11-26;9(1):17610.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
The HIV protease inhibitor darunavir prevents kidney injury via HIV-independent mechanisms
Authors: X Gao, A Rosales, H Karttunen, GM Bommana, B Tandoh, Z Yi, Z Habib, V Agati, W Zhang, MJ Ross
Sci Rep, 2019-11-01;9(1):15857.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Dysregulated expression but redundant function of the long non-coding RNA HOTAIR in diabetic kidney disease
Authors: Syamantak Majumder, Mitchell J. Hadden, Karina Thieme, Sri N. Batchu, Divya Niveditha, Shibasish Chowdhury et al.
Diabetologia
-
Compression of morbidity in a progeroid mouse model through the attenuation of myostatin/activin signalling
Authors: Khalid Alyodawi, Wilbert P. Vermeij, Saleh Omairi, Oliver Kretz, Mark Hopkinson, Francesca Solagna et al.
Journal of Cachexia, Sarcopenia and Muscle
-
A KDM6A-KLF10 reinforcing feedback mechanism aggravates diabetic podocyte dysfunction
Authors: CL Lin, YC Hsu, YT Huang, YH Shih, CJ Wang, WC Chiang, PJ Chang
EMBO Mol Med, 2019-05-01;11(5):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Urine podoplanin heralds the onset of ischemia-reperfusion injury of the kidney
Authors: Vivek Kasinath, Osman Arif Yilmam, Mayuko Uehara, Merve Yonar, Liwei Jiang, Xiaofei Li et al.
American Journal of Physiology-Renal Physiology
-
Targeting VE-PTP phosphatase protects the kidney from diabetic injury
Authors: Isabel A. Carota, Yael Kenig-Kozlovsky, Tuncer Onay, Rizaldy Scott, Benjamin R. Thomson, Tomokazu Souma et al.
Journal of Experimental Medicine
-
Change in Renal Glomerular Collagens and Glomerular Filtration Barrier-Related Proteins in a Dextran Sulfate Sodium-Induced Colitis Mouse Model
Authors: CJ Chang, PC Wang, TC Huang, A Taniguchi
Int J Mol Sci, 2019-03-22;20(6):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Inhibiting 4E-BP1 re-activation represses podocyte cell cycle re-entry and apoptosis induced by adriamycin
Authors: F Li, X Mao, Q Zhuang, Z Zhao, Z Zhang, H Wu
Cell Death Dis, 2019-03-11;10(3):241.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-P -
Generation of pluripotent stem cell-derived mouse kidneys in Sall1-targeted anephric rats
Authors: T Goto, H Hara, M Sanbo, H Masaki, H Sato, T Yamaguchi, S Hochi, T Kobayashi, H Nakauchi, M Hirabayash
Nat Commun, 2019-02-05;10(1):451.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Accelerated Glomerular Cell Senescence in Experimental Lupus Nephritis
Authors: C Yang, J Xue, N An, XJ Huang, ZH Wu, L Ye, ZH Li, SJ Wang, QJ Pan, D Liang, HF Liu
Med. Sci. Monit., 2018-09-28;24(0):6882-6891.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Activated renal tubular Wnt/β-catenin signaling�triggers renal inflammation during�overload proteinuria
Authors: DWL Wong, WH Yiu, KW Chan, Y Li, B Li, SWY Lok, MM Taketo, P Igarashi, LYY Chan, JCK Leung, KN Lai, SCW Tang
Kidney Int., 2018-03-28;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Engineered Sialylation of Pathogenic Antibodies In Vivo Attenuates Autoimmune Disease
Authors: Jose D. Pagan, Maya Kitaoka, Robert M. Anthony
Cell
-
Deficient Insulin-mediated Upregulation of the Equilibrative Nucleoside Transporter 2 Contributes to Chronically Increased Adenosine in Diabetic Glomerulopathy
Authors: S Alarcón, W Garrido, C Cappelli, R Suárez, C Oyarzún, C Quezada, R San Martín
Sci Rep, 2017-08-25;7(1):9439.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Natriuretic peptide receptor guanylyl cyclase-A pathway counteracts glomerular injury evoked by aldosterone through p38 mitogen-activated protein kinase inhibition
Authors: Y Kato, K Mori, M Kasahara, K Osaki, A Ishii, KP Mori, N Toda, S Ohno, T Kuwabara, T Tokudome, I Kishimoto, MA Saleem, T Matsusaka, K Nakao, M Mukoyama, M Yanagita, H Yokoi
Sci Rep, 2017-04-21;7(0):46624.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Dependence of Glomerulonephritis Induction on Novel Intraglomerular Alternatively Activated Bone Marrow-Derived Macrophages and Mac-1 and PD-L1 in Lupus-Prone NZM2328 Mice
Authors: SJ Sung, Y Ge, C Dai, H Wang, SM Fu, R Sharma, YS Hahn, J Yu, TH Le, MD Okusa, WK Bolton, JR Lawler
J. Immunol, 2017-02-20;0(0):.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Flow Cytometry, IHC -
Endoplasmic reticulum stress inhibition attenuates hypertensive chronic kidney disease through reduction in proteinuria
Authors: Z Mohammed-A, C Lu, MK Marway, RE Carlisle, K Ask, D Lukic, JC Krepinsky, JG Dickhout
Sci Rep, 2017-02-02;7(0):41572.
