Human C-Reactive Protein/CRP DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human C-Reactive Protein/CRP. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
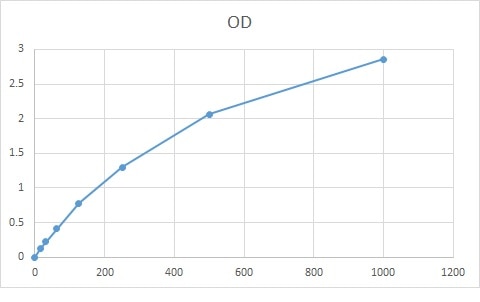
Scientific Data
Product Datasheets
Preparation and Storage
Background: C-Reactive Protein/CRP
C-Reactive Protein (CRP), also known as Pentraxin 1, is a non-glycosylated protein in the Pentraxin family that also includes Pentraxin 2/SAP and Pentraxin 3/TSG-14. CRP functions as a sensor and activator of the innate immune response (1). In humans, it is a major acute-phase protein; its circulating concentration is dramatically elevated at the onset of inflammation (2). In mice, however, serum CRP levels increase only slightly during inflammation, and the analogous acute phase role is filled by Pentraxin 2 (3). CRP assembles non-covalently into a 110-120 kDa cyclical pentamer (4). Mature human CRP shares 71% and 64% amino acid (aa) sequence identity with mouse and rat CRP, respectively (5).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human C-Reactive Protein/CRP DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
75
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Age-Associated Inflammatory Monocytes Are Increased in Menopausal Females and Reversed by Hormone Replacement Therapy
Authors: De Maeyer, RPH;Sikora, J;Bracken, OV;Shih, B;Lloyd, AF;Peckham, H;Hollett, K;Abdelhamid, K;Cai, W;James, M;Pfeffer, PE;Vukmanovic-Stejic, M;Akbar, AN;Chambers, ES;
Aging cell
Species: Human
Sample Types: Serum
-
Complement activation assessed by C3bc and C5b-9 terminal complex as diagnostic biomarkers for deep vein thrombosis
Authors: Wikan, VE;Øverli, Ø;Ueland, T;Mollnes, TE;Brækkan, SK;Michelsen, AE;Yeganeh, GJ;Hansen, JB;Brodin, EE;
PloS one
Species: Human
Sample Types: Plasma
-
BCG-Induced DNA Methylation Changes Improve Coronavirus Disease 2019 Vaccine Immunity Without Decreasing the Risk for Severe Acute Respiratory Syndrome Coronavirus 2 Infection
Authors: Longlax, S;Koster, K;Kamat, A;Lozano, M;Lerner, S;Hannigan, R;Nishiguchi, T;Abhimanyu, A;Sheikh, D;Ladki, M;Portillo, A;Koirala, A;Patel, T;Spieler, Z;Benjamin, A;Lebedev, M;Ofili, T;Hutchison, R;Udeani, G;Opperman, L;Neal, G;Mandalakas, A;Netea, M;Arditi, M;Avalos, P;Grimm, S;Coarfa, C;Cirillo, J;DiNardo, A;
Open forum infectious diseases
Species: Human
Sample Types: Serum
-
Therapeutic Potential of a Natural Blend of Aronia melancarpa, Lonicera caerulea, and Echinacea purpurea Extracts in Treating Upper Respiratory Tract Infections: Preliminary Clinical and In Vitro Immunomodulatory Insights
Authors: Zima, K;Sochocka, M;Ochnik, M;Khaidakov, B;Lemke, K;Kowalczyk, P;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
Commonly Used Dose of Montmorency Tart Cherry Powder Does Not Improve Sleep or Inflammation Outcomes in Individuals with Overweight or Obesity
Authors: Tucker, RM;Kim, N;Gurzell, E;Mathi, S;Chavva, S;Senthilkumar, D;Bartunek, O;Fenton, KC;Herndon-Fenton, SJ;Cardino, VN;Cooney, GM;Young, S;Fenton, JI;
Nutrients
Species: Human
Sample Types: Serum
-
Gastrointestinal complaints after Roux-en-Y gastric bypass surgery. Impact of microbiota and its metabolites
Authors: Custers, E;van der Burgh, YGR;Vreeken, D;Schuren, F;van den Broek, TJ;Verschuren, L;de Blaauw, I;Bouwens, M;Kleemann, R;Kiliaan, AJ;Hazebroek, EJ;
Heliyon
Species: Human
Sample Types: Plasma
-
Metabolic dysfunction-associated steatotic liver disease is associated with effects on cerebral perfusion and white matter integrity
Authors: Seidel, F;Vreeken, D;Custers, E;Wiesmann, M;Özsezen, S;van Duyvenvoorde, W;Caspers, M;Menke, A;Morrison, MC;Verschuren, L;Duering, M;Hazebroek, EJ;Kiliaan, AJ;Kleemann, R;
Heliyon
Species: Human
Sample Types: Plasma
-
Rapid Microfluidic Immuno-Biosensor Detection System for the Point-of-Care Determination of High-Sensitivity Urinary C-Reactive Protein
Authors: Chen, SJ;Lu, SY;Tseng, CC;Huang, KH;Chen, TL;Fu, LM;
Biosensors
Species: N/A
Sample Types: Urine
-
Highly secreted tryptophanyl tRNA synthetase 1 as a potential theranostic target for hypercytokinemic severe sepsis
Authors: Kim, YT;Huh, JW;Choi, YH;Yoon, HK;Nguyen, TT;Chun, E;Jeong, G;Park, S;Ahn, S;Lee, WK;Noh, YW;Lee, KS;Ahn, HS;Lee, C;Lee, SM;Kim, KS;Suh, GJ;Jeon, K;Kim, S;Jin, M;
EMBO molecular medicine
Species: Human
Sample Types: Plasma
-
Disrupted balance between pro-inflammatory lipid mediators and anti-inflammatory specialized pro-resolving mediators is linked to hyperinflammation in patients with alcoholic hepatitis
Authors: Li, W;Xia, Y;Yang, J;Sanyal, AJ;Shah, VH;Chalasani, NP;Yu, Q;
medRxiv : the preprint server for health sciences
Species: Human
Sample Types: Plasma
-
Inflammation and autoimmunity are interrelated in patients with sickle cell disease at a steady-state condition: implications for vaso-occlusive crisis, pain, and sensory sensitivity
Authors: Li, W;Pucka, AQ;Debats, C;Reyes, B;Syed, F;O'Brien, AR;Mehta, R;Manchanda, N;Jacob, SA;Hardesty, BM;Greist, A;Harte, SE;Harris, RE;Yu, Q;Wang, Y;
medRxiv : the preprint server for health sciences
Species: Human
Sample Types: Plasma
-
Changes in the Proteome of Platelets from Patients with Critical Progression of COVID-19
Authors: Wolny, M;Rozanova, S;Knabbe, C;Pfeiffer, K;Barkovits, K;Marcus, K;Birschmann, I;
Cells
Species: Human
Sample Types: Cell Lysates, Plasma
-
Haemoglobin diagnostic cut-offs for anaemia in Indian women of reproductive age
Authors: Ghosh, S;Palika, R;Dasi, T;Varshney, RK;Parasannanavar, DJ;Sen Gupta, S;Chitikineni, A;Banjara, SK;Pullakhandam, R;Thomas, T;Sachdev, HS;Kurpad, AV;Kulkarni, B;
European journal of clinical nutrition
Species: Human
Sample Types: Serum
-
Impact of airway challenges on cardiovascular risk in asthma - a randomized controlled trial
Authors: Moore, LE;Brotto, AR;Fuhr, DP;Rosychuk, RJ;Wong, E;Bhutani, M;Stickland, MK;
PloS one
Species: Human
Sample Types: Serum
-
Pre-pregnancy obesity is associated with greater systemic inflammation and increased risk of antenatal depression
Authors: Sominsky, L;O'Hely, M;Drummond, K;Cao, S;Collier, F;Dhar, P;Loughman, A;Dawson, S;Tang, ML;Mansell, T;Saffery, R;Burgner, D;Ponsonby, AL;Vuillermin, P;Barwon Infant Study Investigator Group, ;
Brain, behavior, and immunity
Species: Human
Sample Types: Serum
-
Central and Peripheral Inflammation in Mild Cognitive Impairment in the Context of Alzheimer's Disease
Authors: Schmidt-Morgenroth, I;Michaud, P;Gasparini, F;Avrameas, A;
International journal of molecular sciences
Species: Human
Sample Types: CSF, Serum
-
Omega-3 polyunsaturated fatty acids (n-3 PUFAs), somatic and fatigue symptoms in cardiovascular diseases comorbid major depressive disorder (MDD): A randomized controlled trial
Authors: Chang, JP;Chang, SS;Chen, HT;Chien, YC;Yang, HT;Huang, SY;Tseng, PT;Chang, CH;Galecki, P;Su, KP;
Brain, behavior, and immunity
Species: Human
Sample Types: Plasma
-
Efficacy of Cooling Centers for Mitigating Physiological Strain in Older Adults during Daylong Heat Exposure: A Laboratory-Based Heat Wave Simulation
Authors: Meade, RD;Notley, SR;Akerman, AP;McCormick, JJ;King, KE;Sigal, RJ;Kenny, GP;
Environmental health perspectives
Species: Human
Sample Types: Serum
-
Increased plasma lipopolysaccharide-binding protein and altered inflammatory mediators in overweight women suggest a state of subclinical endotoxemia
Authors: Metz, CN;Xue, X;Chatterjee, PK;Adelson, R;Brines, M;Tracey, KJ;Gregersen, PK;Pavlov, VA;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Plasma
-
Prognostic accuracy of biomarkers of immune and endothelial activation in Mozambican children hospitalized with pneumonia
Authors: N Balanza, C Erice, M Ngai, CR McDonald, AM Weckman, J Wright, M Richard-Gr, R Varo, E López-Vare, A Sitoe, P Vitorino, J Bramugy, M Lanaspa, S Acácio, L Madrid, B Baro, KC Kain, Q Bassat
PLOS global public health, 2023-02-23;3(2):e0001553.
Species: Human
Sample Types: Plasma
-
Sugar-sweetened beverages exacerbate high-fat diet-induced inflammatory bowel disease by altering the gut microbiome: Sweetened beverages enhance fat-induced IBD
Authors: WJ Shon, MH Jung, Y Kim, GH Kang, EY Choi, DM Shin
The Journal of nutritional biochemistry, 2022-12-24;0(0):109254.
Species: Human
Sample Types: Serum
-
NLRP3-Inflammasome Inhibition with IZD334 Does Not Reduce Cardiac Damage in a Pig Model of Myocardial Infarction
Authors: MJM Silvis, EJ Demkes, L Timmers, F Arslan, SCA de Jager, JPG Sluijter, A Mosterd, DPV de Kleijn, L Bosch, GPJ van Hout
Biomedicines, 2022-11-28;10(12):.
Species: Porcine
Sample Types: Plasma
-
High levels of soluble RAGE are associated with a greater risk of mortality in COVID-19 patients treated with dexamethasone
Authors: L Butcher, JC Zaldua, JA Carnicero, K Hawkins, J Whitley, R Mothukuri, PA Evans, K Morris, S Pillai, JD Erusalimsk
Respiratory Research, 2022-11-05;23(1):303.
Species: Human
Sample Types: Serum
-
Intestinal Injury Biomarkers Predict Mortality in Pediatric Severe Malaria
Authors: ML Sarangam, R Namazzi, D Datta, C Bond, CPB Vanderpool, RO Opoka, CC John, AL Conroy
MBio, 2022-09-07;0(0):e0132522.
