Recombinant Mouse IL-33 Protein Summary
Product Specifications
Ser109-Ile266
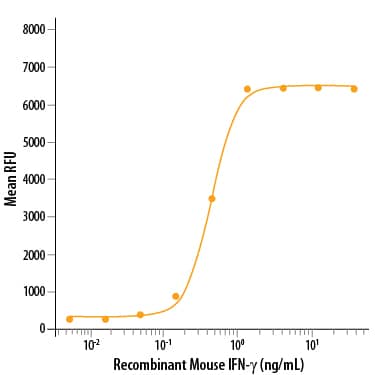
Analysis
Customers also Viewed
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
3626-ML
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS, EDTA and DTT with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
3626-ML/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS, EDTA and DTT. |
| Reconstitution | Reconstitute at 100 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Background: IL-33
IL-33, also known as NF-HEV and DVS 27, is a 30 kDa proinflammatory protein that may also regulate gene transcription (1‑3). DVS 27 was identifed as a gene that is up‑regulated in vasospastic cerebral arteries (1). NF-HEV was described as a nuclear factor that is preferentially expressed in the endothelial cells of high endothelial venules relative to endothelial cells from other tissues (2). IL-33 was identified based on sequence and structural homology with IL-1 family cytokines (3). DVS 27, NF-HEV, and IL-33 share 100% amino acid sequence identity. IL-33 is constitutively expressed in smooth muscle and airway epithelia. It is up‑regulated in arterial smooth muscle, dermal fibroblasts, and keratinocytes following IL-1 alpha or IL‑1 beta stimulation (1, 3). Similar to IL-1, IL-33 can be cleaved in vitro by caspase‑1, generating an N‑terminal fragment that is slightly shorter than the C‑terminal fragment (3, 4). The N‑terminal portion of full length IL-33 contains a predicted bipartite nuclear localization sequence and a homeodomain-like helix-turn-helix DNA binding domain. By immunofluorescence, full length IL-33 localizes to the nucleus in HUVECs and transfectants (2). The C‑terminal fragment, corresponding to mature IL-33, binds and triggers signaling through mast cell IL‑1 R4/ST2L, a longtime orphan receptor involved in the augmentation of Th2 cell responses (3, 5‑7). A ternary signaling complex is formed by the subsequent association of IL-33 and ST2L with IL‑1 RAcP (8). Stimulation of Th2 polarized lymphocytes with mature IL-33 in vitro induces IL-5 and IL-13 secretion (3). In vivo administration of mature IL-33 promotes increased production of IL-5, IL-13, IgE, and IgA, as well as splenomegaly and inflammatory infiltration of mucosal tissues (3). Full length and mature mouse IL-33 share approximately 55% and 90% aa sequence identity with human and rat IL-33, respectively. Mouse IL-33 shares less than 25% aa sequence identity with other IL-1 family proteins.
- Onda, H. et al. (1999) J. Cereb. Blood Flow Metab. 19:1279.
- Baekkevold, E.S. et al. (2003) Am. J. Pathol. 163:69.
- Schmitz, J. et al. (2005) Immunity 23:479.
- Black, R.A. et al. (1989) J. Biol. Chem. 264:5323.
- Xu, D. et al. (1998) J. Exp. Med. 187:787.
- Lohning, M. et al. (1998) Proc. Natl. Acad. Sci. 95:6930.
- Dinarello, C.A. (2005) Immunity 23:461.
- Chackerian, A.A. et al. (2007) J. Immunol. 179:2551.
Citations for Recombinant Mouse IL-33 Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
119
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Polymeric immunoglobulin receptor promotes Th2 immune response in the liver by increasing cholangiocytes derived IL-33: a diagnostic and therapeutic biomarker of biliary atresia
Authors: Li, Y;Li, TY;Qiao, Q;Zhang, MT;Tong, MX;Xu, LF;Zhang, ZB;
EBioMedicine
Species: Mouse
Sample Types:
Applications: In-vivo -
TCF-1 and TOX regulate the memory formation of intestinal group 2 innate lymphoid cells in asthma
Authors: Bao, K;Gu, X;Song, Y;Zhou, Y;Chen, Y;Yu, X;Yuan, W;Shi, L;Zheng, J;Hong, M;
Nature communications
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Cullin5 drives experimental asthma exacerbations by modulating alveolar macrophage antiviral immunity
Authors: Zhang, H;Xue, K;Li, W;Yang, X;Gou, Y;Su, X;Qian, F;Sun, L;
Nature communications
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
JAK3 inhibitor suppresses multipotent ILC2s and attenuates steroid-resistant asthma
Authors: Kim, J;Ham, J;Kang, HR;Bae, YS;Kim, T;Kim, HY;
Science advances
Species: Mouse
Sample Types: In Vivo
Applications: In vivo assay -
Acrylamide, an Air Pollutant, Enhances Allergen-Induced Eosinophilic Lung Inflammation via Group 2 Innate Lymphoid Cells