Species: Human, Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Alteration of histone H3K4 methylation in glomerular podocytes associated with proteinuria in patients with membranous nephropathy
BMC Nephrol, 2016-11-17;17(1):179.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Smad3 deficiency protects mice from obesity-induced podocyte injury that precedes insulin resistance.
Authors: Sun Y, Qu X, Howard V, Dai L, Jiang X, Ren Y, Fu P, Puelles V, Nikolic-Paterson D, Caruana G, Bertram J, Sleeman M, Li J
Kidney Int, 2015-05-06;88(2):286-98.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
TGF-beta-activated kinase 1 is crucial in podocyte differentiation and glomerular capillary formation.
Authors: Kim S, Lee S, Wang Z, Ding Y, Haque N, Zhang J, Zhou J, Choi M
J Am Soc Nephrol, 2014-03-20;25(9):1966-78.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Rac1 Activation in Podocytes Induces Rapid Foot Process Effacement and Proteinuria
Authors: Haiyang Yu, Hani Suleiman, Alfred H. J. Kim, Jeffrey H. Miner, Adish Dani, Andrey S. Shaw et al.
Molecular and Cellular Biology
-
Morphine induces albuminuria by compromising podocyte integrity.
Authors: Lan, Xiqian, Rai, Partab, Chandel, Nirupama, Cheng, Kang, Lederman, Rivka, Saleem, Moin A, Mathieson, Peter W, Husain, Mohammad, Crosson, John T, Gupta, Kalpna, Malhotra, Ashwani, Singhal, Pravin C
PLoS ONE, 2013-03-29;8(3):e55748.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Glomerular endothelial surface layer acts as a barrier against albumin filtration.
Authors: Dane M, van den Berg B, Avramut M, Faas F, van der Vlag J, Rops A, Ravelli R, Koster B, van Zonneveld A, Vink H, Rabelink T
Am J Pathol, 2013-03-19;182(5):1532-40.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Aldosterone deficiency and mineralocorticoid receptor antagonism prevent angiotensin II–induced cardiac, renal, and vascular injury
Authors: James M. Luther, Pengcheng Luo, Zuofei Wang, Samuel E. Cohen, Hyung-Suk Kim, Agnes B. Fogo et al.
Kidney International
-
Divergent roles of Smad3 and PI3-kinase in murine adriamycin nephropathy indicate distinct mechanisms of proteinuria and fibrogenesis.
Kidney Int., 2012-04-25;82(5):525-36.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Activation of NFAT Signaling in Podocytes Causes Glomerulosclerosis
Authors: Yinqiu Wang, George Jarad, Piyush Tripathi, Minggui Pan, Jeanette Cunningham, Daniel R. Martin et al.
Journal of the American Society of Nephrology
-
Proteomic analysis of the slit diaphragm complex: CLIC5 is a protein critical for podocyte morphology and function.
Authors: Pierchala BA, Munoz MR, Tsui CC
Kidney Int., 2010-07-21;78(9):868-82.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Podocytes derived from human induced pluripotent stem cells: characterization, comparison, and modeling of diabetic kidney disease
Authors: Julie Bejoy, Justin M. Farry, Jennifer L. Peek, Mariana C. Cabatu, Felisha M. Williams, Richard C. Welch et al.
Stem Cell Research & Therapy
-
Histone H3 Serine 10 Phosphorylation Facilitates Endothelial Activation in Diabetic Kidney Disease
Authors: Alghamdi TA, Batchu SN, Hadden MJ, et al.
Diabetes
-
TAp73 opposes tumor angiogenesis by promoting hypoxia-inducible factor 1alpha degradation.
Authors: Amelio I, Inoue S, Markert EK et al.
Proc. Natl. Acad. Sci. U.S.A.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse Nephrin Antibody
Average Rating: 5 (Based on 2 Reviews)
Have you used Mouse Nephrin Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: