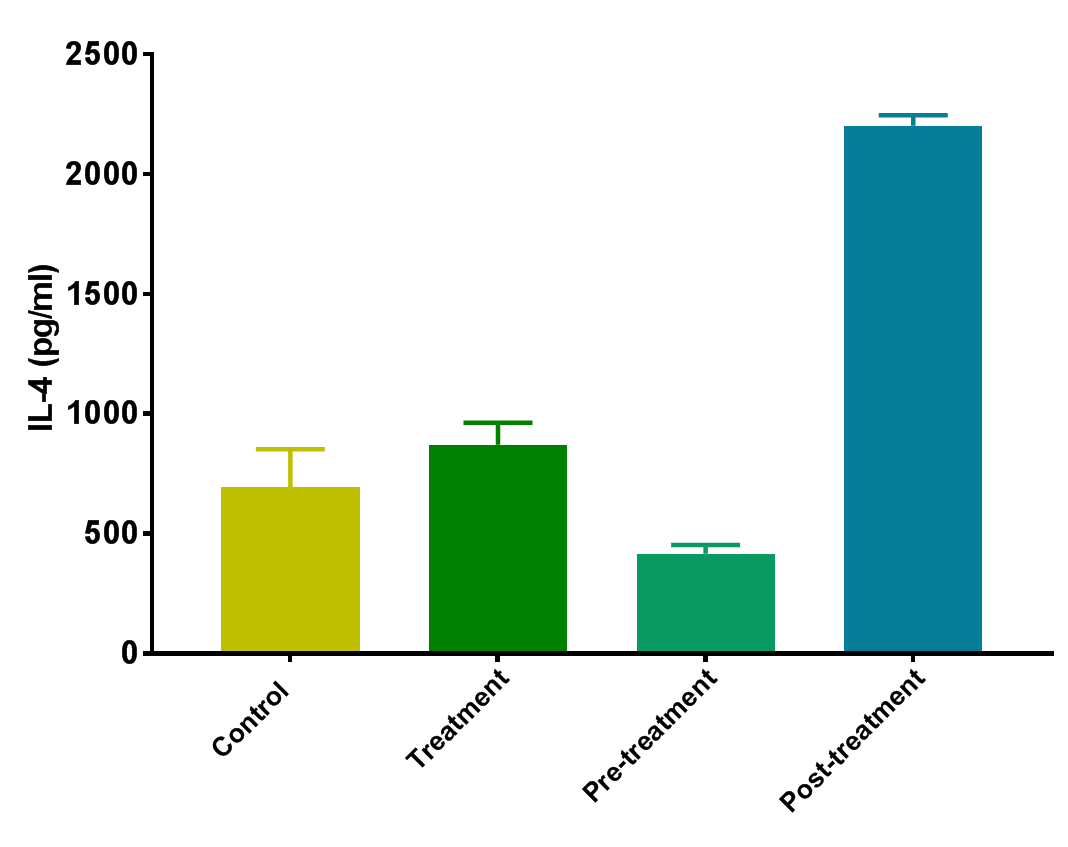Human IL-4 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human IL-4. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Block Buffer: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Reagent Diluent: 0.1% BSA, 0.05% Tween 20 in Tris-buffered Saline (20 mM Trizma base, 150 mM NaCI) pH 7.2-7.4, 0.2 μm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-4
IL-4 (Interleukin-4) is a cytokine that is primarily expressed by Th2-biased CD4+ T cells, mast cells, basophils, and eosinophils. It promotes cell proliferation, survival, and immunoglobulin class switch to IgG4 and IgE in human B cells, acquisition of the Th2 phenotype by naïve CD4+ T cells, priming and chemotaxis of mast cells, eosinophils, and basophils, and the proliferation and activation of epithelial cells. IL-4 plays a dominant role in the development of allergic inflammation and asthma. The type I receptor for IL-4, which is expressed on hematopoietic cells, is a heterodimer of the ligand binding IL-4 R? and the common ? chain (a shared subunit of the receptors for IL-2, -7, -9, -15, and -21). The type II receptor on nonhematopoietic cells consists of IL-4 R? and IL-13 R?1. The type II receptor also transduces IL-13 mediated signals.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL of Block Buffer to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human IL-4 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
48
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Neutrophil extracellular traps promote immunopathogenesis of virus-induced COPD exacerbations
Authors: Katsoulis, O;Toussaint, M;Jackson, MM;Mallia, P;Footitt, J;Mincham, KT;Meyer, GFM;Kebadze, T;Gilmour, A;Long, M;Aswani, AD;Snelgrove, RJ;Johnston, SL;Chalmers, JD;Singanayagam, A;
Nature communications
Species: Human
Sample Types: Sputum
-
Reduced Tolerogenic Program Death-Ligand 1-Expressing Conventional Type 1 Dendritic Cells Are Associated with Rapid Decline in Chronic Obstructive Pulmonary Disease
Authors: Chen, KY;Sun, WL;Wu, SM;Feng, PH;Lin, CF;Chen, TT;Lu, YH;Ho, SC;Chen, YH;Lee, KY;
Cells
Species: Human
Sample Types: Cell Culture Supernates
-
Cognitive function is associated with performance in time up and go test and with leptin blood levels in community-dwelling older women
Authors: da Costa Teixeira, LA;Soares, LA;Lima, LP;Avelar, NCP;de Moura, JA;Leopoldino, AAO;Figueiredo, PHS;Parentoni, AN;Mendonça, VA;Lacerda, ACR;
Scientific reports
Species: Human
Sample Types: Plasma
-
The strong inverse association between plasma concentrations of soluble tumor necrosis factor receptors type 1 with adiponectin/leptin ratio in older women
Authors: Augusto da Costa Teixeira, L;Rocha-Vieira, E;Aparecida Soares, L;Mota de Oliveira, F;Aparecida Oliveira Leopoldino, A;Netto Parentoni, A;Amaral Mendonça, V;Cristina Rodrigues Lacerda, A;
Cytokine
Species: Human
Sample Types: Plasma
-
Suppression of NK Cell Activation by JAK3 Inhibition: Implication in the Treatment of Autoimmune Diseases
Authors: Wu, WC;Shiu, C;Tong, TK;Leung, SO;Hui, CW;
Journal of immunology research
Species: Human
Sample Types: Cell Culture Supernates
-
Neutralizing tumor-related inflammation and reprogramming of cancer-associated fibroblasts by Curcumin in breast cancer therapy
Authors: Jalilian, E;Abolhasani-Zadeh, F;Afgar, A;Samoudi, A;Zeinalynezhad, H;Langroudi, L;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
RNA m6A methylation modulates airway inflammation in allergic asthma via PTX3-dependent macrophage homeostasis
Authors: Han, X;Liu, L;Huang, S;Xiao, W;Gao, Y;Zhou, W;Zhang, C;Zheng, H;Yang, L;Xie, X;Liang, Q;Tu, Z;Yu, H;Fu, J;Wang, L;Zhang, X;Qian, L;Zhou, Y;
Nature communications
Species: Human
Sample Types: Serum, Cell Culture Supernates, Plasma
-
Stratification of Amniotic Fluid Cells and Amniotic Fluid by Sex Opens Up New Perspectives on Fetal Health
Authors: Campesi, I;Capobianco, G;Cano, A;Lodde, V;Cruciani, S;Maioli, M;Sotgiu, G;Idda, ML;Puci, MV;Ruoppolo, M;Costanzo, M;Caterino, M;Cambosu, F;Montella, A;Franconi, F;
Biomedicines
Species: Human
Sample Types: Amniotic Fluid
-
Inflammatory biomarkers at different stages of Sarcopenia in older women
Authors: da Costa Teixeira, LA;Avelar, NCP;Peixoto, MFD;Parentoni, AN;Santos, JMD;Pereira, FSM;Danielewicz, AL;Leopoldino, AAO;Costa, SP;Arrieiro, AN;Soares, LA;da Silva Lage, VK;Prates, ACN;Taiar, R;de Carvalho Bastone, A;Oliveira, VC;Oliveira, MX;Costa, HS;Nobre, JNP;Brant, FP;Duarte, TC;Figueiredo, PHS;Mendonça, VA;Lacerda, ACR;
Scientific reports
Species: Human
Sample Types: Plasma
-
Mild Cognitive Impairment Is Associated with Enhanced Activation of Th17 Lymphocytes in Non-Alcoholic Fatty Liver Disease
Authors: Fiorillo, A;Gallego, JJ;Casanova-Ferrer, F;Giménez-Garzó, C;Urios, A;Ballester, MP;Durbán, L;Rios, MP;Megías, J;San Miguel, T;Kosenko, E;Escudero-García, D;Benlloch, S;Felipo, V;Montoliu, C;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Adiponectin Is a Contributing Factor of Low Appendicular Lean Mass in Older Community-Dwelling Women: A Cross-Sectional Study
Authors: LAC Teixeira, JM Dos Santos, AN Parentoni, LP Lima, TC Duarte, FP Brant, CDC Neves, FSM Pereira, NCP Avelar, AL Danielewic, AAO Leopoldino, SP Costa, AN Arrieiro, LA Soares, ACN Prates, JNP Nobre, A de Carvalh, VC de Oliveir, MX Oliveira, PH Scheidt Fi, HS Costa, V Amaral Men, R Taiar, AC Rodrigues
Journal of Clinical Medicine, 2022-12-02;11(23):.