Authors: Su, HH;Cheng, CM;Yang, YN;Chang, YW;Li, CY;Wu, ST;Lin, CC;Wu, HE;Suen, JL;
Mucosal immunology
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A tissue-intrinsic IL-33/EGF circuit promotes epithelial regeneration after intestinal injury
Authors: Calafiore, M;Fu, YY;Vinci, P;Arnhold, V;Chang, WY;Jansen, SA;Egorova, A;Takashima, S;Kuttiyara, J;Ito, T;Serody, J;Nakae, S;Turnquist, H;van Es, J;Clevers, H;Lindemans, CA;Blazar, BR;Hanash, AM;
Nature communications
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Heat-Labile Enterotoxin Decreases Macrophage Phagocytosis of Enterotoxigenic Escherichia coli
Authors: Hollifield, IE;Motyka, NI;Fernando, KA;Bitoun, JP;
Microorganisms
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Differential and cooperative effects of IL-25 and IL-33 on T helper cells contribute to cryptococcal virulence and brain infection
Authors: Hansakon, A;Jeerawattanawart, S;Angkasekwinai, P;
Scientific reports
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Steady-state memory-phenotype conventional CD4+ T cells exacerbate autoimmune neuroinflammation in a bystander manner via the Bhlhe40/GM-CSF axis
Authors: Cho, MJ;Lee, HG;Yoon, JW;Kim, GR;Koo, JH;Taneja, R;Edelson, BT;Lee, YJ;Choi, JM;
Experimental & molecular medicine
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Maternal gammadelta T�cells shape offspring pulmonary type 2 immunity in a microbiota-dependent manner
Authors: PH Papotto, B Yilmaz, G Pimenta, S Mensurado, C Cunha, GJ Fiala, D Gomes da C, N Gonçalves-, BHK Chan, B Blankenhau, RG Domingues, T Carvalho, MR Hepworth, AJ Macpherson, JE Allen, B Silva-Sant
Cell Reports, 2023-02-07;0(0):112074.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Bioassay, In Vivo -
Crosstalk between ILC2s and Th2 cells varies among mouse models
Authors: RK Gurram, D Wei, Q Yu, MJ Butcher, X Chen, K Cui, G Hu, M Zheng, X Zhu, J Oh, B Sun, JF Urban, K Zhao, WJ Leonard, J Zhu
Cell Reports, 2023-02-02;42(2):112073.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-33 Deficiency Attenuates Lung Inflammation by Inducing Th17 Response and Impacting the Th17/Treg Balance in LPS-Induced ARDS Mice via Dendritic Cells
Authors: L Cheng, Y Jiao, W Jiang, X Zhang, L Zhang, G Jia
Journal of Immunology Research, 2022-12-16;2022(0):9543083.
Species: Mouse, Transgenic Mouse
Sample Types: In Vivo
Applications: In Vivo -
Maturation and specialization of group 2 innate lymphoid cells through the lung-gut axis
Authors: M Zhao, F Shao, D Yu, J Zhang, Z Liu, J Ma, P Xia, S Wang
Nature Communications, 2022-12-09;13(1):7600.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Physiological microbial exposure transiently inhibits mouse lung ILC2 responses to allergens
Authors: KE Block, K Iijima, MJ Pierson, DA Walsh, R Tei, TA Kucaba, J Xu, MH Khan, C Staley, TS Griffith, HJ McSorley, H Kita, SC Jameson
Nature Immunology, 2022-11-21;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
RNA-binding protein RBM3 intrinsically suppresses lung innate lymphoid cell activation and inflammation partially through CysLT1R
Authors: JH Badrani, AN Strohm, L Lacasa, B Civello, K Cavagnero, YA Haung, M Amadeo, LH Naji, SJ Lund, A Leng, H Kim, RE Baum, N Khorram, M Mondal, G Seumois, J Pilotte, PW Vanderklis, HM McGee, TA Doherty
Nature Communications, 2022-07-30;13(1):4435.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
IL-23 plays a significant role in the augmentation of particulate matter-mediated allergic airway inflammation
Authors: HS Lee, HW Park
Journal of Cellular and Molecular Medicine, 2022-07-08;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-7 induces type 2 cytokine response in lung ILC2s and regulates GATA3 and CD25 expression
Authors: A Sheikh, J Lu, E Melese, JH Seo, N Abraham
Journal of leukocyte biology, 2022-05-23;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Stimulation -
Single-cell analysis identifies the interaction of altered renal tubules with basophils orchestrating kidney fibrosis
Authors: T Doke, A Abedini, DL Aldridge, YW Yang, J Park, CM Hernandez, MS Balzer, R Shrestra, G Coppock, JMI Rico, SY Han, J Kim, S Xin, AM Piliponsky, M Angelozzi, V Lefebvre, MC Siracusa, CA Hunter, K Susztak