Species: Human
Sample Types: Serum
-
Association between Leptin (G2548A) and Leptin Receptor (Q223R) Polymorphisms with Plasma Leptin, BMI, Stress, Sleep and Eating Patterns among the Multiethnic Young Malaysian Adult Population from a Healthcare University
Authors: J Mohanraj, UJA D'Souza, SY Fong, IR Karkada, H Jaiprakash
Oncogene, 2022-07-21;19(14):.
Species: Human
Sample Types: Plasma
-
Impact of Zinc to Copper Ratio and Lipocalin 2 in Obese Patients Undergoing Sleeve Gastrectomy
Authors: HM Demerdash, AA Sabry, OE Arida
Oncogene, 2022-06-10;2022(0):9278531.
Species: Human
Sample Types: Serum
-
Infant inflammation predicts childhood emotional and behavioral problems and partially mediates socioeconomic disadvantage
Authors: C Pham, S Bekkering, M O'Hely, D Burgner, S Thomson, P Vuillermin, F Collier, W Marx, T Mansell, C Symeonides, PD Sly, M Lk Tang, R Saffery, AL Ponsonby
Brain, Behavior, and Immunity, 2022-05-23;0(0):.
Species: Human
Sample Types: Serum
-
Early life infection and proinflammatory, atherogenic metabolomic and lipidomic profiles in infancy: a population-based cohort study
Authors: T Mansell, R Saffery, S Burugupall, AL Ponsonby, MLK Tang, M O'Hely, S Bekkering, AAT Smith, R Rowland, S Ranganatha, PD Sly, P Vuillermin, F Collier, P Meikle, D Burgner, Barwon Inf
Elife, 2022-05-10;11(0):.
Species: Human
Sample Types: Plasma
-
Efficacy of iron-folic acid treatment for reducing anemia prevalence and improving iron status in women of reproductive age: A one-year longitudinal study
Authors: R Palika, T Dasi, S Ghosh, R Peter, DJ Parasannan, AS Pradhan, AV Kurpad, HS Sachdev, B Kulkarni, R Pullakhand
Clinical Nutrition ESPEN, 2022-03-23;49(0):390-397.
Species: Human
Sample Types: Plasma
-
The effect of transdermal gender-affirming hormone therapy on markers of inflammation and hemostasis
Authors: MH Schutte, R Kleemann, NM Nota, CM Wiepjes, JM Snabel, G T'Sjoen, A Thijs, M den Heijer
PLoS ONE, 2022-03-15;17(3):e0261312.
Species: Human
Sample Types: Plasma
-
Circulating Interleukin-6 and CD16 positive monocytes increase following angioplasty of an arteriovenous fistula
Authors: S Hakki, EJ Robinson, MG Robson
Scientific Reports, 2022-01-26;12(1):1427.
Species: Human
Sample Types: Plasma
-
Clinical sign and biomarker-based algorithm to identify bacterial pneumonia among outpatients with lower respiratory tract infection in Tanzania
Authors: SKL Hogendoorn, L Lhopitalli, M Richard-Gr, E Tenisch, Z Mbarack, J Samaka, T Mlaganile, A Mamin, B Genton, L Kaiser, V D'Acremont, KC Kain, N Boillat-Bl
BMC infectious diseases, 2022-01-06;22(1):39.
Species: Human
Sample Types: Plasma
-
Maternal inflammatory and omega-3 fatty acid pathways mediate the association between socioeconomic disadvantage and childhood cognition
Authors: W Marx, S Thomson, M O'Hely, C Symeonides, F Collier, MLK Tang, A Loughman, D Burgner, R Saffery, C Pham, T Mansell, PD Sly, P Vuillermin, S Ranganatha, AL Ponsonby, Barwon Inf
Brain, Behavior, and Immunity, 2021-12-08;100(0):211-218.
Species: Human
Sample Types: Serum
-
Cytomegalovirus Immunity, Inflammation and Cognitive Abilities in the Elderly
Authors: J Hesson, N Fudge, M Grant
Viruses, 2021-11-21;13(11):.
