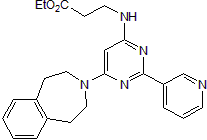
GSK J5
Chemical Name: N-[2-(3-Pyridinyl)-6-(1,2,4,5-tetrahydro-3H-3-benzazepin-3-yl)-4-pyrimidinyl]-β-alanine ethyl ester
Purity: ≥98%
Biological Activity
Inactive isomer of GSK J4; also cell permeable ester derivative of the inactive control, GSK J2.Active Analog also available.
Technical Data
The technical data provided above is for guidance only.
For batch specific data refer to the Certificate of Analysis.
Tocris products are intended for laboratory research use only, unless stated otherwise.
Additional Information
Background References
-
A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response.
Kruidenier et al.
Nature, 2012;488:404
Product Datasheets
Reconstitution Calculator
Molarity Calculator
Citations for GSK J5
The citations listed below are publications that use Tocris products. Selected citations for GSK J5 include:
2 Citations: Showing 1 - 2
-
Jumonji Inhibitors Overcome Radioresistance in Cancer through Changes in H3K4 Methylation at Double-Strand Breaks.
Authors: Bayo Et al.
Cell Rep 2018;25:1040
-
Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells.
Authors: Carey Et al.
Int J Nanomedicine 2015;518:413
FAQs
No product specific FAQs exist for this product, however you may
View all Small Molecule FAQsReviews for GSK J5
There are currently no reviews for this product. Be the first to review GSK J5 and earn rewards!
Have you used GSK J5?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image