Recombinant Human NRG1/HRG1-alpha EGF domain Fc Protein, CF
Recombinant Human NRG1/HRG1-alpha EGF domain Fc Protein, CF Summary
- R&D Systems HEK293-derived Recombinant Human NRG1/HRG1-alpha EGF domain Fc Protein (11343-NR)
- Quality control testing to verify active proteins with lot specific assays by in-house scientists
- All R&D Systems proteins are covered with a 100% guarantee
Product Specifications
| Human NRG1/HRG1-alpha EGF domain (Ser177-Pro226) Accession # NP_039254.1 | IEGRMD | Human IgG1 (Pro100-Lys330) |
| N-terminus | C-terminus | |
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
11343-NR
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with Trehalose. |
| Reconstitution | Reconstitute at 500 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
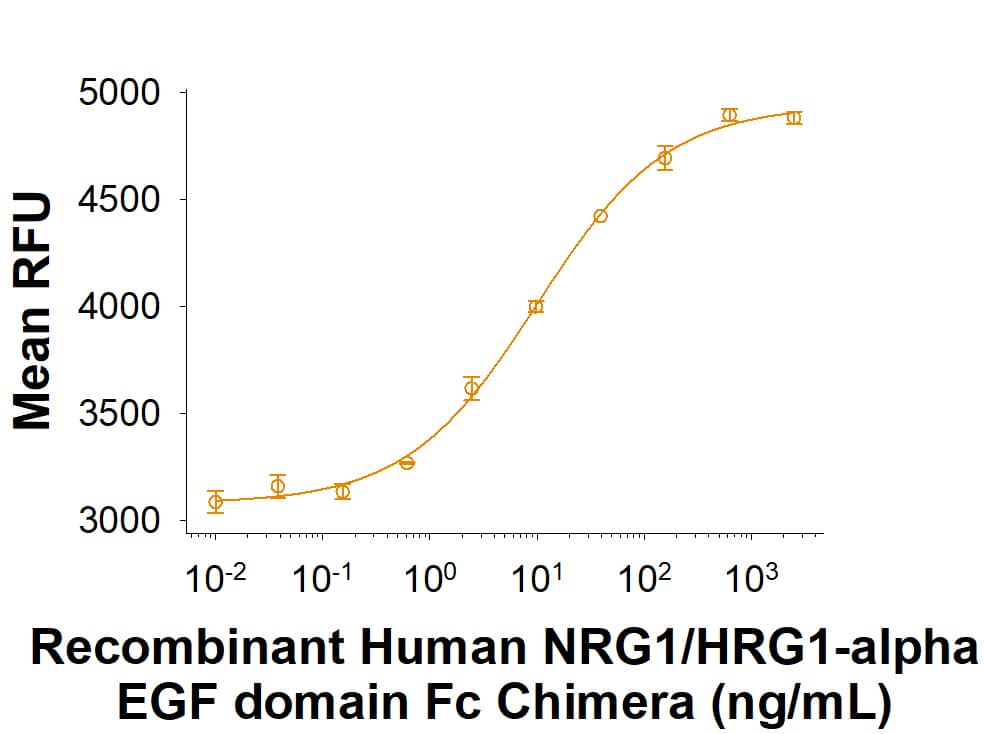 View Larger
View Larger
Recombinant Human NRG1/HRG1-alpha EGF domain Fc Chimera Protein (Catalog # 11343-NR) stimulates proliferation of MCF 7 human breast cancer cells. The ED50 for this effect is 4.00-40.0 ng/mL.
 View Larger
View Larger
2 μg/lane of Recombinant Human NRG1/HRG1-alpha EGF domain Fc Chimera Protein (Catalog # 11343-NR) was resolved with SDS-PAGE under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining, showing bands at 36-43 kDa and 72-86 kDa, respectively.
Reconstitution Calculator
Background: Neuregulin-1/NRG1
Neuregulin-1 (NRG1) belongs to a family of structurally related glycoproteins encoded by four distinct but related genes, Nrg1, Nrg2, Nrg3, and Nrg4. Through alternative splicing or the use of alternative promoters, Nrg1 encodes more than 14 soluble or transmembrane proteins. Type I NRG1 isoforms include Neu Differentiation Factor, Heregulin, and ARIA. These consist of an N-terminal domain, an Ig-like domain, a linker with a Ser/Thr rich region, an EGF-like domain, a transmembrane segment, and a cytoplasmic domain. Type II isoforms such as Glial Growth Factor have a larger N-terminal domain and lack the Ser/Thr rich linker. Type III isoforms such as Sensory and Motor neuron-Derived Factor lack the Ig-like domain but contain a cysteine rich domain (CRD) and a second transmembrane segment (1-5). The alpha and beta splice variants of NRG1 differ in their extracellular juxtamembrane regions (3, 6). This recombinant protein corresponds to the alpha isoforms NRG1 isoforms exhibit distinct expression patterns and functions (7). The EGF-like domain, which is common to all NRG1 isoforms, is required for Neuregulin binding to ErbB3 or ErbB4 receptors (3). ErbB3 or ErbB4 subsequently heterodimerize with ErbB2, resulting in tyrosine phosphorylation and NRG1 induced signaling (1, 2). Soluble growth factors can be released by TACE/ADAM17, BACE, or ADAM19 mediated shedding of the ECD of transmembrane NRG1 (8 - 10). The cytoplasmic region can be cleaved by gamma -secretase, generating a repressor that inhibits the transcription of proapoptotic genes (11). NRG1 regulates multiple nervous system functions including axon guidance, synapse formation and plasticity, glial cell development, and axon myelination (1, 2). In the heart, NRG1 regulates organ morphogenesis and contractility and also plays a cardioprotective role following tissue injury (12). Multiple polymorphisms and aberrant expression of NRG1 isoforms are associated with the development of schizophrenia and many cancers (1, 2, 13). Fusing Fc tag to NRG1 is reported to extend its half life in circulation and improved signal potency (14).
- Mei, L. and W.-C. Xiong (2008) Nat. Rev. Neurosci. 9:437.
- Talmage, D.A. (2008) Novartis Found. Symp. 289:74.
- Holmes, W.E. et al. (1992) Science 256:1205.
- Marchionni, M.A. et al. (1993) Nature 362:312.
- Ho, W.-H. et al. (1995) J. Biol. Chem. 270:14523.
- Wen, D. et al. (1994) Mol. Cell. Biol. 14:1909.
- Meyer, D. et al. (1997) Development 124:3575.
- Hu, X. et al. (2006) Nat. Neurosci. 9:1520.
- Willem, M. et al. (2006) Science 314:664.
- Yokozeki, T. et al. (2007) Genes Cells 12:329.
- Bao, J. et al. (2003) J. Cell Biol. 161:1133.
- Lemmens, K. et al. (2007) Circulation 116:954.
- Breleux, M. (2007) Cell. Mol. Life Sci. 64:2358.
- Zhang, P. et al. (2018) JCI Insight. 3:e98522.
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human NRG1/HRG1-alpha EGF domain Fc Protein, CF
There are currently no reviews for this product. Be the first to review Recombinant Human NRG1/HRG1-alpha EGF domain Fc Protein, CF and earn rewards!
Have you used Recombinant Human NRG1/HRG1-alpha EGF domain Fc Protein, CF?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
