Mouse Podocalyxin Antibody Summary
Ser21-Arg402
Accession # Q9R0M4
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Mouse Podocalyxin by Western Blot. Western blot shows lysates of mouse kidney tissue. PVDF membrane was probed with 1 µg/mL of Goat Anti-Mouse Podocalyxin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1556) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF109). A specific band was detected for Podocalyxin at approximately 130 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Podocalyxin in Mouse Thymus. Podocalyxin was detected in perfusion fixed frozen sections of mouse thymus using Goat Anti-Mouse Podocalyxin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1556) at 15 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; CTS008) and counterstained with hematoxylin (blue). Lower panel shows a lack of labeling when primary antibodies are omitted and tissue is stained only with secondary antibody followed by incubation with detection reagents. Specific staining was localized to high endothelial venules. View our protocol for Chromogenic IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence Defects in TRAF3IP1 mutants are mediated by MAP4.(a) Lateral views of WT zebrafish embryos injected with map4 morpholino at 48 hpf and phenotype distribution in WT embryos injected with control or map4 morpholino. (b) Lateral views of elipsa zebrafish embryos injected with map4 morpholino at 48 hpf and phenotype distribution in elipsa mutant embryos injected with control or map4 morpholino (data shown as combined result of n=3 independent experiments). Scale bars, 1 mm. (c) Relative expression of Map4 normalized to that of Hprt was analysed by qPCR in control and Traf3ip1-KD mIMCD3 cells stably expressing GFP or GFP-IFT54 mutants and Map4 shRNA. (d) Control and Traf3ip1-KD/ Map4-KD mIMCD3 cells expressing either GFP or IFT54-GFP fusions were fixed in MeOH and stained for acetylated alpha -tubulin (red) and gamma -tubulin (light blue). Scale bar, 10 μm. (e) Six hours after Ca2+ switch, mIMCD3 cells grown until confluence on filters were fixed with 4% PFA and stained for the apical marker Gp135 (red). Scale bar, 10 μm. (f) Percentage of normal spheroids of control and Traf3ip1-KD/ Map4-KD mIMCD3 cells expressing either GFP or IFT54-GFP fusions grown on Matrigel for 5 days (mean ± s.d., n≥100 spheroids from 3 independent experiments, ***P≤0,0001, *P<0.012, Bonferonni's multiple-comparison test). Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/ncomms9666), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence Gdf2 deletion decreases tumor perfusion and maturation in the E0771 mammary cancer model. E0771 cells were injected in the 4th mammary gland of WT and Gdf2−/− mice and tumor vascularization was analyzed 9 days after tumor detection. a Representative images of the tumors stained for podocalyxin (red), lectin (green) and cell nuclei (blue, Hoechst). Scale bar 50 μm. b Vascular density quantified by podocalyxin positive area (% of tumor area) and (c) assessment of vessel diameter using Ferret’s theorem (WT n = 7, Gdf2−/−n = 13, 1 representative experiment out of 2). d Quantification of vessel perfusion by lectin staining (% area of lectin/podocalyxin) (WT n = 8, Gdf2−/− n = 7, 1 representative experiment out of 3). e Representative images of the tumors stained for podocalyxin (red), alpha -smooth muscle actin ( alpha -SMA) (green) and cell nuclei (blue, Hoechst). Scale bar 100 μm. f alpha -SMA staining quantification (% area of alpha -SMA/podocalyxin) (WT n = 8, Gdf2−/− n = 7, 1 representative experiment out of 3). b, c, d, f Data are the median ± interquartile range. Statistical analysis: Mann-Whitney test. *p ≤ 0.05 and **p ≤ 0.01 significantly different Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30165893), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence Confocal ExM images of mouse kidney labeled with antibodies or fluorescent proteins. (a–c) Single focal plane of glomerulus immunostained for podocin (a), agrin (b), podocalyxin (Podxl c), and merge (d) of (a–c). (e–g) Confocal maximum intensity projections of glomerulus immunostained for synaptopodin (Synpo, e), acetylated tubulin (acTub, f), podocin (g) highlighting secondary FPs, primary FPs, and slit diaphragms/FP boundaries, respectively. (h) Merge of (e–h). (i–k) Confocal maximum intensity projections of glomerulus immunostained for collagen IV (Coll IV, i), podocalyxin (Podxl, j), and alpha smooth muscle actin ( alpha SMA, k) and highlighting Bowman’s capsule and the mesangium, podocytes, and arterioles and the mesangium, respectively. (l) Merge of (i–k). (m–p) Single focal plane of glomerulus showing native fluorescence from confetti mouse expressing YFP (m) and RFP (o) in separate podocyte cell bodies and FPs as well as GFP (n) in various podocyte nuclei. (p) Merge of (m–o). (q) Zoomed-in view of region highlighted in (p). (r) Further zoomed-in view (top) and cross-sectional profile (bottom) of boxed region highlighted in (q). All distances and scale bars are in pre-expansion units. Scale bars, 2 µm (a–h,q), 25 µm (i–l), 5 µm (m–p). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29991751), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence Endothelial beta -catenin GOF does not affect the ECM of astrocytic endfeet and ECs within the subfornical organ (SFO).Striatal BBB-vessel showing a polarized distribution of Lama2 and Aqp4 in AC endfeet. Lumen is stained by Podxl (asterisk) (A). Coronal overview of the subfornical organ (SFO) (B); rectangular inset demarcates area for higher magnification in (C). Striatal BBB-vessel showing a polarized distribution of ColIV (green) but no Meca32 (white) in ECs (D). Coronal overview SFO, rectangular inset demarcates area for higher magnification in F (E); white dashed lines show Meca32+, red dashed lines show Meca32 vessels (F). Dashed lines outline SFO vessels; scale bars show 2 µm (A), 50 µm (B), 10 µm (C), 2.5 µm (D), 50 µm (E), 10 µm (F). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30932814), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence TRAF3IP1 mutations lead to epithelialization and polarity defects.(a) mIMCD3 cells grown until confluence on filters were subjected to Ca2+-free medium to disrupt the tight junctions. Six hours after Ca2+ addition, cells were analysed by immunofluorescence using the apical marker Gp135 (red) and beta -catenin (light blue) to stain the cell junctions. Scale bar, 10 μm. (b) Following Ca2+ switch, tight junction re-formation was assessed by measurement of trans-epithelial resistance (TER) at different time points (mean ± s.e.m. of n=5 independent experiments, two-way ANOVA; NS: not-significant, ***P<0.001 at 6 h). (c) Height of mIMCD3 cells grown on filters measured as the distance from the base to the top of the cells (GFP staining, not shown; mean ± s.d. of n≥20, from 3 independent experiments, ***P<0.001, Bonferonni's multiple-comparison test). (d) Expression of the apical marker Gp135 was analysed by Western blot with alpha -tubulin as a loading control. (e) mIMCD3 cells grown in matrigel 3D matrix to form spheroids were stained for ZO1 (tight junctions, red) and analysed by confocal microscopy. Arrows indicate ZO-1 at the apical junctions, while arrow heads point to mislocalized ZO-1. Equatorial sections of representative spheres are shown for each cell line. Scale bars, 10 μm. (f) Percentage of abnormal spheroids (no/small lumen filled with cells) (mean ± s.d., n=80 spheroids from 2 independent experiments, ***P≤0,001, **P<0.002, Bonferonni's multiple-comparison test). Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/ncomms9666), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence Bmp10 conditional deletion has no impact on tumor growth, angiogenesis and lung metastasis in the E0771 mammary cancer model. a Schematic representation of the experimental protocol for Bmp10 specific deletion and E0771 cells implantation. Tamoxifen was injected in all 3-week-old mice; 3 weeks later, E0771 cells were injected and tumor growth was analyzed for 3 weeks. b Plasmatic levels of BMP10 in control (CTL, n = 15) and Bmp10 conditional KO (Bmp10-cKO, n = 15) mice assessed by ELISA at the end of the experiment. c Tumor growth was assessed by caliper measurement every 2 to 3 days after tumor detection (CTL n = 7, Bmp10-cKO n = 8, 1 representative experiment out of 3). d Representative images of the tumors stained for podocalyxin (red), lectin (green) and cell nuclei (blue, Hoechst). Scale bar 50 μm. e Vascular density quantified by podocalyxin surface area (% of tumor area) and (f) Quantification of vessel perfusion by lectin staining (% area of lectin/podocalyxin) (CTL n = 7, Bmp10-cKO n = 8, 1 representative experiment out of 3). g Total area, (h) number and (i) mean size of lung metastases per mice bearing metastases (CTL n = 10, Bmp10-cKO n = 9, 2 experiments). c Data are the mean ± SEM. Statistical analysis: Two-way matched ANOVA. b, e, f, g, h, i Data are the median ± interquartile range. Statistical analysis: Mann-Whitney test. ****p ≤ 0.001 significantly different Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30165893), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence Endothelial beta -catenin GOF does not affect astrocytic endfoot polarization of alpha -dystroglycan ( alpha -Dag) and Kir4.1 within the subfornical organ (SFO).Striatal BBB-vessel showing a polarized distribution of alpha -Dag and Kir4.1 in AC endfeet. Lumen is stained by Podxl (asterisk) (A). Coronal overview of the subfornical organ (SFO) (B); rectangular inset demarcates area for higher magnification in (C). Dashed lines outline SFO vessels. Scale bar show 2 µm (A), 50 µm (B), 10 µm (C). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30932814), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Podocalyxin Like by Western Blot TRAF3IP1 mutations lead to epithelialization and polarity defects.(a) mIMCD3 cells grown until confluence on filters were subjected to Ca2+-free medium to disrupt the tight junctions. Six hours after Ca2+ addition, cells were analysed by immunofluorescence using the apical marker Gp135 (red) and beta -catenin (light blue) to stain the cell junctions. Scale bar, 10 μm. (b) Following Ca2+ switch, tight junction re-formation was assessed by measurement of trans-epithelial resistance (TER) at different time points (mean ± s.e.m. of n=5 independent experiments, two-way ANOVA; NS: not-significant, ***P<0.001 at 6 h). (c) Height of mIMCD3 cells grown on filters measured as the distance from the base to the top of the cells (GFP staining, not shown; mean ± s.d. of n≥20, from 3 independent experiments, ***P<0.001, Bonferonni's multiple-comparison test). (d) Expression of the apical marker Gp135 was analysed by Western blot with alpha -tubulin as a loading control. (e) mIMCD3 cells grown in matrigel 3D matrix to form spheroids were stained for ZO1 (tight junctions, red) and analysed by confocal microscopy. Arrows indicate ZO-1 at the apical junctions, while arrow heads point to mislocalized ZO-1. Equatorial sections of representative spheres are shown for each cell line. Scale bars, 10 μm. (f) Percentage of abnormal spheroids (no/small lumen filled with cells) (mean ± s.d., n=80 spheroids from 2 independent experiments, ***P≤0,001, **P<0.002, Bonferonni's multiple-comparison test). Image collected and cropped by CiteAb from the following publication (https://www.nature.com/articles/ncomms9666), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Podocalyxin Like by Immunocytochemistry/Immunofluorescence Gdf2 deletion decreases tumor perfusion and maturation in the E0771 mammary cancer model. E0771 cells were injected in the 4th mammary gland of WT and Gdf2−/− mice and tumor vascularization was analyzed 9 days after tumor detection. a Representative images of the tumors stained for podocalyxin (red), lectin (green) and cell nuclei (blue, Hoechst). Scale bar 50 μm. b Vascular density quantified by podocalyxin positive area (% of tumor area) and (c) assessment of vessel diameter using Ferret’s theorem (WT n = 7, Gdf2−/−n = 13, 1 representative experiment out of 2). d Quantification of vessel perfusion by lectin staining (% area of lectin/podocalyxin) (WT n = 8, Gdf2−/− n = 7, 1 representative experiment out of 3). e Representative images of the tumors stained for podocalyxin (red), alpha -smooth muscle actin ( alpha -SMA) (green) and cell nuclei (blue, Hoechst). Scale bar 100 μm. f alpha -SMA staining quantification (% area of alpha -SMA/podocalyxin) (WT n = 8, Gdf2−/− n = 7, 1 representative experiment out of 3). b, c, d, f Data are the median ± interquartile range. Statistical analysis: Mann-Whitney test. *p ≤ 0.05 and **p ≤ 0.01 significantly different Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30165893), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Podocalyxin in Neuro-2A cells by Flow Cytometry Neuro-2A cells were stained with Goat Anti-Mouse Podocalyxin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1556, filled histogram) or isotype control antibody (Catalog # 4-001-A, open histogram) followed by Allophycocyanin-conjugated Anti-Goat IgG Secondary Antibody (Catalog # F0108). View our protocol for Staining Membrane-associated Proteins.
 View Larger
View Larger
Detection of Mouse Mouse Podocalyxin Antibody by Immunohistochemistry TRAF3IP1 mutations lead to epithelialization and polarity defects.(a) mIMCD3 cells grown until confluence on filters were subjected to Ca2+-free medium to disrupt the tight junctions. Six hours after Ca2+ addition, cells were analysed by immunofluorescence using the apical marker Gp135 (red) and beta -catenin (light blue) to stain the cell junctions. Scale bar, 10 μm. (b) Following Ca2+ switch, tight junction re-formation was assessed by measurement of trans-epithelial resistance (TER) at different time points (mean ± s.e.m. of n=5 independent experiments, two-way ANOVA; NS: not-significant, ***P<0.001 at 6 h). (c) Height of mIMCD3 cells grown on filters measured as the distance from the base to the top of the cells (GFP staining, not shown; mean ± s.d. of n≥20, from 3 independent experiments, ***P<0.001, Bonferonni's multiple-comparison test). (d) Expression of the apical marker Gp135 was analysed by Western blot with alpha -tubulin as a loading control. (e) mIMCD3 cells grown in matrigel 3D matrix to form spheroids were stained for ZO1 (tight junctions, red) and analysed by confocal microscopy. Arrows indicate ZO-1 at the apical junctions, while arrow heads point to mislocalized ZO-1. Equatorial sections of representative spheres are shown for each cell line. Scale bars, 10 μm. (f) Percentage of abnormal spheroids (no/small lumen filled with cells) (mean ± s.d., n=80 spheroids from 2 independent experiments, ***P≤0,001, **P<0.002, Bonferonni's multiple-comparison test). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26487268), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
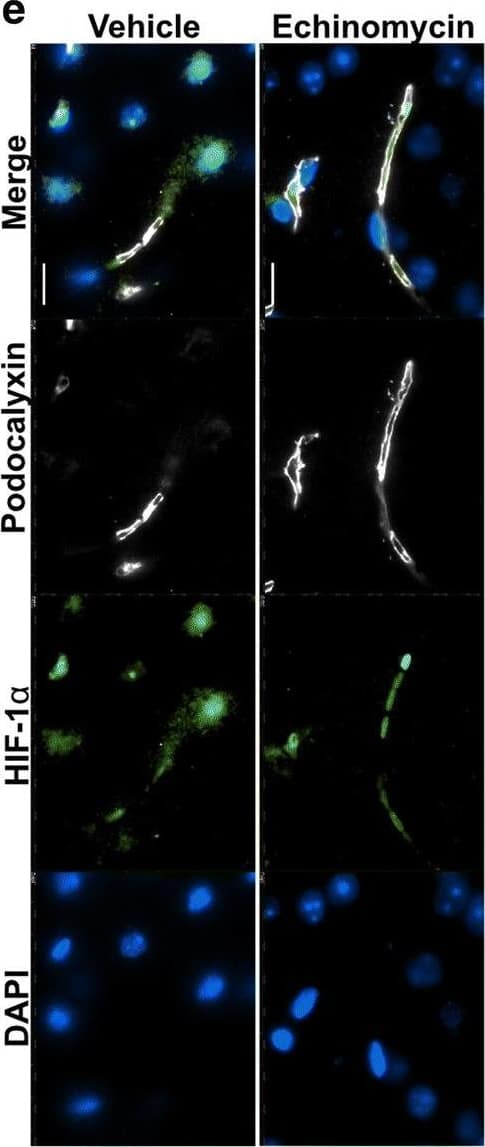
Detection of Mouse Podocalyxin Like by Immunohistochemistry Improved survival&blood–brain barrier function post pneumococcal infection in mice by anti-HIF-1 alpha treatment using echinomycin. e Immunofluorescence staining for HIF-1 alpha, BBB permeability&junctional markers in the echinomycin&vehicle groups at the survival end point. Left panel shows that echinomycin treatment leads to a reduction in HIF-1 alpha -positive nuclei including in ECs co-stained for podocalyxin, a vascular marker which was unchanged by the treatment. Middle panel displays reduced vascular permeability to fibrinogen in the echinomycin group as indicated by stronger intravascular signal compared to the vehicle-treated mice. Increased expression of tight junction proteins—occludin (middle panel)&claudin-5 (right top panel)—in echinomycin-treated mice indicate improved BBB function. Tight junction-associated ZO-1, adherens junction marker VE-Cadherin,&endothelial cell adhesion molecule CD31 unchanged (middle, bottom right panel). There was also no difference in S. pneumoniae (Spn) staining (right top panel) between the two groups. Scale bar 10 μm. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/32529267), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
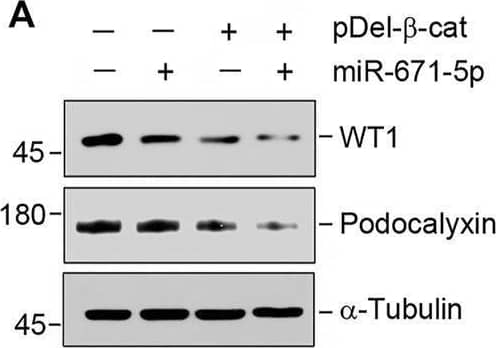
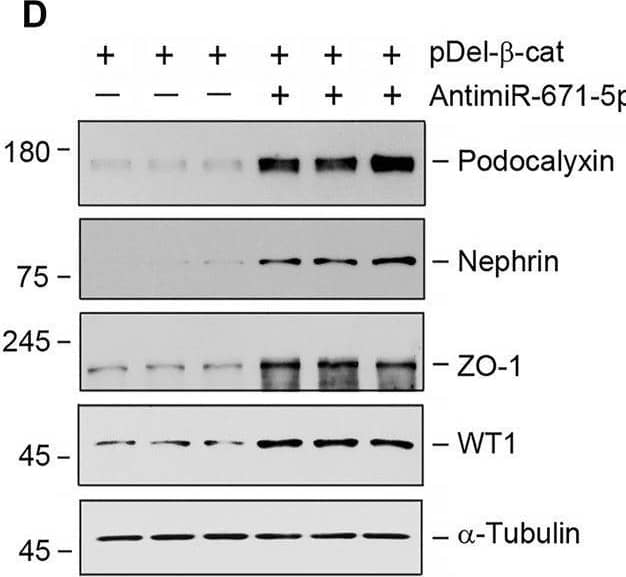
Detection of Podocalyxin by Western Blot miR-671-5p aggravates beta -catenin-induced podocyte injury while miR-671-5p inhibitor ameliorates it in vitro. (A–C) Representative Western blot (A) and graphic presentations of WT1 (B) and podocalyxin (C) were presented. MPC5 cells were transfected with miR-671-5p mimics (miR-671-5p) or/and beta -catenin expression plasmid (pDel-beta -cat) for 24 h *p < 0.05 versus pcDNA3 controls; †p < 0.05 versus pDel-beta -cat (n = 3). (D–H) Representative Western blot (D) and graphic presentations of podocalyxin (E), nephrin (F), ZO-1 (G) and WT1 (H) were presented. MPC5 cells were transfected with beta -catenin expression plasmid (pDel-beta -cat) or/and miR-671-5p inhibitor (AntimiR-671-5p) for 24 h *p < 0.05 (n = 3). (I) Representative micrographs show the expression of ZO-1 in different groups as indicated. MPC5 cells were transfected with beta -catenin expression plasmid (pDel-beta -cat) and miR-671-5p mimics (miR-671-5p)/miR-671-5p inhibitor (AntimiR-671-5p) for 24 h, respectively. Scale bar, 50 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35111054), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
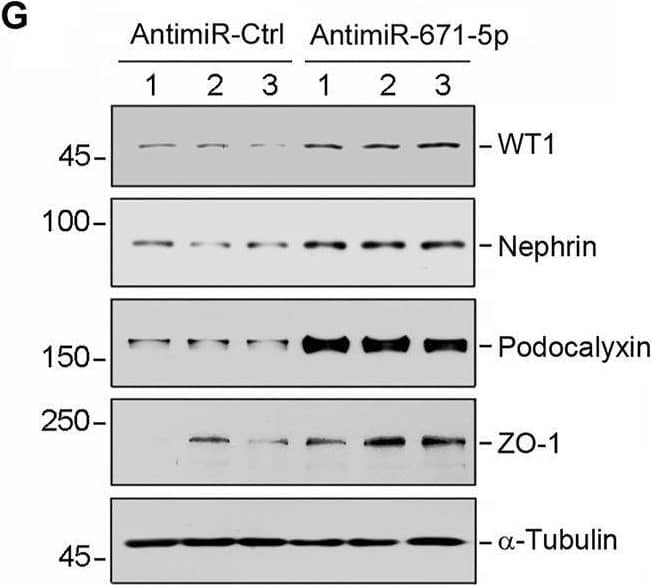
Detection of Podocalyxin by Western Blot Overexpression of miR-671-5p impairs but knockdown of miR-671-5p protects podocyte integrity in vitro. Mouse podocytes (MPC5) were transfected with miR-671-5p mimics (miR-671-5p) or negative control (miR-Ctrl) for 24 h. (A) qRT-PCR analysis shows the relative levels of miR-671-5p after transfection. *p < 0.05 (n = 3). (B–E) Representative Western blot (B) and graphic presentations of WT1 (C), ZO-1 (D) and podocalyxin (E) were presented. *p < 0.05 (n = 3). (F) Representative micrographs show the expression and distribution of ZO-1 in podocytes after miR-671-5p overexpression. Scale bar, 50 µm. (G–K) Inhibition of miR-671-5p protects podocyte integrity. MPC5 cells were transfected with miR-671-5p inhibitor (AntimiR-671-5p) or control (AntimiR-Ctrl) or for 24 h. Representative Western blot (G) and graphic presentations of WT1 (H), nephrin (I), podocalyxin (J) and ZO-1 (K) were presented. *p < 0.05 (n = 3). Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35111054), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Podocalyxin by Western Blot miR-671-5p aggravates beta -catenin-induced podocyte injury while miR-671-5p inhibitor ameliorates it in vitro. (A–C) Representative Western blot (A) and graphic presentations of WT1 (B) and podocalyxin (C) were presented. MPC5 cells were transfected with miR-671-5p mimics (miR-671-5p) or/and beta -catenin expression plasmid (pDel-beta -cat) for 24 h *p < 0.05 versus pcDNA3 controls; †p < 0.05 versus pDel-beta -cat (n = 3). (D–H) Representative Western blot (D) and graphic presentations of podocalyxin (E), nephrin (F), ZO-1 (G) and WT1 (H) were presented. MPC5 cells were transfected with beta -catenin expression plasmid (pDel-beta -cat) or/and miR-671-5p inhibitor (AntimiR-671-5p) for 24 h *p < 0.05 (n = 3). (I) Representative micrographs show the expression of ZO-1 in different groups as indicated. MPC5 cells were transfected with beta -catenin expression plasmid (pDel-beta -cat) and miR-671-5p mimics (miR-671-5p)/miR-671-5p inhibitor (AntimiR-671-5p) for 24 h, respectively. Scale bar, 50 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35111054), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
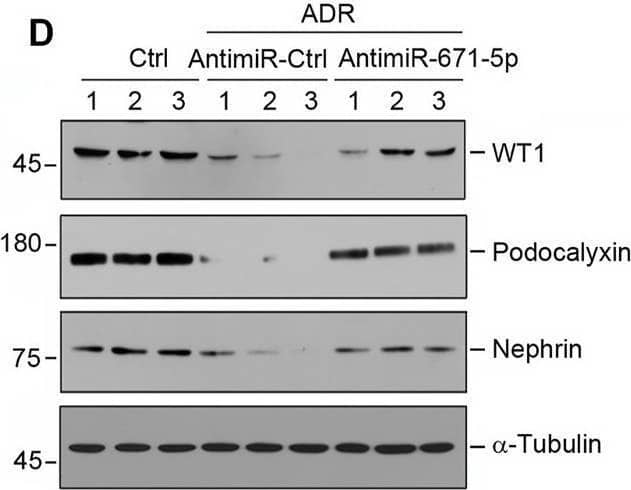
Detection of Podocalyxin by Western Blot Inhibition of miR-671-5p reduces proteinuria and renal fibrotic lesions in ADR nephropathy. (A) Experimental design. Red Arrows indicate the time of ADR injection. Green arrows indicate the different time points of antagomir injections. (B) qRT-PCR analysis shows that miR-671-5p level was increased in ADR group compared with control, and injections of antimiR-671-5p decreased miR-671-5p level. *p < 0.05 versus normal controls; †p < 0.05 versus ADR (n = 5–6). (C) Inhibition of miR-671-5p reduces proteinuria in ADR nephropathy. Urinary albumin levels were assessed in mice at 2 weeks after ADR injection and expressed as mg/mg creatinine. *p < 0.05 versus normal controls; †p < 0.05 versus ADR (n = 5–6). (D–G) Representative Western blots (D) and graphic presentations of WT1 (E), podocalyxin (F) and nephrin (G) were presented. *p < 0.05 versus normal controls, †p < 0.05 versus ADR alone (n = 5–6). (H) Immunofluorescence staining shows that antimiR-671-5p preserved renal podocalyxin expression in ADR nephropathy. Arrow indicate positive staining. Scale bar, 20 µm. (I,J) Representative Western blots (I) and graphic presentations of fibronectin and alpha -SMA (J) were presented. *p < 0.05 versus normal controls, †p < 0.05 versus ADR alone (n = 5–6). (K) Representative micrographs show that antimiR-671-5p inhibited alpha -SMA expression (upper panel) and renal fibrotic lesions (bottom panel) in different groups as indicated. Scale bar, 50 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35111054), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Podocalyxin
Podocalyxin, also known as Podocalyxin-like protein-1 (PCLP1 or PODXL), is a type I transmembrane glycoprotein. It belongs to the CD34/Podocalyxin family of sialomucins that share structural similarity and sequence homology. Podocalyxin is a major sialoprotein in the podocytes of the kidney glomerulus and is also expressed by both endothelium and multipotent hematopoietic progenitors. It has been identified as a novel cell surface marker for hemangioblasts, the common precursors of hematopoietic and endothelial cells (1, 2).