Nature Immunology, 2022-05-12;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Fasting impairs type 2 helper T cell infiltration in the lung of an eosinophilic asthma mouse model
Authors: Y Suzuki, T Hayashi, R Yokoyama, F Nakagawa, J Inoue, T Higashi, R Onodera, K Motoyama
FEBS Open Bio, 2021-08-20;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Bioassay -
Neuro-mesenchymal units control ILC2 and obesity via a brain-adipose circuit
Authors: F Cardoso, RGJ Klein Wolt, C Godinho-Si, RG Domingues, H Ribeiro, JA da Silva, I Mahú, AI Domingos, H Veiga-Fern
Nature, 2021-08-18;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Bioassay -
Myeloid-IL4Ralpha is an indispensable link in IL-33-ILCs-IL-13-IL4Ralpha axis of eosinophil recruitment in murine lungs
Authors: S Patial, BW Lewis, T Vo, I Choudhary, K Paudel, Y Mao, D Singamsett, F Brombacher, Y Saini
Scientific Reports, 2021-07-29;11(1):15465.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A role for IL-33-activated ILC2s in eosinophilic vasculitis
Authors: ME Kotas, J Dion, S Van Dyken, RR Ricardo-Go, CJ Danel, C Taillé, L Mouthon, RM Locksley, B Terrier
JCI Insight, 2021-06-22;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Bioassay -
Serum free culture for the expansion and study of type 2 innate lymphoid cells
Authors: P de Lucía F, C Sherwood, I Saranchova, W Xia, L Munro, CG Pfeifer, JM Piret, WA Jefferies
Scientific Reports, 2021-06-10;11(1):12233.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Loss of IL-33 enhances elastase-induced and cigarette smoke extract-induced emphysema in mice
Authors: D Morichika, A Taniguchi, N Oda, U Fujii, S Senoo, J Itano, A Kanehiro, Y Kitaguchi, M Yasuo, M Hanaoka, T Satoh, S Akira, K Kiura, Y Maeda, N Miyahara
Respiratory Research, 2021-05-15;22(1):150.
Species: Mouse
Sample Types: In Vivo
Applications: Bioassay -
Exogenous oxidative stress suppresses IL-33 -driven proliferation programming in group 2 innate lymphoid cells
Authors: C Zheng, Z Lu, H Wu, L Cui, J Bi, X Wan
International immunopharmacology, 2021-03-20;95(0):107541.
Species: Mouse
Sample Types: In Vivo
Applications: Bioassay -
Interleukin-33 and thymic stromal lymphopoietin, but not interleukin-25, are crucial for development of airway eosinophilia induced by chitin
Authors: K Arae, M Ikutani, K Horiguchi, S Yamaguchi, Y Okada, H Sugiyama, K Orimo, H Morita, H Suto, K Okumura, H Taguchi, K Matsumoto, H Saito, K Sudo, S Nakae
Scientific Reports, 2021-03-15;11(1):5913.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A gene-environment-induced epigenetic program initiates tumorigenesis
Authors: D Alonso-Cur, YJ Ho, C Burdziak, JLV Maag, JP Morris, R Chandwani, HA Chen, KM Tsanov, FM Barriga, W Luan, N Tasdemir, G Livshits, E Azizi, J Chun, JE Wilkinson, L Mazutis, SD Leach, R Koche, D Pe'er, SW Lowe
Nature, 2021-02-03;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Bioassay -
AMPK induces regulatory innate lymphoid cells after traumatic brain injury
Authors: B Baban, M Braun, H Khodadadi, A Ward, K Alverson, A Malik, K Nguyen, S Nazarian, DC Hess, S Forseen, AF Post, FL Vale, JR Vender, MN Hoda, O Akbari, K Vaibhav, KM Dhandapani
JCI Insight, 2021-01-11;6(1):.
Species: Mouse, Transgenic Mouse
Sample Types: In Vivo
Applications: In Vivo -
Hyperactivity of Innate Immunity Triggers Pain via TLR2-IL-33-Mediated Neuroimmune Crosstalk
Authors: J Huang, MA Gandini, L Chen, S M'Dahoma, PL Stemkowski, H Chung, DA Muruve, GW Zamponi
Cell Rep, 2020-10-06;33(1):108233.
Species: Mouse
Sample Types: In Vivo
Applications: Bioassay -
Hippocampal interleukin-33 mediates neuroinflammation-induced cognitive impairments
Authors: F Reverchon, V de Concini, V Larrigaldi, S Benmerzoug, S Briault, D Togbé, B Ryffel, VFJ Quesniaux, A Menuet
J Neuroinflammation, 2020-09-11;17(1):268.
Species: Mouse
Sample Types: In Vivo
Applications: Bioassay -
Basophils prime group 2 innate lymphoid cells for neuropeptide-mediated inhibition
Authors: JM Inclan-Ric, JJ Ponessa, N Valero-Pac, CM Hernandez, CB Sy, AD Lemenze, AM Beaulieu, MC Siracusa
Nat. Immunol., 2020-08-17;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Inflammatory Type 2 cDCs Acquire Features of cDC1s and Macrophages to Orchestrate Immunity to Respiratory Virus Infection.