Species: Human
Sample Types: Plasma
-
Risky family climates presage increased cellular aging in young adulthood
Authors: GH Brody, T Yu, E Chen, M Kobor, SRH Beach, MK Lei, A Barr, DT Lin, GE Miller
Psychoneuroendocrinology, 2021-05-14;130(0):105256.
Species: Human
Sample Types: Serum
-
Changes in liver steatosis in HIV-positive women are associated with the BMI, but not with biomarkers
Authors: R Fernandez-, MW Plankey, D Ware, J Bordon
Cytokine, 2021-05-12;0(0):155573.
Species: Human
Sample Types: Plasma
-
Cross-Sectional Estimation of Endogenous Biomarker Associations with Prenatal Phenols, Phthalates, Metals, and Polycyclic Aromatic Hydrocarbons in Single-Pollutant and Mixtures Analysis Approaches
Authors: MT Aung, Y Yu, KK Ferguson, DE Cantonwine, L Zeng, TF McElrath, S Pennathur, B Mukherjee, JD Meeker
Environmental health perspectives, 2021-03-24;129(3):37007.
Species: Human
Sample Types: Plasma
-
Effect of Anti-Rheumatic Treatment on the Periodontal Condition of Rheumatoid Arthritis Patients
Authors: MJ de Smit, J Westra, MD Posthumus, G Springer, AJ van Winkel, A Vissink, E Brouwer, M Bijl
International Journal of Environmental Research and Public Health, 2021-03-04;18(5):.
Species: Human
Sample Types: Serum
-
Association of circulating proprotein convertase subtilisin/kexin type 9 levels and the risk of incident type 2 diabetes in subjects with prediabetes: a population-based cohort study
Authors: J Shi, W Zhang, Y Niu, N Lin, X Li, H Zhang, R Hu, G Ning, J Fan, L Qin, Q Su, Z Yang
Cardiovascular Diabetology, 2020-12-10;19(1):209.
Species: Human
Sample Types:
-
Reduced concentrations of the B cell cytokine Interleukin 38 are associated with cardiovascular disease risk in overweight subjects
Authors: DM de Graaf, M Jaeger, ICL van den Mu, RT Horst, K Schraa, J Zwaag, M Kox, M Fujita, T Yamauchi, L Mercurio, S Madonna, JHW Rutten, J de Graaf, NP Riksen, FL van de Vee, MG Netea, LAB Joosten, CA Dinarello
Eur J Immunol, 2020-11-19;0(0):.
Species: Human
Sample Types: Plasma
-
Application of an analytical framework for multivariate mediation analysis of environmental data
Authors: MT Aung, Y Song, KK Ferguson, DE Cantonwine, L Zeng, TF McElrath, S Pennathur, JD Meeker, B Mukherjee
Nat Commun, 2020-11-06;11(1):5624.
Species: Human
Sample Types: Plasma
-
Circulating heat shock protein 27 as a novel marker of subclinical atherosclerosis in type 2 diabetes: a cross-sectional community-based study
Authors: X Wang, J Shi, B Lu, W Zhang, Y Yang, J Wen, R Hu, Z Yang, X Wang
BMC Cardiovasc Disord, 2020-04-25;20(1):198.
Species: Human
Sample Types: Serum
-
Enhanced Adipose Expression of Interferon Regulatory Factor (IRF)-5 Associates with the Signatures of Metabolic Inflammation in Diabetic Obese Patients
Authors: S Sindhu, S Kochumon, R Thomas, A Bennakhi, F Al-Mulla, R Ahmad
Cells, 2020-03-16;9(3):.
Species: Human
Sample Types: Plasma
-
Elevation of C-reactive protein, P-selectin and Resistin as potential inflammatory biomarkers of urogenital Schistosomiasis exposure in preschool children
Authors: TN Chimponda, C Mushayi, DNM Osakunor, A Vengesai, E Enwono, S Amanfo, J Murray, C Tshuma, F Mutapi, T Mduluza
BMC Infect. Dis., 2019-12-19;19(1):1071.