Product Datasheets
Citations for Mouse Podocalyxin Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
109
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Rescue of a lysosomal storage disorder caused by Grn loss of function with a brain penetrant progranulin biologic
Authors: Logan T, Simon MJ, Rana A Et al.
Cell
-
HIF-1 alpha is involved in blood-brain barrier dysfunction and paracellular migration of bacteria in pneumococcal meningitis.
Authors: Devraj, G, GuErit, S Et al.
Acta Neuropathol
-
Single cell analysis of the cellular heterogeneity and interactions in the injured mouse spinal cord
Authors: Milich LM, Choi JS, Ryan CB et al.
J Exp Med
-
Circadian Clock Regulation of Developmental Time in the Kidney
Authors: Dan H, Ruan T, Sampogna RV
Cell Rep
-
Parenchymal pericytes are not the major contributor of extracellular matrix in the fibrotic scar after stroke in male mice
Authors: Michaela Roth, Andreas Enström, Candice Aghabeick, Robert Carlsson, Guillem Genové, Gesine Paul
Journal of Neuroscience Research
-
Low wnt/beta-catenin signaling determines leaky vessels in the subfornical organ and affects water homeostasis in mice
Authors: Benz, F;Wichitnaowarat, V;Lehmann, M;Germano, RF;Mihova, D;Macas, J;Adams, RH;Taketo, MM;Plate, KH;Guerit, S;Vanhollebeke, B;Liebner, S;
Elife
-
Chemokine Receptor CXCR4 Plays a Crucial Role in Mediating Oxidative Stress-Induced Podocyte Injury
Authors: Hongyan Mo
Antioxid. Redox Signal, 2017-03-28;0(0):.
-
In vivo imaging of the barrier properties of the glia limitans during health and neuroinflammation
Authors: Hélie-Legoupil, P;Kloster, F;Pareja, J;Vladymyrov, M;Mapunda, JA;Bouillet, E;Oetiker, Y;Spera, I;Barcos, S;Brenna, A;Odriozola, A;Baert, A;Fankhauser, C;Haenni, B;Proulx, ST;Zuber, B;Deutsch, U;Engelhardt, B;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Pexidartinib and Nintedanib Combination Therapy Targets Macrophage Polarization to Reverse Pulmonary Fibrosis: A Preclinical Study
Authors: Kim, JH;Nam, JK;Park, MS;Seo, S;Ryu, HC;Lee, HJ;Lee, J;Lee, YJ;
International journal of molecular sciences
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Depth-Variant Deconvolution Applied to Widefield Microscopy for Rapid Large-Volume Tissue Imaging
Authors: Lee, DD;Telfer, KA;Koenis, MAJ;Lee, YK;Namink, KW;Saunders, BT;Lee, H;Kelley, HK;Ruiz, HS;Gaut, JP;Randolph, GJ;Zinselmeyer, BH;
Research square
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
KRIT1 heterozygous mutations are sufficient to induce a pathological phenotype in patient-derived iPSC models of cerebral cavernous malformation
Authors: Arce, M;Erzar, I;Yang, F;Senthilkumar, N;Onyeogaziri, FC;Ronchi, D;Ahlstrand, FC;Noll, N;Lugano, R;Richards, M;Scola, E;Corada, M;Lazzaroni, F;Meggiolaro, L;Schuster, J;Dahl, N;Niemelä, M;Jahromi, BR;Dimberg, A;Lanfraconi, S;Latini, R;Magnusson, PU;
Cell reports
Species: Human, Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Immunohistochemistry -
Brain pericytes and perivascular fibroblasts are stromal progenitors with dual functions in cerebrovascular regeneration after stroke
Authors: Bernier, LP;Hefendehl, JK;Scott, RW;Tung, LW;Lewis, CA;Soliman, H;Simm, S;Dissing-Olesen, L;Hofmann, J;Guo, D;DeMeglio, M;Rossi, FM;Underhill, TM;MacVicar, BA;
Nature neuroscience
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
The interaction of tPA with NMDAR1 drives neuroinflammation and neurodegeneration in ?-synuclein-mediated neurotoxicity
Authors: Torrente, D;Su, E;Citalán?Madrid, A;Schielke, G;Magaoay, D;Warnock, M;Stevenson, T;Mann, K;Lesept, F;Delétage, N;Blanc, M;Norris, E;Vivien, D;Lawrence, D;
Journal of neuroinflammation
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-F -
Genetic deletion of calcium-independent phospholipase A2? protects mice from diabetic nephropathy
Authors: Cybulsky, AV;Papillon, J;Guillemette, J;Navarro-Betancourt, JR;Elimam, H;Fantus, IG;
PloS one
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Employing Multi-Omics Analyses to Understand Changes during Kidney Development in Perinatal Interleukin-6 Animal Model
Authors: Panzade, G;Srivastava, T;Heruth, DP;Rezaiekhaligh, MH;Zhou, J;Lyu, Z;Sharma, M;Joshi, T;
Cells
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
MicroRNA-29b Plays a Vital Role in Podocyte Injury and Glomerular Diseases through Inducing Mitochondrial Dysfunction
Authors: Liu, J;Xiong, Y;Mo, H;Niu, H;Miao, J;Shen, W;Zhou, S;Wang, X;Li, X;Zhang, Y;Ma, K;Zhou, L;
International journal of biological sciences
Species: Human
Sample Types: Tissue Lysates, Whole Tissue
Applications: Immunohistochemistry, Western Blot -
An Anti-VEGF-B Antibody Reduces Abnormal Tumor Vasculature and Enhances the Effects of Chemotherapy
Authors: Janes, PW;Parslow, AC;Cao, D;Rigopoulos, A;Lee, FT;Gong, SJ;Cartwright, GA;Burvenich, IJG;Eriksson, U;Johns, TG;Scott, FE;Scott, AM;
Cancers
Species: Xenograft
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Protective Role of the Podocyte IL-15 / STAT5 Pathway in Focal Segmental Glomerulosclerosis
Authors: Niasse, A;Louis, K;Lenoir, O;Schwarz, C;Xu, X;Couturier, A;Dobosziewicz, H;Corchia, A;Placier, S;Vandermeersch, S;Hennighausen, L;Frère, P;Galichon, P;Surin, B;Ouchelouche, S;Louedec, L;Migeon, T;Verpont, MC;Yousfi, N;Buob, D;Xu-Dubois, YC;François, H;Rondeau, E;Mesnard, L;Hadchouel, J;Luque, Y;
Kidney international reports
Species: Mouse, Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
TIMP-2 and IGFBP7 in human kidney biopsies in renal disease
Authors: Moritz Schanz, Martin Kimmel, Mark Dominik Alscher, Kerstin Amann, Christoph Daniel
Clinical Kidney Journal
-
Partial Mural Cell Ablation Disrupts Coronary Vasculature Integrity and Induces Systolic Dysfunction
Authors: Cornuault, L;Hérion, FX;Bourguignon, C;Rouault, P;Foussard, N;Alzieu, P;Chapouly, C;Gadeau, AP;Couffinhal, T;Renault, MA;
Journal of the American Heart Association
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Functional gene delivery to and across brain vasculature of systemic AAVs with endothelial-specific tropism in rodents and broad tropism in primates
Authors: Chen, X;Wolfe, DA;Bindu, DS;Zhang, M;Taskin, N;Goertsen, D;Shay, TF;Sullivan, EE;Huang, SF;Ravindra Kumar, S;Arokiaraj, CM;Plattner, VM;Campos, LJ;Mich, JK;Monet, D;Ngo, V;Ding, X;Omstead, V;Weed, N;Bishaw, Y;Gore, BB;Lein, ES;Akrami, A;Miller, C;Levi, BP;Keller, A;Ting, JT;Fox, AS;Eroglu, C;Gradinaru, V;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Scalable projected Light Sheet Microscopy for high-resolution imaging of large samples
Authors: Chen, Y;Gong, C;Chauhan, S;De La Cruz, ED;Datta, MS;Tomer, R;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Whole-mount immunofluorescence staining of blood and lymphatic vessels in murine small intestine
Authors: Satu Paavonsalo, Yelin Subashi, Madeleine H. Lackman, Sinem Karaman
STAR Protocols
-
Generation and characterization of an inducible renal proximal tubule-specific CreERT2 mouse
Authors: Shiting Liang, Youliang Wang, Meixia Kang, Juan Deng, Liting Chen, Xizhen Hong et al.