Authors: Bosteels C, Neyt K, Vanheerswynghels M, van Helden M, Sichien D, Debeuf N, De Prijck S, Bosteels V, Vandamme N, Martens L, Saeys Y, Louagie E, Lesage M, Williams D, Tang S, Mayer J, Ronchese F, Scott C, Hammad H, Guilliams M, Lambrecht B
Immunity, 2020-05-08;0(0):.
Species: Mouse
Sample Types: Primary Cells
-
Cancer cell-derived interleukin-33 decoy receptor sST2 enhances orthotopic tumor growth in a murine pancreatic cancer model
Authors: K Takenaga, M Akimoto, N Koshikawa, H Nagase
PLoS ONE, 2020-04-27;15(4):e0232230.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Sex-specific adipose tissue imprinting of regulatory T cells
Authors: A Vasanthaku, D Chisanga, J Blume, R Gloury, K Britt, DC Henstridge, Y Zhan, SV Torres, S Liene, N Collins, E Cao, T Sidwell, C Li, RG Spallanzan, Y Liao, PA Beavis, T Gebhardt, N Trevaskis, SL Nutt, JD Zajac, RA Davey, MA Febbraio, D Mathis, W Shi, A Kallies
Nature, 2020-02-26;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Tumor-Derived Lactic Acid Contributes to the Paucity of Intratumoral ILC2s
Authors: M Wagner, KN Ealey, H Tetsu, T Kiniwa, Y Motomura, K Moro, S Koyasu
Cell Rep, 2020-02-25;30(8):2743-2757.e5.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Bioassay, In Vivo -
ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity
Authors: JA Moral, J Leung, LA Rojas, J Ruan, J Zhao, Z Sethna, A Ramnarain, B Gasmi, M Gururajan, D Redmond, G Askan, U Bhanot, E Elyada, Y Park, DA Tuveson, M Gönen, SD Leach, JD Wolchok, RP DeMatteo, T Merghoub, VP Balachandr
Nature, 2020-02-19;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Nuocytes from mesenteric lymph node promote allergic responses in a mouse model
Authors: L Lin, X Tang, Z Chen, J Wei, F Dai, G Sun
Braz J Otorhinolaryngol, 2020-02-12;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Allergic inflammation is initiated by IL-33-dependent crosstalk between mast cells and basophils
Authors: CL Hsu, KD Chhiba, R Krier-Burr, S Hosakoppal, S Berdnikovs, ML Miller, PJ Bryce
PLoS ONE, 2020-01-15;15(1):e0226701.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The Prostaglandin D2 Receptor CRTH2 Promotes IL-33-Induced ILC2 Accumulation in the Lung
Authors: OO Oyesola, C Duque, LC Huang, EM Larson, SP Früh, LM Webb, SA Peng, ED Tait Wojno
J. Immunol., 2020-01-03;204(4):1001-1011.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Allergin-1 Immunoreceptor Suppresses House Dust Mite-Induced Allergic Airway Inflammation
Authors: H Miki, S Tahara-Han, MS Almeida, K Hitomi, S Shibagaki, K Kanemaru, YH Lin, K Iwata, S Miyake, S Shibayama, T Sumida, K Shibuya, A Shibuya
J. Immunol., 2020-01-03;204(4):753-762.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The Putatively Specific Synthetic REV-ERB Agonist SR9009 Inhibits IgE- and IL-33-Mediated Mast Cell Activation Independently of the Circadian Clock
Authors: K Ishimaru, S Nakajima, G Yu, Y Nakamura, A Nakao
Int J Mol Sci, 2019-12-14;20(24):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
ILC2 transfers to apolipoprotein E deficient mice reduce the lipid content of atherosclerotic lesions
Authors: PT Mantani, P Dunér, I Ljungcrant, J Nilsson, H Björkbacka, GN Fredrikson
BMC Immunol., 2019-12-10;20(1):47.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-33 delays recovery of mucosal inflammation via downregulation of homeostatic ABCG5/8 in the colon
Authors: Y Mishima, H Sonoyama, S Ishihara, N Oshima, I Moriyama, K Kawashima, Y Kinoshita
Lab. Invest., 2019-10-22;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Innate Lymphoid Cells in the Induction of Obesity
Authors: T Sasaki, K Moro, T Kubota, N Kubota, T Kato, H Ohno, S Nakae, H Saito, S Koyasu
Cell Rep, 2019-07-02;28(1):202-217.e7.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Th2 cell-derived IL-4/IL-13 promote ILC2 accumulation in the lung by ILC2-intrinsic STAT6 signaling in mice
Authors: C Symowski, D Voehringer
Eur. J. Immunol., 2019-06-05;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Sex differences in IL-17 contribute to chronicity in male versus female urinary tract infection
Authors: A Zychlinsky, M Rousseau, L Lacerda Ma, T Canton, CR Consiglio, ML Albert, M Fontes, D Duffy, MA Ingersoll
JCI Insight, 2019-05-30;5(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Costimulation Induces CD4�T Cell Antitumor Immunity via an Innate-like Mechanism
Authors: C Morales De, JR Maxwell, MM Xu, A Menoret, P Mittal, N Tsurutani, AJ Adler, AT Vella
Cell Rep, 2019-04-30;27(5):1434-1445.e3.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-1 and TNF? Contribute to the Inflammatory Niche to Enhance Alveolar Regeneration
Authors: H Katsura, Y Kobayashi, PR Tata, BLM Hogan
Stem Cell Reports, 2019-03-28;0(0):.