Species: Human
Sample Types: Serum
-
Prediction and associations of preterm birth and its subtypes with eicosanoid enzymatic pathways and inflammatory markers
Authors: MT Aung, Y Yu, KK Ferguson, DE Cantonwine, L Zeng, TF McElrath, S Pennathur, B Mukherjee, JD Meeker
Sci Rep, 2019-11-19;9(1):17049.
Species: Human
Sample Types: Plasma
-
Increased Adipose Tissue Expression of Interferon Regulatory Factor (IRF)-5 in Obesity: Association with Metabolic Inflammation
Authors: S Sindhu, R Thomas, S Kochumon, A Wilson, M Abu-Farha, A Bennakhi, F Al-Mulla, R Ahmad
Cells, 2019-11-11;8(11):.
Species: Human
Sample Types: Plasma
-
Average and time-specific maternal prenatal inflammatory biomarkers and the risk of labor epidural associated fever
Authors: DY Arce, A Bellavia, DE Cantonwine, OJ Napoli, JD Meeker, T James-Todd, TF McElrath, LC Tsen
PLoS ONE, 2019-11-05;14(11):e0222958.
Species: Human
Sample Types: Plasma
-
Development of a multiplex and sensitive lateral flow immunoassay for the diagnosis of periprosthetic joint infection
Authors: TT Tsai, TH Huang, NY Ho, YP Chen, CA Chen, CF Chen
Sci Rep, 2019-10-30;9(1):15679.
Species: Human
Sample Types: Synovial Fluid
-
Early malaria infection, dysregulation of angiogenesis, metabolism and inflammation across pregnancy, and risk of preterm birth in Malawi: A cohort study
Authors: RE Elphinston, AM Weckman, CR McDonald, V Tran, K Zhong, M Madanitsa, L Kalilani-P, C Khairallah, SM Taylor, SR Meshnick, V Mwapasa, FO Ter Kuile, AL Conroy, KC Kain
PLoS Med., 2019-10-01;16(10):e1002914.
Species: Human
Sample Types: Plasma
-
Systemic inflammation is associated with malaria and preterm birth in women living with HIV on antiretrovirals and co-trimoxazole
Authors: CR McDonald, AM Weckman, AL Conroy, P Olwoch, P Natureeba, MR Kamya, DV Havlir, G Dorsey, KC Kain
Sci Rep, 2019-05-01;9(1):6758.
Species: Human
Sample Types: Plasma
-
Association of antenatal depression with oxidative stress and impact on spontaneous preterm birth
Authors: KK Venkatesh, JD Meeker, DE Cantonwine, TF McElrath, KK Ferguson
J Perinatol, 2019-02-05;0(0):.
Species: Human
Sample Types: Plasma
-
Effects of continuous moderate exercise with partial blood flow restriction during hemodialysis: A protocol for a randomized clinical trial
Authors: RK Cardoso, AM Araujo, RB Orcy, M Bohlke, JP Oses, FB Del Vecchi, FC Barcellos, MC Gonzalez, AJ Rombaldi
MethodsX, 2019-01-23;6(0):190-198.
Species: Human
Sample Types: Serum
-
Plasma hepcidin is associated with future risk of venous thromboembolism.
Authors: Ellingsen T, Lappegard J, Ueland T, Aukrust P, Braekkan S, Hansen J
Blood Adv, 2018-06-12;2(11):1191-1197.
Species: Human
Sample Types: Plasma
-
Relationship of the Adherence to a Mediterranean Diet and Its Main Components with CRP Levels in the Spanish Population
Authors: C Lahoz, E Castillo, JM Mostaza, O de Dios, MA Salinero-F, T González-A, F García-Igl, E Estirado, F Laguna, V Sanchez, C Sabín, S López, V Cornejo, C de Burgos, C Garcés
Nutrients, 2018-03-20;10(3):.
Species: Human
Sample Types: Plasma
-
High serum levels of C-reactive protein (CRP) predict beneficial decrease of visceral fat in obese females after sleeve gastrectomy
Authors: F Carbone, E Nulli Migl, A Bonaventur, A Vecchié, S De Vuono, MA Ricci, G Vaudo, M Boni, F Dallegri, F Montecucco, G Lupattelli
Nutr Metab Cardiovasc Dis, 2018-02-02;0(0):.
Species: Human
Sample Types: Serum
-
Effects of freezer storage time on levels of complement biomarkers
Authors: AR Morgan, C O'Hagan, S Touchard, S Lovestone, B Paul Morga
BMC Res Notes, 2017-11-06;10(1):559.
Species: Human
Sample Types: Plasma
-
Monocyte/macrophage and T cell activation markers are not independently associated with MI risk in healthy individuals - results from the HUNT Study
Authors: T Ueland, LE Laugsand, LJ Vatten, I Janszky, C Platou, AE Michelsen, JK Damås, P Aukrust, BO Åsvold
Int. J. Cardiol., 2017-06-12;0(0):.
Species: Human
Sample Types: Serum
-
Association of calf circumference with insulin resistance and non-alcohol fatty liver disease: the REACTION study
Authors: W Zhang, Z Yang, Y Niu, X Li, L Zhu, S Lu, H Zhang, J Fan, G Ning, L Qin, Q Su
BMC Endocr Disord, 2017-05-30;17(1):28.
Species: Human
Sample Types: Serum
-
Penicillinase-resistant antibiotics induce non-immune-mediated cholestasis through HSP27 activation associated with PKC/P38 and PI3K/AKT signaling pathways
Authors: A Burban, A Sharanek, R Hüe, M Gay, S Routier, A Guillouzo, C Guguen-Gui
Sci Rep, 2017-05-12;7(1):1815.
Species: Human
Sample Types: Cell Culture Supernates
-
Experimental type 2 diabetes induction reduces serum vaspin, but not serum omentin, in Wistar rats
Authors: CA Castro, KA da Silva, MM Buffo, KNZ Pinto, FO Duarte, KO Nonaka, FF Aníbal, ACGO Duarte
Int J Exp Pathol, 2017-04-25;0(0):.
Species: Human
Sample Types: Serum
-
The association between osteopontin gene polymorphisms, osteopontin expression and sarcoidosis
Authors: H Lavi, M Assayag, A Schwartz, N Arish, ZG Fridlender, N Berkman
PLoS ONE, 2017-03-02;12(3):e0171945.
Species: Human
Sample Types: Plasma
-
Improvement of cardiometabolic markers after fish oil intervention in young Mexican adults and the role of PPAR? L162V and PPAR?2 P12A
Authors: A Binia, C Vargas-Mar, M Ancira-Mor, LM Gosoniu, I Montoliu, E Gámez-Vald, DC Soria-Cont, A Angeles-Qu, R Gonzalez-A, S Fernández, D Martínez-C, B Hernández-, M Ramírez-So, C Pérez-Orte, Y Rodríguez-, I Castan, I Rubio-Alia, F Vadillo-Or, R Márquez-Ve, R Bojalil, JC López-Alva, P Valet, M Kussmann, I Silva-Zole, ME Tejero
J. Nutr. Biochem., 2017-02-10;43(0):98-106.
Species: Human
Sample Types: Serum
-
Circulating periostin in relation to insulin resistance and nonalcoholic fatty liver disease among overweight and obese subjects
Sci Rep, 2016-11-25;6(0):37886.
Species: Human
Sample Types: Serum
-
Plasma selenium levels and nonalcoholic fatty liver disease in Chinese adults: a cross-sectional analysis
Sci Rep, 2016-11-17;6(0):37288.
Species: Human
Sample Types: Serum
-
Intraplaque Expression of C-Reactive Protein Predicts Cardiovascular Events in Patients with Severe Atherosclerotic Carotid Artery Stenosis
Authors: Aldo Bonaventur
Mediators Inflamm, 2016-09-21;2016(0):9153673.