Frontiers in Cell and Developmental Biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Altered hemodynamics and vascular reactivity in a mouse model with severe pericyte deficiency
Authors: Stobart JL, Erlebach E, Gl�ck C et al.
Journal of Cerebral Blood Flow & Metabolism
-
Functional gene delivery to and across brain vasculature of systemic AAVs with endothelial-specific tropism in rodents and broad tropism in primates.
Authors: Xinhong Chen, Damien A. Wolfe, Dhanesh Sivadasan Sivadasan Bindu, Mengying Zhang, Naz Taskin, David Goertsen et al.
bioRxiv
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Assessment of Choroidal Vasculature and Innate Immune Cells in the Eyes of Albino and Pigmented Mice
Authors: IS Zaitoun, YS Song, HB Zaitoun, CM Sorenson, N Sheibani
Cells, 2022-10-21;11(20):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Imaging Blood Vessels and Lymphatics in Mouse Trachea Wholemounts
Authors: Peter Baluk, Donald M. McDonald
Methods in Molecular Biology
-
Severe cerebellar malformations in mutant mice demonstrate a role for PDGF-C/PDGFR alpha signalling in cerebellar development
Authors: Sara Gillnäs, Radiosa Gallini, Liqun He, Christer Betsholtz, Johanna Andrae
Biology Open
-
Conserved meningeal lymphatic drainage circuits in mice and humans
Authors: Laurent Jacob, Jose de Brito Neto, Stephanie Lenck, Celine Corcy, Farhat Benbelkacem, Luiz Henrique Geraldo et al.
Journal of Experimental Medicine
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Interplay of vascular endothelial growth factor receptors in organ-specific vessel maintenance
Authors: Sinem Karaman, Satu Paavonsalo, Krista Heinolainen, Madeleine H. Lackman, Amanda Ranta, Karthik A. Hemanthakumar et al.
Journal of Experimental Medicine
-
Role of miRNA-671-5p in Mediating Wnt/ beta -Catenin-Triggered Podocyte Injury
Authors: Chunhong Wang, Jiafeng Liu, Xiaoyao Zhang, Qiyan Chen, Xiaoyan Bai, Xue Hong et al.
Frontiers in Pharmacology
-
High-resolution fluorescence-guided transcranial ultrasound mapping in the live mouse brain
Authors: H Estrada, J Robin, A Özbek, Z Chen, A Marowsky, Q Zhou, D Beck, B le Roy, M Arand, S Shoham, D Razansky
Science Advances, 2021-12-08;7(50):eabi5464.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Anisotropic expansion of hepatocyte lumina enforced by apical bulkheads
Authors: Belicova L, Repnik U, Delpierre J et al.
Journal of Cell Biology
-
Differentiation of mouse fetal lung alveolar progenitors in serum-free organotypic cultures
Authors: K Gkatzis, P Panza, S Peruzzo, DY Stainier
Elife, 2021-09-29;10(0):.
Species: Mouse
Sample Types: Organoids
Applications: IHC -
Imbalanced Activation of Wnt-/ beta -Catenin-Signaling in Liver Endothelium Alters Normal Sinusoidal Differentiation
Authors: Philipp-Sebastian Koch, Kajetan Sandorski, Joschka Heil, Christian D. Schmid, Sina W. Kürschner, Johannes Hoffmann et al.
Frontiers in Physiology
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Characterization of the blood–brain barrier in genetically diverse laboratory mouse strains
Authors: Johanna Schaffenrath, Sheng-Fu Huang, Tania Wyss, Mauro Delorenzi, Annika Keller
Fluids and Barriers of the CNS
-
A mouse model of prenatal exposure to Interleukin-6 to study the developmental origin of health and disease
Authors: T Srivastava, T Joshi, DP Heruth, MH Rezaiekhal, RE Garola, J Zhou, VC Boinpelly, MF Ali, US Alon, M Sharma, GB Vanden Heu, P Mahajan, L Priya, Y Jiang, ET McCarthy, VJ Savin, R Sharma, M Sharma
Scientific Reports, 2021-06-24;11(1):13260.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mast Cells Are the Trigger of Small Vessel Disease and Diastolic Dysfunction in Diabetic Obese Mice
Authors: Sarah Guimbal, Lauriane Cornuault, Paul Rouault, Pierre-Louis Hollier, Candice Chapouly, Marie-Lise Bats et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Talin-dependent integrin activation is required for endothelial proliferation and postnatal angiogenesis
Authors: Fadi E. Pulous, Jamie C. Carnevale, Zaki Al-Yafeai, Brenna H. Pearson, Jamie A. G. Hamilton, Curtis J. Henry et al.
Angiogenesis
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Hepatocyte growth factor-regulated tyrosine kinase substrate is essential for endothelial cell polarity and cerebrovascular stability
Authors: Zhenyang Yu, Jian Zeng, Jun Wang, Yaxiong Cui, Xiaopeng Song, Yizhe Zhang et al.
Cardiovascular Research
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Role of IRE1 alpha in podocyte proteostasis and mitochondrial health
Authors: José R. Navarro-Betancourt, Joan Papillon, Julie Guillemette, Takao Iwawaki, Chen-Fang Chung, Andrey V. Cybulsky
Cell Death Discovery
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Mapping endothelial-cell diversity in cerebral cavernous malformations at single-cell resolution
Authors: Fabrizio Orsenigo, Lei Liu Conze, Suvi Jauhiainen, Monica Corada, Francesca Lazzaroni, Matteo Malinverno et al.
eLife
-
Single nuclei RNA-seq of mouse placental labyrinth development
Authors: Bryan Marsh, Robert Blelloch
eLife
-
Cytotoxic T-cells mediate exercise-induced reductions in tumor growth
Authors: Helene Rundqvist, Pedro Veliça, Laura Barbieri, Paulo A Gameiro, David Bargiela, Milos Gojkovic et al.
eLife
-
Circulating Levels of Epirubicin Cause Endothelial Senescence While Compromising Metabolic Activity and Vascular Function
Authors: Amanda J. Eakin, Tamara Mc Erlain, Aileen Burke, Amy Eaton, Nuala Tipping, Gloria Allocca et al.
Frontiers in Cell and Developmental Biology
-
Feature-rich covalent stains for super-resolution and cleared tissue fluorescence microscopy
Authors: C Mao, MY Lee, JR Jhan, AR Halpern, MA Woodworth, AK Glaser, TJ Chozinski, L Shin, JW Pippin, SJ Shankland, JTC Liu, JC Vaughan
Sci Adv, 2020-05-27;6(22):eaba4542.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Digenic inheritance of mutations in EPHA2 and SLC26A4 in Pendred syndrome
Authors: M Li, SY Nishio, C Naruse, M Riddell, S Sapski, T Katsuno, T Hikita, F Mizapoursh, FM Smith, LT Cooper, MG Lee, M Asano, T Boettger, M Krueger, A Wietelmann, J Graumann, BW Day, AW Boyd, S Offermanns, SI Kitajiri, SI Usami, M Nakayama
Nat Commun, 2020-03-12;11(1):1343.
Species: Mouse
Sample Types: Cells
Applications: ICC -
Genetic Ablation of Calcium-independent Phospholipase A2 gamma Exacerbates Glomerular Injury in Adriamycin Nephrosis in Mice
Authors: Hanan Elimam, Joan Papillon, Julie Guillemette, José R. Navarro-Betancourt, Andrey V. Cybulsky
Scientific Reports
-
The coronary artery disease risk-associated Plpp3 gene and its product lipid phosphate phosphatase 3 regulate experimental atherosclerosis
Authors: Paul A. Mueller, Liping Yang, Margo Ubele, Guogen Mao, Jason Brandon, Julia Vandra et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Retinoic acid receptor alpha as a novel contributor to adrenal cortex structure and function through interactions with Wnt and Vegfa signalling
Authors: RM El Zein, AH Soria, JF Golib Dzib, AJ Rickard, FL Fernandes-, B Samson-Cou, I Giscos-Dou, A Rocha, M Poglitsch, CE Gomez-Sanc, L Amar, NB Ghyselinck, A Benecke, MC Zennaro, S Boulkroun
Sci Rep, 2019-10-11;9(1):14677.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Vascular regression precedes motor neuron loss in the FUS (1-359) ALS mouse model
Authors: Martin Crivello, Marion C. Hogg, Elisabeth Jirström, Luise Halang, Ina Woods, Megan Rayner et al.
Disease Models & Mechanisms
-
Acute and chronic hypoxia differentially predispose lungs for metastases
Authors: M Reiterer, R Colaço, P Emrouzneja, A Jensen, H Rundqvist, RS Johnson, C Branco
Sci Rep, 2019-07-15;9(1):10246.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Evaporative cooling provides a major metabolic energy sink
Authors: I Kasza, D Adler, DW Nelson, CL Eric Yen, S Dumas, JM Ntambi, OA MacDougald, D Hernando, WP Porter, FA Best, CM Alexander
Mol Metab, 2019-07-01;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Loss of the transcription factor RBPJ induces disease-promoting properties in brain pericytes
Authors: R Diéguez-Hu, K Kato, BD Giaimo, M Nieminen-K, H Arf, F Ferrante, M Bartkuhn, T Zimmermann, MG Bixel, HM Eilken, S Adams, T Borggrefe, P Vajkoczy, RH Adams
Nat Commun, 2019-06-27;10(1):2817.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endothelial cell clonal expansion in the development of cerebral cavernous malformations
Authors: M Malinverno, C Maderna, A Abu Taha, M Corada, F Orsenigo, M Valentino, F Pisati, C Fusco, P Graziano, M Giannotta, QC Yu, YA Zeng, MG Lampugnani, PU Magnusson, E Dejana
Nat Commun, 2019-06-24;10(1):2761.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endophilin-A2 dependent VEGFR2 endocytosis promotes sprouting angiogenesis
Authors: G Genet, K Boyé, T Mathivet, R Ola, F Zhang, A Dubrac, J Li, N Genet, L Henrique G, L Benedetti, S Künzel, L Pibouin-Fr, JL Thomas, A Eichmann
Nat Commun, 2019-05-28;10(1):2350.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Foxc2 is essential for podocyte function
Authors: D Nilsson, M Heglind, Z Arani, S Enerbäck
Physiol Rep, 2019-05-01;7(9):e14083.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Wnt/ beta -catenin links oxidative stress to podocyte injury and proteinuria
Authors: Lili Zhou, Xiaowen Chen, Meizhi Lu, Qinyu Wu, Qian Yuan, Chengxiao Hu et al.
Kidney International
Species: Mouse
Sample Types: Cell Lysates, Whole Cells, Whole Tissue
Applications: Immunohistochemistry, Western Blot, Immunocytochemistry -
Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders
Authors: T. Yang, G. Xu, P.T. Newton, A.S. Chagin, S. Mkrtchian, M. Carlström et al.
British Journal of Anaesthesia
-
aPKC controls endothelial growth by modulating c-Myc via FoxO1 DNA-binding ability
Authors: M Riddell, A Nakayama, T Hikita, F Mirzapours, T Kawamura, A Pasha, M Li, M Masuzawa, M Looso, T Steinbache, K Ebnet, M Potente, T Hirose, S Ohno, I Fleming, S Gattenlöhn, PP Aung, T Phung, O Yamasaki, T Yanagi, H Umemura, M Nakayama
Nat Commun, 2018-12-17;9(1):5357.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
APOL1 risk allele RNA contributes to renal toxicity by activating protein kinase R
Authors: Koji Okamoto, Jason W. Rausch, Hidefumi Wakashin, Yulong Fu, Joon-Yong Chung, Patrick D. Dummer et al.
Communications Biology
-
Mice doubly deficient in Six4 and Six5 show ventral body wall defects reproducing human omphalocele
Authors: M Takahashi, M Tamura, S Sato, K Kawakami
Dis Model Mech, 2018-10-25;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
PAR ‐3 controls endothelial planar polarity and vascular inflammation under laminar flow
Authors: Takao Hikita, Fatemeh Mirzapourshafiyi, Pedro Barbacena, Meghan Riddell, Ayesha Pasha, Mengnan Li et al.
EMBO reports
-
BMP9, but not BMP10, acts as a quiescence factor on tumor growth, vessel normalization and metastasis in a mouse model of breast cancer
Authors: M Ouarné, C Bouvard, G Boneva, C Mallet, J Ribeiro, A Desroches-, E Soleilhac, E Tillet, O Peyruchaud, S Bailly
J. Exp. Clin. Cancer Res., 2018-08-30;37(1):209.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Volumetric, Nanoscale Optical Imaging of Mouse and Human Kidney via Expansion Microscopy
Authors: TJ Chozinski, C Mao, AR Halpern, JW Pippin, SJ Shankland, CE Alpers, B Najafian, JC Vaughan
Sci Rep, 2018-07-10;8(1):10396.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Ste20-like kinase, SLK, a novel mediator of podocyte integrity
Authors: Andrey V. Cybulsky, Joan Papillon, Julie Guillemette, Natalya Belkina, Genaro Patino-Lopez, Elena Torban
American Journal of Physiology-Renal Physiology
-
EphrinB2/EphB4 signaling regulates non-sprouting angiogenesis by VEGF
Authors: E Groppa, S Brkic, A Uccelli, G Wirth, P Korpisalo-, M Filippova, B Dasen, V Sacchi, MG Muraro, M Trani, S Reginato, R Gianni-Bar, S Ylä-Herttu, A Banfi
EMBO Rep., 2018-04-11;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Extracellular retention of PDGF-B directs vascular remodeling in mouse hypoxia-induced pulmonary hypertension
Authors: Philip Tannenberg, Ya-Ting Chang, Lars Muhl, Bàrbara Laviña, Hanna Gladh, Guillem Genové et al.
American Journal of Physiology-Lung Cellular and Molecular Physiology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Loss of ezrin expression reduced the susceptibility to the glomerular injury in mice
Authors: R Hatano, A Takeda, Y Abe, K Kawaguchi, I Kazama, M Matsubara, S Asano
Sci Rep, 2018-03-14;8(1):4512.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury
Authors: DO Dias, H Kim, D Holl, B Werne Soln, J Lundeberg, M Carlén, C Göritz, J Frisén
Cell, 2018-03-01;173(1):153-165.e22.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Pleiotropic activity of systemically delivered angiogenin in the SOD1G93Amouse model
Authors: M Crivello, SL O'Riordan, I Woods, S Cannon, L Halang, KS Coughlan, MC Hogg, SA Lewandowsk, JHM Prehn
Neuropharmacology, 2018-02-25;133(0):503-511.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Neurog3-dependent pancreas dysgenesis causes ectopic pancreas in Hes1 mutant mice
Authors: Mette C. Jørgensen, Kristian H. de Lichtenberg, Caitlin A. Collin, Rasmus Klinck, Jeppe H. Ekberg, Maja S. Engelstoft et al.
Development
-
Development and plasticity of meningeal lymphatic vessels
Authors: Salli Antila, Sinem Karaman, Harri Nurmi, Mikko Airavaara, Merja H. Voutilainen, Thomas Mathivet et al.
Journal of Experimental Medicine
-
Loss of Vascular CD34 Results in Increased Sensitivity to Lung Injury
Authors: Bernard C. Lo, Matthew J. Gold, Sebastian Scheer, Michael R. Hughes, Jessica Cait, Erin Debruin et al.
American Journal of Respiratory Cell and Molecular Biology
-
Genetic and pharmacological inhibition of microRNA-92a maintains podocyte cell cycle quiescence and limits crescentic glomerulonephritis
Authors: Carole Henique, Guillaume Bollée, Xavier Loyer, Florian Grahammer, Neeraj Dhaun, Marine Camus et al.
Nature Communications
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
HS3ST1 genotype regulates antithrombin's inflammomodulatory tone and associates with atherosclerosis
Authors: Nicole C. Smits, Takashi Kobayashi, Pratyaksh K. Srivastava, Sladjana Skopelja, Julianne A. Ivy, Dustin J. Elwood et al.
Matrix Biology
-
Smooth muscle cell recruitment to lymphatic vessels requires PDGFB and impacts vessel size but not identity
Authors: Yixin Wang, Yi Jin, Maarja Andaloussi Mäe, Yang Zhang, Henrik Ortsäter, Christer Betsholtz et al.