Species: Mouse
Sample Types: Organoids
Applications: Bioassay -
The Tec kinase ITK is essential for ILC2 survival and epithelial integrity in the intestine
Authors: HS Cho, A Reboldi, JA Hall, LJ Berg
Nat Commun, 2019-02-15;10(1):784.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-33 induction and signaling are controlled by glutaredoxin-1 in mouse macrophages
Authors: EO Weinberg, B Ferran, Y Tsukahara, MMS Hatch, J Han, CE Murdoch, R Matsui
PLoS ONE, 2019-01-25;14(1):e0210827.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Innate Sensing through Mesenchymal TLR4/MyD88 Signals Promotes Spontaneous Intestinal Tumorigenesis
Authors: V Koliaraki, N Chalkidi, A Henriques, C Tzaferis, A Polykratis, A Waisman, W Muller, DJ Hackam, M Pasparakis, G Kollias
Cell Rep, 2019-01-15;26(3):536-545.e4.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
L-type calcium channel-mediated Zinc wave is involved in the regulation of IL-6 by stimulating non-IgE with LPS and IL-33 in mast cells and dendritic cells
Authors: R Uchida, H Xiang, H Arai, H Kitamura, K Nishida
Biol. Pharm. Bull., 2018-11-10;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Intestinal helminth infection promotes IL-5- and CD4+ T cell-dependent immunity in the lung against migrating parasites
Authors: KJ Filbey, M Camberis, J Chandler, R Turner, AJ Kettle, RM Eichenberg, P Giacomin, G Le Gros
Mucosal Immunol, 2018-11-06;0(0):.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Bioassay, In Vivo -
IL-33 drives the antitumor effects of dendritic cells via the induction of Tc9 cells
Authors: N Liu, Y Jiang, J Chen, H Nan, Y Zhao, X Chu, A Wang, D Wang, T Qin, S Gao, Q Yi, Y Yue, S Wang
Cell. Mol. Immunol., 2018-10-01;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Tissue signals imprint ILC2 identity with anticipatory function
Authors: RR Ricardo-Go, SJ Van Dyken, C Schneider, J Lee, JC Nussbaum, HE Liang, D Vaka, WL Eckalbar, AB Molofsky, DJ Erle, RM Locksley
Nat. Immunol., 2018-09-10;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Role of IL-18 induced Amphiregulin expression on virus induced ocular lesions
Authors: SK Varanasi, NK Rajasagi, U Jaggi, BT Rouse
Mucosal Immunol, 2018-08-07;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-33/ST2 plays a critical role in endothelial cell activation and microglia-mediated neuroinflammation modulation
Authors: K Cao, X Liao, J Lu, S Yao, F Wu, X Zhu, D Shi, S Wen, L Liu, H Zhou
J Neuroinflammation, 2018-05-04;15(1):136.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-18 Amplifies Macrophage Polarization and Morphological Alteration, Leading to Excessive Angiogenesis
Authors: T Kobori, S Hamasaki, A Kitaura, Y Yamazaki, T Nishinaka, A Niwa, S Nakao, H Wake, S Mori, T Yoshino, M Nishibori, H Takahashi
Front Immunol, 2018-03-06;9(0):334.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Characterization and allergic role of IL-33-induced neutrophil polarization
Authors: B Sun, L Zhu, Y Tao, HX Sun, Y Li, P Wang, Y Hou, Y Zhao, X Zhang, L Zhang, N Na, Y Zhao
Cell. Mol. Immunol., 2018-03-05;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
The effects of IL-33 on airways collagen deposition and matrix metalloproteinase expression in a murine surrogate of asthma
Authors: G An, X Zhang, W Wang, Q Huang, Y Li, S Shan, CJ Corrigan, W Wang, S Ying
Immunology, 2018-02-18;0(0):.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Bioassay, In Vivo -
E3 Ligase VHL Promotes Group 2 Innate Lymphoid Cell Maturation and Function via Glycolysis Inhibition and Induction of Interleukin-33 Receptor
Authors: Q Li, D Li, X Zhang, Q Wan, W Zhang, M Zheng, L Zou, C Elly, JH Lee, YC Liu
Immunity, 2018-02-13;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Airway inflammation after epicutaneous sensitization of mice requires protease activity of low-dose allergen inhalation
Authors: I Nishioka, T Takai, N Maruyama, S Kamijo, P Suchiva, M Suzuki, S Kunimine, H Ochi, S Shimura, K Sudo, H Ogawa, K Okumura, S Ikeda
J. Allergy Clin. Immunol., 2018-02-06;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Cell Culture -
Modulation of the IL-33/IL-13 Axis in Obesity by IL-13R?2
Authors: J Duffen, M Zhang, K Masek-Hamm, A Nunez, A Brennan, JEC Jones, J Morin, K Nocka, M Kasaian
J. Immunol., 2018-01-05;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Macrophages regulate lung ILC2 activation via Pla2g5-dependent mechanisms
Authors: M Yamaguchi, SK Samuchiwal, O Quehenberg, JA Boyce, B Balestrier