Species: Human
Sample Types: Plasma
-
T Lymphocyte Serotonin 5-HT7 Receptor Is Dysregulated in Natalizumab-Treated Multiple Sclerosis Patients
Authors: F Reverchon, C Guillard, L Mollet, P Auzou, D Gosset, F Madouri, A Valéry, A Menuet, C Ozsancak, M Pallix-Guy, S Morisset-L
Biomedicines, 2022-09-27;10(10):.
Species: Human
Sample Types: Plasma
-
Cytokine Profile and Anti-Inflammatory Activity of a Standardized Conditioned Medium Obtained by Coculture of Monocytes and Mesenchymal Stromal Cells (PRS CK STORM)
Authors: JP Lapuente, A Blázquez-M, J Marco-Brua, G Gómez, P Desportes, J Sanz, P Fernández, M García-Gil, F Bermejo, JV San Martín, A Algaba, JC De Gregori, D Lapuente, A De Gregori, B Lapuente, MV Andrés, A Anel
Biomolecules, 2022-03-31;12(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Reduced Interleukin-17-Expressing Cells in Cutaneous Melanoma
Authors: A Tosi, L Nardinocch, ML Carbone, L Capriotti, E Pagani, S Mastroeni, C Fortes, F Scopelliti, C Cattani, F Passarelli, A Rosato, S D'Atri, CM Failla, A Cavani
Biomedicines, 2021-12-16;9(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Neutrophils promote T-cell activation through the regulated release of CD44-bound Galectin-9 from the cell surface during HIV infection
Authors: G Dunsmore, EP Rosero, S Shahbaz, DM Santer, J Jovel, P Lacy, S Houston, S Elahi
PloS Biology, 2021-08-19;19(8):e3001387.
Species: Human
Sample Types: Plasma
-
Impact of the Uncoupling Protein 1 on Cardiovascular Risk in Patients with Rheumatoid Arthritis
Authors: LI Lyngfelt, MC Erlandsson, M Nadali, S Hedjazifar, R Pullerits, KM Andersson, P Brembeck, ST Silfverswä, U Smith, MI Bokarewa
Cells, 2021-05-07;10(5):.
Species: Human
Sample Types: Serum
-
New Biomarker in Chagas Disease: Extracellular Vesicles Isolated from Peripheral Blood in Chronic Chagas Disease Patients Modulate the Human Immune Response
Authors: RP Madeira, LM Dal'Mas Ro, P de Cássia, C Mady, BM Ianni, AC Torrecilha
Journal of Immunology Research, 2021-01-11;2021(0):6650670.
Species: Human
Sample Types: Cell Culture Supernates
-
Altered plasma levels of &betaC and &gammaC chain cytokines and post-treatment modulation in tuberculous lymphadenitis
Authors: GR Kathamuthu, K Moideen, R Sridhar, D Baskaran, S Babu
Cytokine, 2020-12-17;138(0):155405.
Species: Human
Sample Types: Plasma
-
Monocytes differentiated into macrophages and dendritic cells in the presence of human IFN-&lambda3 or IFN-&lambda4 show distinct phenotypes
Authors: M De, A Bhushan, S Chinnaswam
J Leukoc Biol, 2020-11-17;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating growth/differentiation factor 15 is associated with human CD56bright natural killer cell dysfunction and nosocomial infection in severe systemic inflammation
Authors: H Kleinertz, M Hepner-Sch, S Ehnert, M Claus, R Halbgebaue, L Boller, M Huber-Lang, P Cinelli, C Kirschning, S Flohé, A Sander, C Waydhas, S Vonderhage, M Jäger, M Dudda, C Watzl, SB Flohé
EBioMedicine, 2019-04-13;0(0):.
Species: Human
Sample Types: Serum
-
In Vitro Induction of T Helper 17 Cells by Synergistic Activation of Human Monocyte-Derived Langerhans Cell-Like Cells with Bacterial Agonists
Authors: R Gramlich, E Aliahmadi, M Peiser
Int J Mol Sci, 2019-03-19;20(6):.