Development
-
Loss of mucin-type O-glycans impairs the integrity of the glomerular filtration barrier in the mouse kidney
Authors: K Song, J Fu, J Song, BH Herzog, K Bergstrom, Y Kondo, JM McDaniel, S McGee, R Silasi-Man, F Lupu, H Chen, H Bagavant, L Xia
J. Biol. Chem., 2017-08-25;292(40):16491-16497.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Efficient activation of the lymphangiogenic growth factor VEGF-C requires the C-terminal domain of VEGF-C and the N-terminal domain of CCBE1
Authors: SK Jha, K Rauniyar, T Karpanen, VM Leppänen, P Brouillard, M Vikkula, K Alitalo, M Jeltsch
Sci Rep, 2017-07-07;7(1):4916.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Deletion of Inositol-Requiring Enzyme-1? in Podocytes Disrupts Glomerular Capillary Integrity and Autophagy
Authors: DR Kaufman, J Papillon, L Larose, T Iwawaki, AV Cybulsky
Mol. Biol. Cell, 2017-04-20;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Compound genetically engineered mouse models of cancer reveal dual targeting of ALK1 and endoglin as a synergistic opportunity to impinge on angiogenic TGF-beta signaling
Authors: Nikolas M. Eleftheriou, Jonas Sjölund, Matteo Bocci, Eliane Cortez, Se-Jin Lee, Sara I. Cunha et al.
Oncotarget
-
Vascular development in the vertebrate pancreas
Authors: D. Berfin Azizoglu, Diana C. Chong, Alethia Villasenor, Judith Magenheim, David M. Barry, Simon Lee et al.
Developmental Biology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Simultaneous targeted activation of Notch1 and Vhl-disruption in the kidney proximal epithelial tubular cells in mice
Sci Rep, 2016-08-05;6(0):30739.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Loss of endothelial barrier integrity in mice with conditional ablation of podocalyxin (Podxl) in endothelial cells
Authors: Angélica Horrillo, Gracia Porras, Matilde S. Ayuso, Consuelo González-Manchón
European Journal of Cell Biology
-
EphrinB2 repression through ZEB2 mediates tumour invasion and anti-angiogenic resistance
Nat Commun, 2016-07-29;7(0):12329.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
CD34 Promotes Pathological Epi-Retinal Neovascularization in a Mouse Model of Oxygen-Induced Retinopathy
Authors: Martin J. Siemerink, Michael R. Hughes, Marchien G. Dallinga, Tomek Gora, Jessica Cait, Ilse M. C. Vogels et al.
PLOS ONE
-
Anti-metastatic action of FAK inhibitor OXA-11 in combination with VEGFR-2 signaling blockade in pancreatic neuroendocrine tumors
Authors: Ingrid Moen, Matthew Gebre, Vanesa Alonso-Camino, Debbie Chen, David Epstein, Donald M. McDonald
Clinical & Experimental Metastasis
-
tPA Deficiency in Mice Leads to Rearrangement in the Cerebrovascular Tree and Cerebroventricular Malformations
Authors: Christina Stefanitsch, Anna-Lisa E. Lawrence, Anna Olverling, Ingrid Nilsson, Linda Fredriksson
Frontiers in Cellular Neuroscience
-
The development and plasticity of alveolar type 1 cells
Authors: J Yang, BJ Hernandez, D Martinez A, O Narvaez de, L Vila-Ellis, H Akiyama, SE Evans, EJ Ostrin, J Chen
Development, 2015-11-19;143(1):54-65.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Wt1 and beta -catenin cooperatively regulate diaphragm development in the mouse
Authors: Nicole D. Paris, Garry L. Coles, Kate G. Ackerman
Developmental Biology
-
Identification of a neurovascular signaling pathway regulating seizures in mice
Authors: Linda Fredriksson, Tamara K. Stevenson, Enming J. Su, Margaret Ragsdale, Shannon Moore, Stefan Craciun et al.
Annals of Clinical and Translational Neurology
-
Endothelial ALK1 Is a Therapeutic Target to Block Metastatic Dissemination of Breast Cancer.
Authors: Cunha S, Bocci M, Lovrot J, Eleftheriou N, Roswall P, Cordero E, Lindstrom L, Bartoschek M, Haller B, Pearsall R, Mulivor A, Kumar R, Larsson C, Bergh J, Pietras K
Cancer Res, 2015-06-15;75(12):2445-56.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC-Fr -
Mutual Antagonism of Wilms’ Tumor 1 and beta -Catenin Dictates Podocyte Health and Disease
Authors: Lili Zhou, Yingjian Li, Weichun He, Dong Zhou, Roderick J. Tan, Jing Nie et al.
Journal of the American Society of Nephrology
-
Clustered PI(4,5)P(2) accumulation and ezrin phosphorylation in response to CLIC5A.
Authors: Al-Momany A, Li L, Alexander R, Ballermann B
J Cell Sci, 2014-10-24;127(24):5164-78.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Protein tyrosine phosphatase 1B inhibition protects against podocyte injury and proteinuria.
Authors: Kumagai T, Baldwin C, Aoudjit L, Nezvitsky L, Robins R, Jiang R, Takano T
Am J Pathol, 2014-06-18;184(8):2211-24.
Species: Mouse, Rat
Sample Types: Whole Tissue
Applications: ICC -
Direct action of endothelin-1 on podocytes promotes diabetic glomerulosclerosis.
Authors: Lenoir O, Milon M, Virsolvy A, Henique C, Schmitt A, Masse J, Kotelevtsev Y, Yanagisawa M, Webb D, Richard S, Tharaux P
J Am Soc Nephrol, 2014-04-10;25(5):1050-62.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Endothelial Notch activity promotes angiogenesis and osteogenesis in bone.
Authors: Ramasamy S, Kusumbe A, Wang L, Adams R
Nature, 2014-03-12;507(7492):376-80.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Crim1 maintains retinal vascular stability during development by regulating endothelial cell Vegfa autocrine signaling.
Authors: Fan J, Ponferrada V, Sato T, Vemaraju S, Fruttiger M, Gerhardt H, Ferrara N, Lang R
Development, 2013-12-18;141(2):448-59.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Def-6, a novel regulator of small GTPases in podocytes, acts downstream of atypical protein kinase C (aPKC) lambda/iota.
Authors: Worthmann K, Leitges M, Teng B, Sestu M, Tossidou I, Samson T, Haller H, Huber T, Schiffer M
Am J Pathol, 2013-10-03;183(6):1945-59.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Deficiency for endoglin in tumor vasculature weakens the endothelial barrier to metastatic dissemination.
Authors: Anderberg C, Cunha S, Zhai Z, Cortez E, Pardali E, Johnson J, Franco M, Paez-Ribes M, Cordiner R, Fuxe J, Johansson B, Goumans M, Casanovas O, ten Dijke P, Arthur H, Pietras K
J Exp Med, 2013-02-11;210(3):563-79.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Integrin Beta 1 Suppresses Multilayering of a Simple Epithelium
Authors: Jichao Chen, Mark A. Krasnow
PLoS ONE
-
Oral monosaccharide therapies to reverse renal and muscle hyposialylation in a mouse model of GNE myopathy
Authors: Terren K. Niethamer, Tal Yardeni, Petcharat Leoyklang, Carla Ciccone, Adrian Astiz-Martinez, Katherine Jacobs et al.
Molecular Genetics and Metabolism
-
Proteomic analysis of the slit diaphragm complex: CLIC5 is a protein critical for podocyte morphology and function.
Authors: Pierchala BA, Munoz MR, Tsui CC
Kidney Int., 2010-07-21;78(9):868-82.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Novel regulators of kidney development from the tips of the ureteric bud.
Authors: Schmidt-Ott KM, Yang J, Chen X, Wang H, Paragas N, Mori K, Li JY, Lu B, Costantini F, Schiffer M, Bottinger E, Barasch J
J. Am. Soc. Nephrol., 2005-05-25;16(7):1993-2002.
Species: Rat
Sample Types: Whole Tissue
Applications: IHC -
Bone marrow sinusoidal endothelium controls terminal erythroid differentiation and reticulocyte maturation
Authors: J Heil, V Olsavszky, K Busch, K Klapproth, C de la Torr, C Sticht, K Sandorski, J Hoffmann, H Schönhaber, J Zierow, M Winkler, CD Schmid, T Staniczek, DE Daniels, J Frayne, G Metzgeroth, D Nowak, S Schneider, M Neumaier, V Weyer, C Groden, HJ Gröne, K Richter, C Mogler, MM Taketo, K Schledzews, C Géraud, S Goerdt, PS Koch
Nature Communications, 2021-11-29;12(1):6963.
-
Lung epithelial branching program antagonizes alveolar differentiation.
Authors: Chang DR et al.
Proc Natl Acad Sci U S A
-
Anisotropic expansion of hepatocyte lumina enforced by apical bulkheads
Authors: Belicova L, Repnik U, Delpierre J et al.