Mucosal Immunol, 2017-12-20;11(3):615-626.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Bioassay, In Vivo -
Neuronal regulation of type 2 innate lymphoid cells via neuromedin U
Authors: V Cardoso, J Chesné, H Ribeiro, B García-Cas, T Carvalho, T Bouchery, K Shah, NL Barbosa-Mo, N Harris, H Veiga-Fern
Nature, 2017-09-06;549(7671):277-281.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-33 Signaling Regulates Innate IL-17A and IL-22 Production via Suppression of Prostaglandin E2 during Lung Fungal Infection
Authors: JM Garth, KM Reeder, MS Godwin, JJ Mackel, CW Dunaway, JP Blackburn, C Steele
J. Immunol., 2017-08-07;199(6):2140-2148.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
The Innate Cytokines IL-25, IL-33, and TSLP Cooperate in the Induction of Type 2 Innate Lymphoid Cell Expansion and Mucous Metaplasia in Rhinovirus-Infected Immature Mice
Authors: M Han, C Rajput, JY Hong, J Lei, JL Hinde, Q Wu, JK Bentley, MB Hershenson
J. Immunol., 2017-07-12;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
PD-1 regulates KLRG1(+) group 2 innate lymphoid cells
Authors: S Taylor, Y Huang, G Mallett, C Stathopoul, TC Felizardo, MA Sun, EL Martin, N Zhu, EL Woodward, MS Elias, J Scott, NJ Reynolds, WE Paul, DH Fowler, S Amarnath
J. Exp. Med., 2017-05-10;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
IL-33 receptor ST2 regulates the cognitive impairments associated with experimental cerebral malaria
Authors: F Reverchon, S Mortaud, M Sivoyon, I Maillet, A Laugeray, J Palomo, C Montécot, A Herzine, S Meme, W Meme, F Erard, B Ryffel, A Menuet, VFJ Quesniaux
PLoS Pathog., 2017-04-27;13(4):e1006322.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Disruption of the TWEAK/Fn14 pathway prevents 5-fluorouracil-induced diarrhea in mice
Authors: T Sezaki, Y Hirata, T Hagiwara, YI Kawamura, T Okamura, R Takanashi, K Nakano, M Tamura-Nak, LC Burkly, T Dohi
World J. Gastroenterol., 2017-04-07;23(13):2294-2307.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Prolonged activation of IL-5-producing ILC2 causes pulmonary arterial hypertrophy
Authors: M Ikutani, K Tsuneyama, M Kawaguchi, J Fukuoka, F Kudo, S Nakae, M Arita, Y Nagai, S Takaki, K Takatsu
JCI Insight, 2017-04-06;2(7):e90721.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
NK Cells Alleviate Lung Inflammation by Negatively Regulating Group 2 Innate Lymphoid Cells
Authors: J Bi, L Cui, G Yu, X Yang, Y Chen, X Wan
J. Immunol, 2017-03-08;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Notch Inhibition Enhances Cardiac Reprogramming by Increasing MEF2C Transcriptional Activity
Authors: M Abad, H Hashimoto, H Zhou, MG Morales, B Chen, R Bassel-Dub, EN Olson
Stem Cell Reports, 2017-03-02;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-33 drives hepatic fibrosis through activation of hepatic stellate cells
Authors: Z Tan, Q Liu, R Jiang, L Lv, SS Shoto, I Maillet, V Quesniaux, J Tang, W Zhang, B Sun, B Ryffel
Cell. Mol. Immunol, 2017-02-13;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s
Authors: Jakob von Moltke
J. Exp. Med, 2016-12-23;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Interleukin-33 and RANK-L Interplay in the Alveolar Bone Loss Associated to Periodontitis
PLoS ONE, 2016-12-19;11(12):e0168080.
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
Basophils promote tumor rejection via chemotaxis and infiltration of CD8+ T cells
Cancer Res., 2016-11-22;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A Proinflammatory Role of Type 2 Innate Lymphoid Cells in Murine Immune-Mediated Hepatitis
Authors: Gisa Tiegs
J. Immunol., 2016-11-21;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy
Proc. Natl. Acad. Sci. U.S.A., 2016-11-07;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
In Vivo Expansion of Activated Foxp3+ Regulatory T Cells and Establishment of a Type 2 Immune Response upon IL-33 Treatment Protect against Experimental Arthritis
J Immunol, 2016-07-29;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-33 Contributes to Schistosoma japonicum-induced Hepatic Pathology through Induction of M2 Macrophages
Sci Rep, 2016-07-21;6(0):29844.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence
Nature, 2016-07-13;535(7612):440-3.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Dopaminergic Toxin 1-Methyl-4-Phenylpyridinium, Proteins alpha-Synuclein and Glia Maturation Factor Activate Mast Cells and Release Inflammatory Mediators.
Authors: Kempuraj D, Thangavel R, Yang E, Pattani S, Zaheer S, Santillan D, Santillan M, Zaheer A
PLoS ONE, 2015-08-14;10(8):e0135776.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions.
Authors: Monticelli L, Osborne L, Noti M, Tran S, Zaiss D, Artis D
Proc Natl Acad Sci U S A, 2015-08-04;112(34):10762-7.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Group 2 innate lymphoid cells utilize the IRF4-IL-9 module to coordinate epithelial cell maintenance of lung homeostasis.
Authors: Mohapatra A, Van Dyken S, Schneider C, Nussbaum J, Liang H, Locksley R
Mucosal Immunol, 2015-07-01;9(1):275-86.
Species: Mouse
Sample Types: In Vivo, Tissue Homogenates
Applications: In Vivo, Western Blot -
IL-33 receptor ST2 amplifies the expansion of NK cells and enhances host defense during mouse cytomegalovirus infection.
Authors: Nabekura T, Girard J, Lanier L
J Immunol, 2015-04-29;194(12):5948-52.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Eosinophil-associated ribonuclease 11 is a macrophage chemoattractant.
Authors: Yamada K, Barker T, Dyer K, Rice T, Percopo C, Garcia-Crespo K, Cho S, Lee J, Druey K, Rosenberg H
J Biol Chem, 2015-02-20;290(14):8863-75.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity.
Authors: Brestoff, Jonathan, Kim, Brian S, Saenz, Steven A, Stine, Rachel R, Monticelli, Laurel A, Sonnenberg, Gregory, Thome, Joseph J, Farber, Donna L, Lutfy, Kabirull, Seale, Patrick, Artis, David
Nature, 2014-12-22;519(7542):242-6.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Platelet-derived growth factor primes cancer-associated fibroblasts for apoptosis.
Authors: Rizvi S, Mertens J, Bronk S, Hirsova P, Dai H, Roberts L, Kaufmann S, Gores G
J Biol Chem, 2014-06-27;289(33):22835-49.
Species: Mouse
Sample Types: Whole Organism
Applications: In Vivo -
Airway uric acid is a sensor of inhaled protease allergens and initiates type 2 immune responses in respiratory mucosa.
Authors: Hara K, Iijima K, Elias M, Seno S, Tojima I, Kobayashi T, Kephart G, Kurabayashi M, Kita H
J Immunol, 2014-03-24;192(9):4032-42.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy.
Authors: McSorley H, Blair N, Smith K, McKenzie A, Maizels R
Mucosal Immunol, 2014-02-05;7(5):1068-78.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers.
Authors: Yu, X, Pappu, R, Ramirez-Carrozzi, V, Ota, N, Caplazi, P, Zhang, J, Yan, D, Xu, M, Lee, W P, Grogan, J L
Mucosal Immunol, 2013-11-13;7(3):730-40.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Bioassay, In Vivo -
Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity.
Authors: Palm N, Rosenstein R, Yu S, Schenten D, Florsheim E, Medzhitov R
Immunity, 2013-10-24;39(5):976-85.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells.
Authors: Jovanovic I, Pejnovic N, Radosavljevic G, Pantic J, Milovanovic M, Arsenijevic N, Lukic M
Int J Cancer, 2013-10-10;134(7):1669-82.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells.
Authors: Gao Y, Nish S, Jiang R, Hou L, Licona-Limon P, Weinstein J, Zhao H, Medzhitov R
Immunity, 2013-09-26;39(4):722-32.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Murine mast cells secrete and respond to interleukin-33.
Authors: Tung, Hui-Ying, Plunkett, Beverly, Huang, Shau-Ku, Zhou, Yufeng
J Interferon Cytokine Res, 2013-09-12;34(3):141-7.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-33 and alveolar macrophages contribute to the mechanisms underlying the exacerbation of IgE-mediated airway inflammation and remodelling in mice.
Authors: Mizutani N, Nabe T, Yoshino S
Immunology, 2013-06-01;139(2):205-18.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Macrophages as IL-25/IL-33-responsive cells play an important role in the induction of type 2 immunity.
Authors: Yang Z, Grinchuk V, Urban J, Bohl J, Sun R, Notari L, Yan S, Ramalingam T, Keegan A, Wynn T, Shea-Donohue T, Zhao A
2013-03-25;0(0):.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
IL-33 causes selective mast cell tolerance to bacterial cell wall products by inducing IRAK1 degradation.
Authors: Sandig H, Jobbings C, Roldan N, Whittingham-Dowd J, Orinska Z, Takeuchi O, Akira S, Bulfone-Paus S
Eur J Immunol, 2013-03-06;43(4):979-88.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-33 primes mast cells for activation by IgG immune complexes.
Authors: Kaieda S, Wang J, Shnayder R, Fishgal N, Hei H, Lee R, Stevens R, Nigrovic P
PLoS ONE, 2012-10-11;7(10):e47252.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33.
J. Exp. Med., 2012-07-16;209(8):1505-17.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Regulation of innate CD8+ T-cell activation mediated by cytokines.
Proc Natl Acad Sci U S A, 2012-06-04;109(25):9971-6.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma.
Authors: Wolterink RG, KleinJan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, Hendriks RW
Eur. J. Immunol., 2012-05-01;42(5):1106-16.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues.
Authors: Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, Masopust D
J. Immunol., 2012-04-13;188(10):4866-75.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs.
Authors: Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H
J. Immunol., 2011-12-23;188(3):1503-13.
Species: Mouse
Sample Types: In Vivo, Whole Cells
Applications: Bioassay, In Vivo -
Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus.
Authors: Monticelli LA, Sonnenberg GF, Abt MC
Nat. Immunol., 2011-11-01;12(11):1045-54.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Intravenous gammaglobulin suppresses inflammation through a novel Th2 pathway.
Authors: Anthony RM, Kobayashi T, Wermeling F, Ravetch JV
Nature, 2011-06-19;475(7354):110-3.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
IL-33-activated dendritic cells are critical for allergic airway inflammation.
Authors: Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B
Eur. J. Immunol., 2011-05-25;41(6):1675-86.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation.
Authors: Louten J, Rankin AL, Li Y
Int. Immunol., 2011-03-21;23(5):307-15.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Production and functions of IL-33 in the central nervous system.
Authors: Yasuoka S, Kawanokuchi J, Parajuli B, Jin S, Doi Y, Noda M, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A
Brain Res., 2011-02-22;1385(0):8-17.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
A natural protective function of invariant NKT cells in a mouse model of innate-cell-driven lung inflammation.
Authors: Bourgeois EA, Levescot A, Diem S, Chauvineau A, Berges H, Milpied P, Lehuen A, Damotte D, Gombert JM, Schneider E, Girard JP, Gourdy P, Herbelin A
Eur. J. Immunol., 2011-01-11;41(2):299-305.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus.
Authors: Kayamuro H, Yoshioka Y, Abe Y, Arita S, Katayama K, Nomura T, Yoshikawa T, Kubota-Koketsu R, Ikuta K, Okamoto S, Mori Y, Kunisawa J, Kiyono H, Itoh N, Nagano K, Kamada H, Tsutsumi Y, Tsunoda S
J. Virol., 2010-09-29;84(24):12703-12.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Systemically dispersed innate IL-13-expressing cells in type 2 immunity.
Authors: Price AE, Liang HE, Sullivan BM
Proc. Natl. Acad. Sci. U.S.A., 2010-06-07;107(25):11489-94.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity.
Authors: Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN
Nature, 2010-03-03;464(7293):1367-70.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages.
Authors: Ohno T, Oboki K, Kajiwara N, Morii E, Aozasa K, Flavell RA, Okumura K, Saito H, Nakae S
J. Immunol., 2009-12-15;183(12):7890-7.
Species: Mouse
Sample Types: N/A, Whole Cells
Applications: Bioassay, ELISA (Standard) -
MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity.
Authors: Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, Van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D
Nat. Immunol., 2009-05-24;10(7):697-705.
Species: Mouse
Sample Types: In Vivo
Applications: In Vivo -
The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production.
Authors: Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, Girard JP, Herbelin A
Eur. J. Immunol., 2009-04-01;39(4):1046-55.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis.
Authors: Palmer G, Talabot-Ayer D, Lamacchia C, Toy D, Seemayer CA, Viatte S, Finckh A, Smith DE, Gabay C
Arthritis Rheum., 2009-03-01;60(3):738-49.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Induction of IL-33 expression and activity in central nervous system glia.
Authors: Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT
J. Leukoc. Biol., 2008-06-13;84(3):631-43.
Species: Mouse
Sample Types: N/A, Whole Cells
Applications: Bioassay, ELISA (Standard)
FAQs
-
Has R&D Systems conducted testing to determine the half life or other details, such as administration or dosing, of this protein in an in vivo setting?
We do not perform any in vivo testing, therefore, details relating to half-life, administration, or dosing should be verified within the available literature. Specific references pertaining to this product used in an in vivo setting can be found in the Citations tab on this product page - the results can be filtered by application of interest.
Reviews for Recombinant Mouse IL-33 Protein
Average Rating: 4.7 (Based on 9 Reviews)
Have you used Recombinant Mouse IL-33 Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Reason for Rating: Works as described.
Reason for Rating: Used as a positive control on western blot; able to detect as little as 0.25ug; higher concentrations result in very large, saturated bands
Western blot used to confirm IL-33 knockout in mice; kidney samples from WT, +/-, and -/- IL-33 mice were prepared and blotted
Reason for Rating: Single peak at correct MW observed.