Species: Human
Sample Types: Serum
-
Silk-based blood stabilization for diagnostics
Proc Natl Acad Sci USA, 2016-05-09;113(21):5892-7.
Species: Human
Sample Types: Plasma
-
Biomarkers of Host Response Predict Primary End-Point Radiological Pneumonia in Tanzanian Children with Clinical Pneumonia: A Prospective Cohort Study.
Authors: Erdman L, D'Acremont V, Hayford K, Rajwans N, Kilowoko M, Kyungu E, Hongoa P, Alamo L, Streiner D, Genton B, Kain K
PLoS ONE, 2015-09-14;10(9):e0137592.
Species: Human
Sample Types: Plasma
-
Inflammatory and Angiogenic Factors at Mid-Pregnancy Are Associated with Spontaneous Preterm Birth in a Cohort of Tanzanian Women.
Authors: McDonald C, Darling A, Conroy A, Tran V, Cabrera A, Liles W, Wang M, Aboud S, Urassa W, Fawzi W, Kain K
PLoS ONE, 2015-08-06;10(8):e0134619.
Species: Human
Sample Types: Plasma
-
The effect of smoking on DNA methylation of peripheral blood mononuclear cells from African American women.
Authors: Dogan, Meeshant, Shields, Bridget, Cutrona, Carolyn, Gao, Long, Gibbons, Frederic, Simons, Ronald, Monick, Martha, Brody, Gene H, Tan, Kai, Beach, Steven R, Philibert, Robert A
BMC Genomics, 2014-02-22;15(0):151.
Species: Human
Sample Types: Serum
-
Plasma sCD14 as a biomarker to predict pulmonary exacerbations in cystic fibrosis.
Authors: Quon B, Ngan D, Wilcox P, Man S, Sin D
PLoS ONE, 2014-02-20;9(2):e89341.
Species: Human
Sample Types: Plasma
-
Host biomarkers distinguish dengue from leptospirosis in Colombia: a case-control study.
Authors: Conroy A, Gelvez M, Hawkes M, Rajwans N, Liles W, Villar-Centeno L, Kain K
BMC Infect Dis, 2014-01-20;14(0):35.
Species: Human
Sample Types: Serum
-
Differences in insulin sensitivity, lipid metabolism and inflammation between young adult Pakistani and Norwegian patients with type 2 diabetes: a cross sectional study.
Authors: Wium C, Aasheim E, Ueland T, Michelsen A, Thorsby P, Larsen I, Torjesen P, Aukrust P, Birkeland K
BMC Endocr Disord, 2013-10-22;13(0):49.
Species: Human
Sample Types: Plasma
-
Performance characteristics of combinations of host biomarkers to identify women with occult placental malaria: a case-control study from Malawi.
Authors: Conroy AL, Liles WC, Molyneux ME
PLoS ONE, 2011-12-12;6(12):e28540.
Species: Human
Sample Types: Plasma
-
Combinations of Host Biomarkers Predict Mortality among Ugandan Children with Severe Malaria: A Retrospective Case-Control Study.
Authors: Erdman LK, Dhabangi A, Musoke C, Conroy AL, Hawkes M, Higgins S, Rajwans N, Wolofsky KT, Streiner DL, Liles WC, Cserti-Gazdewich CM, Kain KC
PLoS ONE, 2011-02-25;6(2):e17440.
Species: Human
Sample Types: Plasma
-
Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers.
Authors: Shyamsundar M, McKeown ST, O'Kane CM, O'Kane CM, O'Kane CM, O'Kane CM, O'Kane CM, Craig TR, Brown V, Thickett DR, Matthay MA, Taggart CC, Backman JT, Elborn JS, McAuley DF
Am. J. Respir. Crit. Care Med., 2009-03-26;179(12):1107-14.
Species: Human
Sample Types: BALF
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human C-Reactive Protein/CRP DuoSet ELISA
Average Rating: 5 (Based on 6 Reviews)
Have you used Human C-Reactive Protein/CRP DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Very good results, standard curve with good range and sensitivity, EDTA plasma samples diluted 1:4000!