Journal of Cell Biology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse Podocalyxin Antibody
Average Rating: 4.4 (Based on 5 Reviews)
Have you used Mouse Podocalyxin Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
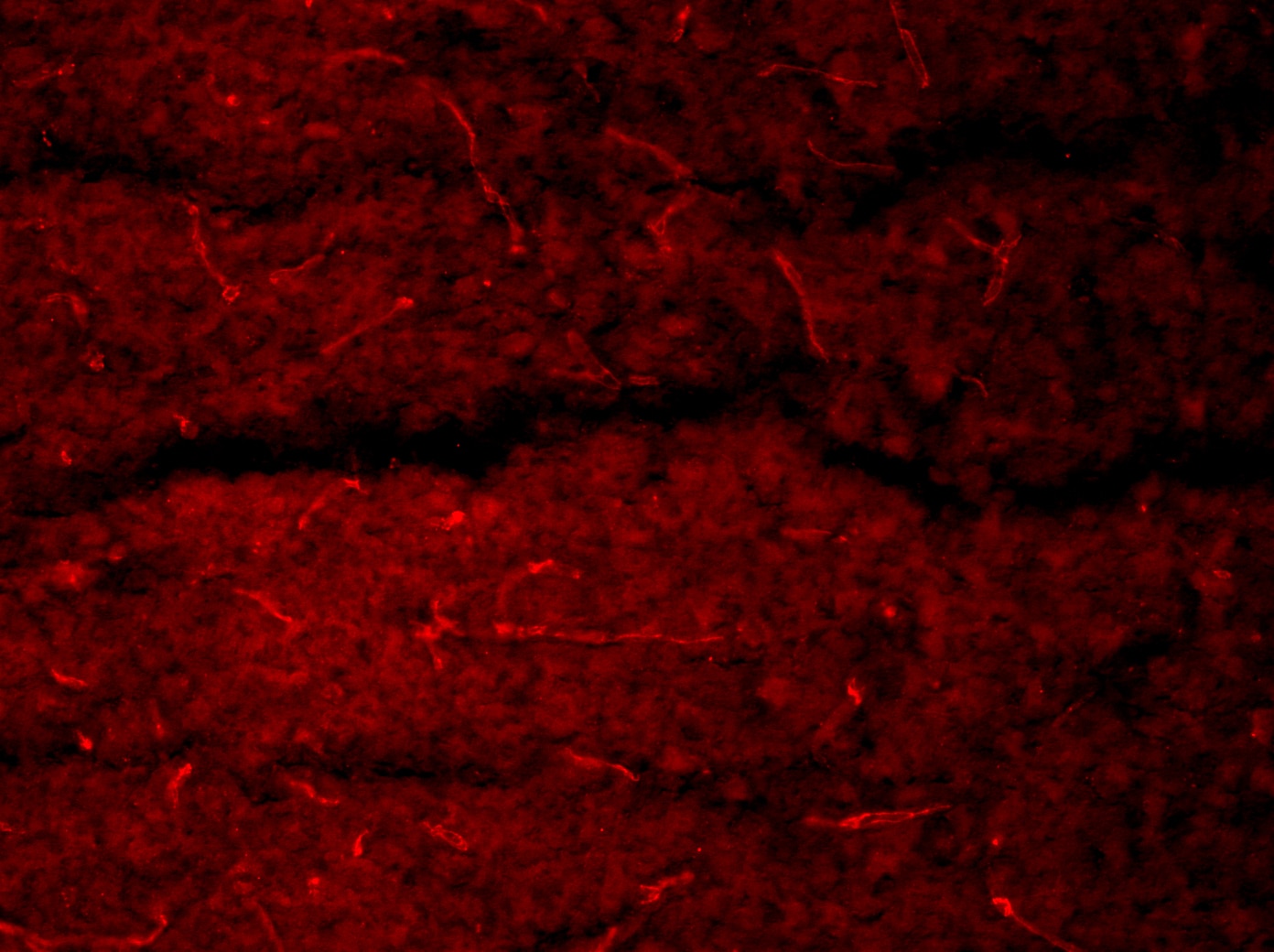
Podocalyxin detection in formalin fixed paraffin embedded sections of mouse brain using Goat Anti-Mouse Podocalyxin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1556) at a dilution of 1:500 overnight at 4 °C. DAPI and Hoechst 33342 were used together for nuclear stain. The podocalyxin staining denoted micro vessels in brain sections for endothelial cell barrier disruption analysis by probing for serum protein deposition proximal to brain microvasculature. This image was taken on a Zeiss LSM 710 Confocal Microscope (META) with Zeiss Plan Apo 63x/1.40 oil
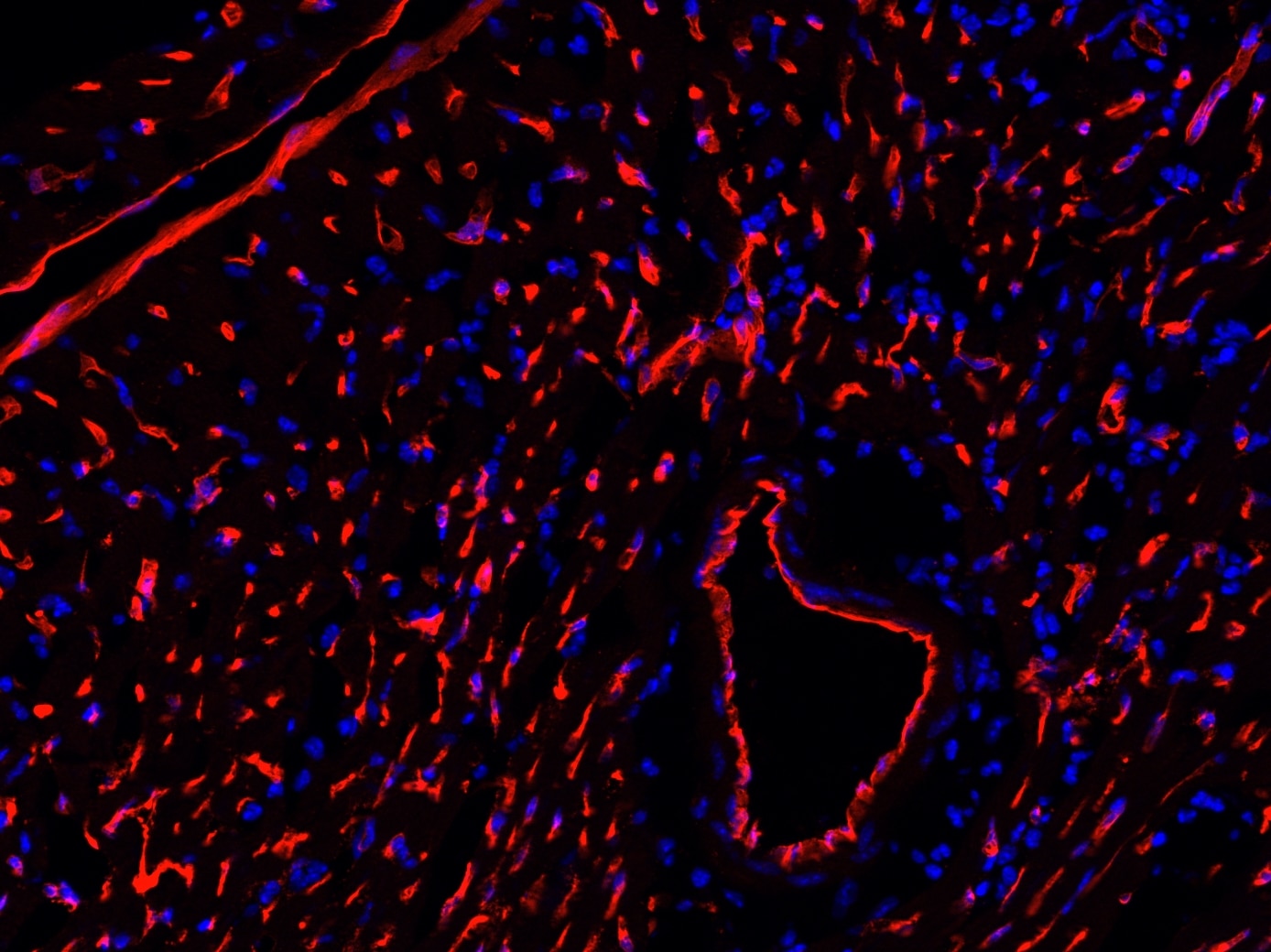
Dilution 1:200
10um think hearts sections. Fixed with 4% PFA at RT for 10mins, permeabilized using 0.3% triton for 30mins at RT followed by 1% BSA block. Incubated with Goat Podocalyxin (1/100 concentration) at 4’C – O/N. Washed using 1xPBS, Detected using Donkey anti-goat alexa 568 (1/200 concentration). Washed and mounted using prolong antifade reagent with DAPI