Species: Human
Sample Types: Cell Culture Supernates
-
Large-scale secretome analyses unveil the superior immunosuppressive phenotype of umbilical cord stromal cells as compared to other adult mesenchymal stromal cells
Authors: A Islam, I Urbarova, JA Bruun, I Martinez-Z
Eur Cell Mater, 2019-02-20;37(0):153-174.
Species: Human
Sample Types: Cell Culture Supernates
-
Annexin A1, FPR2/ALX, and inflammatory cytokine expression in peritoneal endometriosis
Authors: LK Volpato, VV Horewicz, F Bobinski, DF Martins, AP Piovezan
J. Reprod. Immunol., 2018-08-03;129(0):30-35.
Species: Human
Sample Types: Peritoneal Fluid
-
An Autocrine Cytokine/JAK/STAT-Signaling Induces Kynurenine Synthesis in Multidrug Resistant Human Cancer Cells.
Authors: Campia I, Buondonno I, Castella B, Rolando B, Kopecka J, Gazzano E, Ghigo D, Riganti C
PLoS ONE, 2015-05-08;10(5):e0126159.
Species: Human
Sample Types: Cell Culture Supernates
-
Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model.
Authors: Guirado E, Mbawuike U, Keiser T, Arcos J, Azad A, Wang S, Schlesinger L
MBio, 2015-02-17;6(1):e02537-14.
Species: Human
Sample Types: Cell Culture Supernates
-
Human CD4+ T cell responses to the dog major allergen Can f 1 and its human homologue tear lipocalin resemble each other.
Authors: Liukko A, Kinnunen T, Rytkonen-Nissinen M, Kailaanmaki A, Randell J, Maillere B, Virtanen T
PLoS ONE, 2014-05-29;9(5):e98461.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated adaptive immune responses are associated with latent infections of Wuchereria bancrofti.
Authors: Arndts K, Deininger S, Specht S, Klarmann U, Mand S, Adjobimey T, Debrah AY, Batsa L, Kwarteng A, Epp C, Taylor M, Adjei O, Layland LE, Hoerauf A
PLoS Negl Trop Dis, 2012-04-03;6(4):e1611.
Species: Human
Sample Types: Cell Culture Supernates
-
Possible involvement of IL-21 and IL-10 on salivary IgA levels in chronic periodontitis subjects.
Authors: Napimoga MH, Nunes LH, Maciel AA, Demasi AP, Benatti BB, Santos VR, Bastos MF, de Miranda TS, Duarte PM
Scand. J. Immunol., 2011-12-01;74(6):596-602.
Species: Human
Sample Types: Tissue Homogenates
-
IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes.
Authors: Pennino D, Eyerich K, Scarponi C, Carbone T, Eyerich S, Nasorri F, Garcovich S, Traidl-Hoffmann C, Albanesi C, Cavani A
J. Immunol., 2010-03-31;184(9):4880-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunoregulation of autocrine prolactin: suppressing the expression of costimulatory molecules and cytokines in T lymphocytes by prolactin receptor knockdown.
Authors: Xu D, Lin L, Lin X
Cell. Immunol., 2010-03-02;263(1):71-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Interferon-beta treatment in multiple sclerosis attenuates inflammatory gene expression through inducible activity of the phosphatase SHP-1.
Authors: Christophi GP, Panos M, Hudson CA, Tsikkou C, Mihai C, Mejico LJ, Jubelt B, Massa PT
Clin. Immunol., 2009-06-25;133(1):27-44.
Species: Human
Sample Types: Plasma
-
TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23.
Authors: Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M
Eur. J. Immunol., 2009-05-01;39(5):1221-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Der p 1 suppresses indoleamine 2, 3-dioxygenase in dendritic cells from house dust mite-sensitive patients with asthma.
Authors: Maneechotesuwan K, Wamanuttajinda V, Kasetsinsombat K, Huabprasert S, Yaikwawong M, Barnes PJ, Wongkajornsilp A
J. Allergy Clin. Immunol., 2008-12-05;123(1):239-48.
Species: Human
Sample Types: Cell Culture Supernates
-
Helminth infections associated with multiple sclerosis induce regulatory B cells.
Authors: Correale J, Farez M, Razzitte G
Ann. Neurol., 2008-08-01;64(2):187-99.
Species: Human
Sample Types: Cell Culture Supernates
-
Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12.
Authors: Lamhamedi-Cherradi SE, Martin RE, Ito T, Kheradmand F, Corry DB, Liu YJ, Moyle M
J. Immunol., 2008-05-01;180(9):6000-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling.
Authors: Weindl G, Naglik JR, Kaesler S, Biedermann T, Hube B, Korting HC, Schaller M
J. Clin. Invest., 2007-12-01;117(12):3664-3672.
Species: Human
Sample Types: Cell Culture Supernates
-
Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells.
Authors: Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, Descamps P, Gamelin E, Gascan H, Hebbar M, Jeannin P
Blood, 2007-09-11;110(13):4319-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Neuroimmune crosstalk in asthma: dual role of the neurotrophin receptor p75NTR.
Authors: Nassenstein C, Kammertoens T, Veres TZ, Uckert W, Spies E, Fuchs B, Krug N, Braun A
J. Allergy Clin. Immunol., 2007-08-22;120(5):1089-96.
Species: Mouse
Sample Types: BALF
-
Respiratory syncytial virus synergizes with Th2 cytokines to induce optimal levels of TARC/CCL17.
Authors: Monick MM, Powers LS, Hassan I, Groskreutz D, Yarovinsky TO, Barrett CW, Castilow EM, Tifrea D, Varga SM, Hunninghake GW
J. Immunol., 2007-08-01;179(3):1648-58.
Species: Human
Sample Types: Cell Culture Supernates
-
An investigation of auto-reactivity after head injury.
Authors: Cox AL, Coles AJ, Nortje J, Bradley PG, Chatfield DA, Thompson SJ, Menon DK
J. Neuroimmunol., 2006-03-06;174(1):180-6.
Species: Human
Sample Types: Cell Culture Supernates
-
Mast cells regulate procollagen I (alpha 1) production by bronchial fibroblasts derived from subjects with asthma through IL-4/IL-4 delta 2 ratio.
Authors: Plante S, Semlali A, Joubert P, Bissonnette E, Laviolette M, Hamid Q, Chakir J
J. Allergy Clin. Immunol., 2006-02-21;117(6):1321-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Manipulation of host cytokine network by ticks: a potential gateway for pathogen transmission.
Authors: Hajnicka V, Vancova I, Kocakova P, Slovak M, Gasperik J, Slavikova M, Hails RS, Labuda M, Nuttall PA
Parasitology, 2005-03-01;130(0):333-42.
Species: Human
Sample Types: Saliva
-
Microbial exposure, symptoms and inflammatory mediators in nasal lavage fluid of kitchen and clerical personnel in schools.
Authors: Lignell U, Meklin T, Putus T, Vepsalainen A, Roponen M, Torvinen E, Reeslev M, Pennanen S, Hirvonen MR, Kalliokoski P, Nevalainen A
Int J Occup Med Environ Health, 2005-01-01;18(2):139-50.
Species: Human
Sample Types: Nasal Lavage Fluid
-
The Toll-like receptor-2/6 agonist macrophage-activating lipopeptide-2 cooperates with IFN-gamma to reverse the Th2 skew in an in vitro allergy model.
Authors: Weigt H, Muhlradt PF, Larbig M, Krug N, Braun A
J. Immunol., 2004-05-15;172(10):6080-6.
Species: Human
Sample Types: Cell Culture Supernates
-
Immune responses in normal Indian langur monkeys (Presbytis entellus)--a primate model for visceral leishmaniasis.
Authors: Misra A, Dube A, Naik S
J. Med. Primatol., 2004-04-01;33(2):65-9.
Species: Primate - Presbytis entellus (Indian langur)
Sample Types: Cell Culture Supernates
-
Comparison of inflammatory elements in nasal lavage and induced sputum following occupational exposure to moldy-building microbes.
Authors: Purokivi M, Hirvonen MR, Roponen M, Randell J, Vahteristo M, Tukiainen H
Inhal Toxicol, 2002-06-01;14(6):653-62.
Species: Human
Sample Types: Sputum
-
Inflammatory mediators in nasal lavage, induced sputum and serum of employees with rheumatic and respiratory disorders.
Authors: Roponen M, Kiviranta J, Seuri M, Tukiainen H, Myllykangas-Luosujarvi R, Hirvonen MR
Eur. Respir. J., 2001-09-01;18(3):542-8.
Species: Human
Sample Types: Serum
-
Reproducibility of measurements of exhaled NO, and cell count and cytokine concentrations in induced sputum.
Authors: Purokivi M, Randell J, Hirvonen MR, Tukiainen H
Eur. Respir. J., 2000-08-01;16(2):242-6.
Species: Human
Sample Types: Sputum
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human IL-4 DuoSet ELISA
Average Rating: 4.8 (Based on 6 Reviews)
Have you used Human IL-4 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: