Human CFTR C-Terminus Antibody Summary
aa 1377-1480
Accession # P13569
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
CFTR in Human Placenta. CFTR was detected in immersion fixed paraffin-embedded sections of human placenta using 8 µg/mL Mouse Anti-Human CFTR C-Terminus Monoclonal Antibody (Catalog # MAB25031) overnight at 4 °C. Tissue was stained with the Anti-Mouse HRP-AEC Cell & Tissue Staining Kit (red; Catalog # CTS003) and counterstained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
CFTR in Human Placenta. CFTR was detected in immersion fixed paraffin-embedded sections of human placenta using Mouse Anti-Human CFTR C-Terminus Monoclonal Antibody (Catalog # MAB25031) at 25 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Mouse HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS002) and counterstained with hematoxylin (blue). Lower panel shows a lack of labeling if primary antibodies are omitted and tissue is stained only with secondary antibody followed by incubation with detection reagents. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
Detection of Human CFTR by Western Blot Chronic exposure to cigarette smoke (CS) decreases airway surface liquid (ASL) height. Primary human airway epithelial cells from 4 donors (n = 8) were exposed to 30 puffs of whole cigarette smoke (2 cigarettes) every day for 5 days (120 hrs). (A) ASL height was measured one hour after each exposure to CS. ASL height was undisturbed over the course of the reading. *p < 0.05. (B) CFTR present at the plasma membrane was detected by immunoblotting after biotinylation of cell surface proteins (see Methods). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24957904), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CFTR by Immunocytochemistry/Immunofluorescence Effect of S. aureus supernatant on CFTR localisation and expression. (A, B) Immunolocalisation of CFTR (green staining) and Dapi nuclei staining (blue) in lateral view of successive z level images. In control cells, we noticed an apical staining of CFTR (arrow heads in A). In 2%S. aureus supernatant-treated cells (B), the CFTR staining was more diffuse in the cytoplasm. (C) Western blotting analysis of airway glandular cell membrane proteins showed the presence of CFTR in control cells and in fewer amount in cells incubated with 2%S. aureus supernatant. (D) Quantitative measurement showed a significant (*, p < 0.05) decrease in CFTR expression in cell membranes when cells were incubated with 2% S. aureus supernatant. Data represent the mean ± SEM of 5 different experiments. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/20089165), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CFTR by Western Blot Cigarette smoke extract (CSE) decreases the expression of CFTR but not Na+/K+-ATPase in human bronchial epithelial cells. 16HBE14o- cells were treated with 10% CSE for up to 48 hours (A) or increasing concentrations of CSE prepared from commercial grade cigarettes (Camel) for 48 hours (B). CFTR and Na+/K+-ATPase were detected by immunoblotting. The same amount of protein was loaded in each lane as indicated by detection of beta -actin. The blots are representative of at least three independent experiments. (C) Detection of CFTR mRNA transcript levels using quantitative RT-PCR analysis after treatment of 16HBE14o- cells with 10% CSE for 24 hours. Results are expressed as fold change and are representative of three independent experiments. *p < 0.05. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24957904), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fish CFTR by Immunocytochemistry/Immunofluorescence Immunohistochemical localization of transporters in gills of tilapia acclimated to different salinities.Representative micrographs of immunohistolocalization of Nka (green) with either Nkcc/Ncc (a–c; red) or Cftr (d–f; red) in the gills of tilapia acclimated to FW (a,d), SW (b,e) or HSW (c,f). Co-localization of red and green fluorochromes results in yellow-orange staining. Higher magnification (10×) of boxed areas in (a–f) correspond to panels (a′–f′). Sections are counter stained with the nuclear stain DAPI and overlaid with the DIC image for tissue orientation. Arrows indicate apical staining and arrowheads tubular system (basolateral) staining. Scale bar 100 µm (a–f), 10 µm (a′–c′). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0087591), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CFTR by Western Blot COMMD1 regulates CFTR ubiquitination.(A) Representative gels for the same CFTR IP experiment with MAB25031 from HeLa cells stably expressing wt-CFTR and separated on 8% SDS-PAGE transferred to PVDF membrane. Half of the membrane was probed with anti-CFTR mAb and the other half with anti-ubiquitin mAb. Lysates were loaded onto an 11% SDS-PAGE and sequential probing of the membrane was performed (COMMD1, alpha -tubulin and lastly ubiquitin). Filled and empty arrowheads indicate the fully- (170 kDa) and core-glycosylated (140 kDa) CFTR, respectively. (B) Quantification of ubiquitinated CFTR. Ratio of ubiquitinated CFTR to total CFTR in each condition is shown, endogenous COMMD1 expression is referred as 100%. The means ± S.D. were obtained from five independent experiments.* P<0.05 was determined by t-test. (C) Stability of the mature wt-CFTR was determined upon inhibition of protein biosynthesis with cycloheximide (CHX). Cells were incubated in the presence of cycloheximide for the indicated time intervals. (D) Quantification of mature CFTR was normalized to alpha -tubulin level. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0018334), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CFTR by Immunocytochemistry/Immunofluorescence Co-localisation by immunofluorescence of CFTR and actin. (A) The pattern of CFTR (green staining) and actin (red staining) stainings was essentially apical in control cells as well as in cells treated with Sal/FP (B). (C) The incubation of cells with S. aureus supernatant induced alteration of the localisation of CFTR that appeared to be cytoplasmic, in parallel with a disorganization of the actin network. (D) Treatment of S. aureus supernatant pre-incubated cells with Sal/FP restored CFTR and actin apical stainings. (E) Quantification of the co-localisation of CFTR and actin showed that 2% S. aureus supernatant decreased the co-localisation index compared to the index in control cells, but the difference was not significant; the treatment with Sal/FP alone or after S. aureus supernatant incubation significantly enhanced the co-localisation of the 2 proteins compared with control or with S. aureus supernatant-treated cells (*, p < 0.05). Data represent the mean ± SEM of 3 different experiments. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/20089165), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CFTR by Immunoprecipitation COMMD1 and CFTR interact in mammalian cells.(A) Sequences of ICL3 in other species from fish to primates. Asterisks indicate the position of two class II mutations: S945L and D979A. Identity of amino acids between the different proteins are boxed in black, conserved residues are boxed in dark gray and semi-conserved substitutions in light gray. (B) Representative gels for the same co-immunoprecipitation experiments in HT-29 cells expressing endogenous CFTR and COMMD1. Lysates from HT-29 cells were immunoprecipitated (IP) with either 0.8 µg of anti-COMMD1 mAb (Abnova), 0.8 µg of anti-CFTR mAb (MAB25031, R&D Systems) or with 0.8 µg anti-mouse IgG as a control. Each immunoprecipitation sample was then split in half and loaded onto an 8% SDS-PAGE for CFTR detection and 11% SDS-PAGE for COMMD1 detection. Both gels were transferred to PVDF membrane and subjected to immunoblotting (IB). The 8% SDS-PAGE membrane was probed with anti-CFTR mAb (MM13-4) and the 11% SDS-PAGE membrane with a rabbit anti-COMMD1 pAb (Proteintech Group). Both membranes were probed with anti-alpha -tubulin as control (11% gel is shown). Filled and empty arrowheads indicate the fully- (170 kDa) and core-glycosylated (140 kDa) CFTR, respectively. * indicates mouse IgG light chain from the antibody used for immunoprecipitation. (C) COMMD1 constructions in pcDNA3.1/Topo plasmid. Two COMMD1 constructs were generated by adding a Myc-tag at the N-terminus of COMMD1: Myc-COMMD1 and a construct with a deletion of the COMM domain named Myc-COMMD1 delta COMM. (D) Representative gels for the same co-immunoprecipitation experiment between COMMD1 and wt- in heterologous system. HeLa cells stably expressing wt- (spTCF-wt) or empty CFTR vector (spTracer) as control were transfected with Myc-COMMD1. spTCF-wt were transfected with Myc-COMMD1 delta COMM. Lysates from all these experiments were subjected to SDS-PAGE, as in (B) after CFTR IP. The 8% SDS-PAGE membrane was probed with anti-CFTR mAb and the 11% SDS-PAGE membrane with anti-c-Myc mAb. Both membranes were probed with anti-alpha -tubulin as control (11% gel is shown). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0018334), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CFTR by Western Blot Manganese and cadmium decrease the expression of CFTR in bronchial epithelial cells. 16HBE14o- cells were incubated with cadmium chloride (CdCl2) or manganese chloride (MnCl2) at the doses indicated for 24 hours. CFTR protein was detected by immunobloting using a monoclonal antibody as described in Materials and Methods. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24957904), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CFTR by Immunocytochemistry/Immunofluorescence Effect of S. aureus supernatant on CFTR localisation and expression. (A, B) Immunolocalisation of CFTR (green staining) and Dapi nuclei staining (blue) in lateral view of successive z level images. In control cells, we noticed an apical staining of CFTR (arrow heads in A). In 2%S. aureus supernatant-treated cells (B), the CFTR staining was more diffuse in the cytoplasm. (C) Western blotting analysis of airway glandular cell membrane proteins showed the presence of CFTR in control cells and in fewer amount in cells incubated with 2%S. aureus supernatant. (D) Quantitative measurement showed a significant (*, p < 0.05) decrease in CFTR expression in cell membranes when cells were incubated with 2% S. aureus supernatant. Data represent the mean ± SEM of 5 different experiments. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/20089165), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CFTR by Western Blot Cigarette smoke extract (CSE) decreases the expression of CFTR but not Na+/K+-ATPase in human bronchial epithelial cells. 16HBE14o- cells were treated with 10% CSE for up to 48 hours (A) or increasing concentrations of CSE prepared from commercial grade cigarettes (Camel) for 48 hours (B). CFTR and Na+/K+-ATPase were detected by immunoblotting. The same amount of protein was loaded in each lane as indicated by detection of beta -actin. The blots are representative of at least three independent experiments. (C) Detection of CFTR mRNA transcript levels using quantitative RT-PCR analysis after treatment of 16HBE14o- cells with 10% CSE for 24 hours. Results are expressed as fold change and are representative of three independent experiments. *p < 0.05. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24957904), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fish CFTR by Immunocytochemistry/Immunofluorescence Immunohistochemical localization of transporters in gills of tilapia acclimated to different salinities.Representative micrographs of immunohistolocalization of Nka (green) with either Nkcc/Ncc (a–c; red) or Cftr (d–f; red) in the gills of tilapia acclimated to FW (a,d), SW (b,e) or HSW (c,f). Co-localization of red and green fluorochromes results in yellow-orange staining. Higher magnification (10×) of boxed areas in (a–f) correspond to panels (a′–f′). Sections are counter stained with the nuclear stain DAPI and overlaid with the DIC image for tissue orientation. Arrows indicate apical staining and arrowheads tubular system (basolateral) staining. Scale bar 100 µm (a–f), 10 µm (a′–c′). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0087591), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CFTR by Western Blot Metals present in CSE regulate CFTR expression. 16HBE14o- cells were incubated with 10% CSE before and after incubation with Chelex-100 beads, in absence or presence of 10 μM cadmium chloride. CFTR protein was detected by immunoblotting 48 hours after treatment. Blots are representative of at least three independent experiments. *p < 0.05. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24957904), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fish CFTR by Immunocytochemistry/Immunofluorescence Immunohistochemical localization of transporters in gills of tilapia acclimated to different salinities.Representative micrographs of immunohistolocalization of Nka (green) with either Nkcc/Ncc (a–c; red) or Cftr (d–f; red) in the gills of tilapia acclimated to FW (a,d), SW (b,e) or HSW (c,f). Co-localization of red and green fluorochromes results in yellow-orange staining. Higher magnification (10×) of boxed areas in (a–f) correspond to panels (a′–f′). Sections are counter stained with the nuclear stain DAPI and overlaid with the DIC image for tissue orientation. Arrows indicate apical staining and arrowheads tubular system (basolateral) staining. Scale bar 100 µm (a–f), 10 µm (a′–c′). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0087591), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Fish CFTR by Immunocytochemistry/Immunofluorescence Immunohistochemical localization of transporters in anterior and posterior intestine of tilapia acclimated to different salinities.Representative micrographs of immunolocalization of Nka (green) with Nkcc/Ncc (red) (a–d, f–h) from FW (a, f), SW (b, g) and HSW (c, h) acclimated tilapia. (d) A representative higher magnification micrograph of Nkcc/Ncc staining of the brush border of enterocytes with basolateral Nka staining from the anterior intestine of SW-acclimated fish. (e) Apical Cftr (red) double labeling with Nka (green) in the anterior intestine of a FW-acclimated fish. Panels (a–e) are sections of anterior intestine (AI) while panels (f–h) are sections of posterior intestine (PI). Sections are counter stained with the nuclear stain DAPI and overlaid with the DIC image for tissue orientation. Scale bar 100 µm (a–c, f–h); 25 µm (d,e). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0087591), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of CFTR by Western Blot COMMD1 regulates CFTR ubiquitination.(A) Representative gels for the same CFTR IP experiment with MAB25031 from HeLa cells stably expressing wt-CFTR and separated on 8% SDS-PAGE transferred to PVDF membrane. Half of the membrane was probed with anti-CFTR mAb and the other half with anti-ubiquitin mAb. Lysates were loaded onto an 11% SDS-PAGE and sequential probing of the membrane was performed (COMMD1, alpha -tubulin and lastly ubiquitin). Filled and empty arrowheads indicate the fully- (170 kDa) and core-glycosylated (140 kDa) CFTR, respectively. (B) Quantification of ubiquitinated CFTR. Ratio of ubiquitinated CFTR to total CFTR in each condition is shown, endogenous COMMD1 expression is referred as 100%. The means ± S.D. were obtained from five independent experiments.* P<0.05 was determined by t-test. (C) Stability of the mature wt-CFTR was determined upon inhibition of protein biosynthesis with cycloheximide (CHX). Cells were incubated in the presence of cycloheximide for the indicated time intervals. (D) Quantification of mature CFTR was normalized to alpha -tubulin level. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/21483833), licensed under a CC-BY license. Not internally tested by R&D Systems.
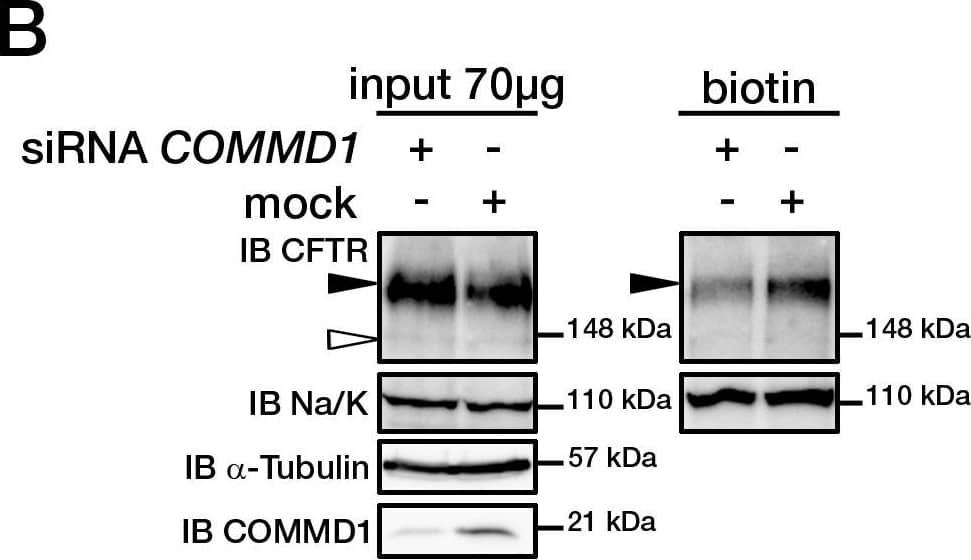
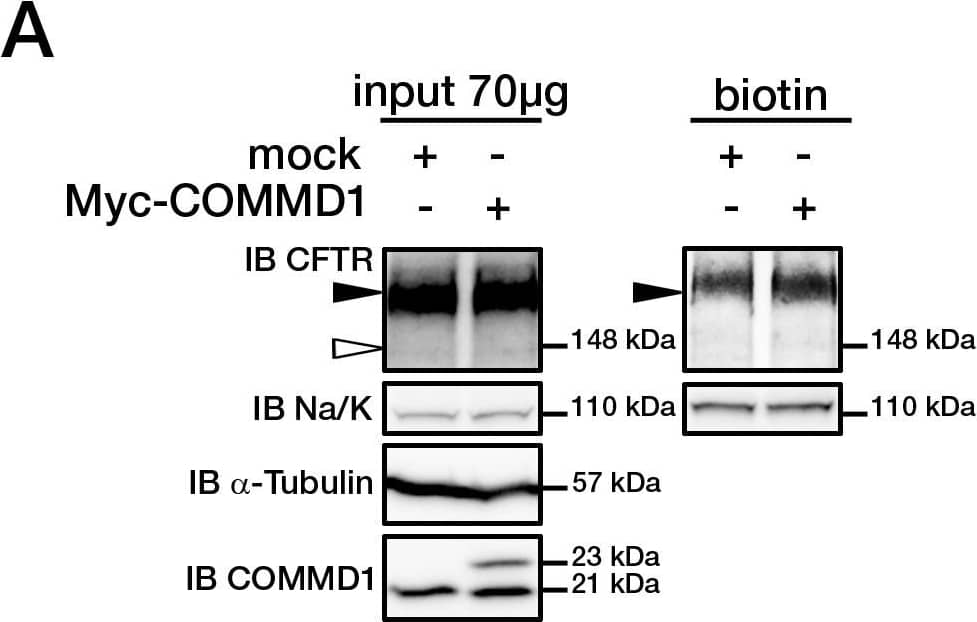 View Larger
View Larger
Detection of CFTR by Western Blot COMMD1 regulates CFTR cell surface expression.(A) HeLa cells stably expressing wt-CFTR were transiently transfected with an empty COMMD1 vector (mock, pcDNA3.1/Topo) or Myc-COMMD1, and were biotinylated with Sulfo-NHS-LC-biotin. Lysates from all these experiments were subjected to SDS-PAGE directly (input) or pulled-down with streptavidin-agarose (biotin). Representative gels for the same samples were separated by 8% SDS-PAGE for CFTR, Na/K-ATPase detection and 11% SDS-PAGE for COMMD1, alpha -tubulin detection. (B) HeLa cells stably expressing wt-CFTR were transiently transfected with a siCONTROL Non-Targeting siRNA (mock) or COMMD1 siRNA and further processed as in (A). Filled and empty arrowheads indicate the fully- (170 kDa) and core-glycosylated (140 kDa) CFTR, respectively. (C) Quantification of CFTR cell surface expression. The biotinylated CFTR level is normalized to the biotinylated Na/K-ATPase level. Endogenous COMMD1 expression is referred as 100%, with mock being pcDNA3.1/Topo for overexpression experiments (A), whereas mock was siCONTROL for silencing experiments (B). The means ± S.D. were obtained from three independent experiments.* P<0.05 was determined by t-test. (D) Immunofluorescence microscopy of COMMD1 and CFTR in HeLa cells stably expressing wt-CFTR. Cells were transfected with Myc-COMMD1 or COMMD1 siRNA for overexpression and silencing studies, respectively, and not transfected for endogenous expression studies. Two types of light exposure microscopy (short and normal) are shown to visualize all expression conditions. Scale bars: 10 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/21483833), licensed under a CC-BY license. Not internally tested by R&D Systems.
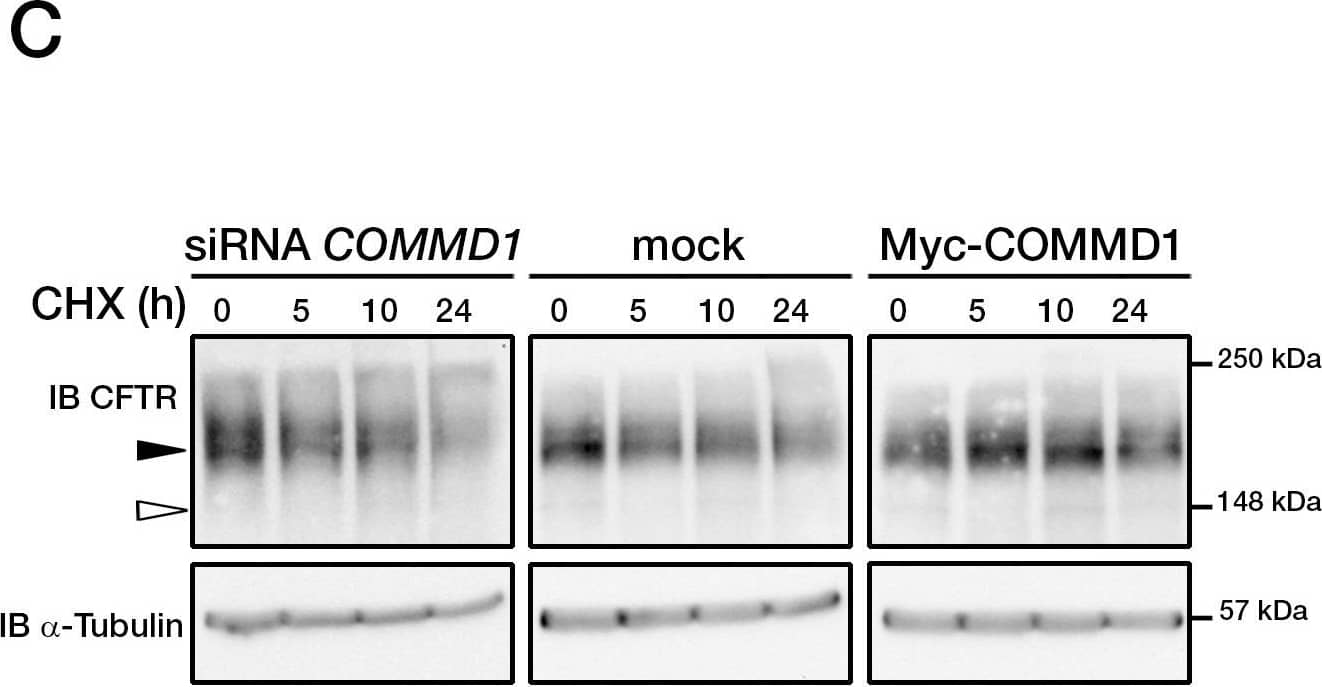
 View Larger
View Larger
Detection of CFTR by Western Blot COMMD1 regulates CFTR cell surface expression.(A) HeLa cells stably expressing wt-CFTR were transiently transfected with an empty COMMD1 vector (mock, pcDNA3.1/Topo) or Myc-COMMD1, and were biotinylated with Sulfo-NHS-LC-biotin. Lysates from all these experiments were subjected to SDS-PAGE directly (input) or pulled-down with streptavidin-agarose (biotin). Representative gels for the same samples were separated by 8% SDS-PAGE for CFTR, Na/K-ATPase detection and 11% SDS-PAGE for COMMD1, alpha -tubulin detection. (B) HeLa cells stably expressing wt-CFTR were transiently transfected with a siCONTROL Non-Targeting siRNA (mock) or COMMD1 siRNA and further processed as in (A). Filled and empty arrowheads indicate the fully- (170 kDa) and core-glycosylated (140 kDa) CFTR, respectively. (C) Quantification of CFTR cell surface expression. The biotinylated CFTR level is normalized to the biotinylated Na/K-ATPase level. Endogenous COMMD1 expression is referred as 100%, with mock being pcDNA3.1/Topo for overexpression experiments (A), whereas mock was siCONTROL for silencing experiments (B). The means ± S.D. were obtained from three independent experiments.* P<0.05 was determined by t-test. (D) Immunofluorescence microscopy of COMMD1 and CFTR in HeLa cells stably expressing wt-CFTR. Cells were transfected with Myc-COMMD1 or COMMD1 siRNA for overexpression and silencing studies, respectively, and not transfected for endogenous expression studies. Two types of light exposure microscopy (short and normal) are shown to visualize all expression conditions. Scale bars: 10 µm. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/21483833), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: CFTR
Cystic fibrosis transmembrane conductance regulator (CFTR) is a multi-pass transmembrane protein that functions as a chloride channel. CFTR belongs to the ATP‑binding cassette (ABC) superfamily. Mutations in CFTR cause the pulmonary disease, cystic fibrosis (CF). Specifically, deletion of phenyalanine at position 508 (DeltaF508-CFTR) results in a folding defect which impairs chloride channel function. The mechanism by which channel dysfunction relates to disease symptoms is a focus of intense research. CFTR dysfunction results in disruption of ion transport and subsequent blockage of airways by secreted mucus. CFTR may also play a role in the skeletal muscle atrophy and dysfunction that characterizes CF. In addition, CFTR-mediated chloride secretion underlies fluid accumulation and cyst growth in autosomal dominant polycystic kidney disease (ADPKD).
Product Datasheets
Citations for Human CFTR C-Terminus Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
76
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
TNFalpha and IL-17 Alkalinize Airway Surface Liquid through CFTR and Pendrin
Authors: Rehman T, Thornell IM, Pezzulo AA et al.
Am. J. Physiol., Cell Physiol.
-
COMMD1-Mediated Ubiquitination Regulates CFTR Trafficking.
Authors: Drevillon L, Tanguy G, Hinzpeter A et al.
PLoS One.
-
Inflammation in the COVID-19 airway is due to inhibition of CFTR signaling by the SARS-CoV-2 spike protein
Authors: Caohuy, H;Eidelman, O;Chen, T;Mungunsukh, O;Yang, Q;Walton, NI;Pollard, BS;Khanal, S;Hentschel, S;Florez, C;Herbert, AS;Pollard, HB;
Scientific reports
Species: Primate - Cercopithecus aethiops (African Green Monkey)
Sample Types: Cell Lysates
Applications: Western Blot -
Prime editing-mediated correction of the CFTR W1282X mutation in iPSCs and derived airway epithelial cells
Authors: Li, C;Liu, Z;Anderson, J;Liu, Z;Tang, L;Li, Y;Peng, N;Chen, J;Liu, X;Fu, L;Townes, TM;Rowe, SM;Bedwell, DM;Guimbellot, J;Zhao, R;
PloS one
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Loss-of-function CFTR p.G970D missense mutation might cause congenital bilateral absence of the vas deferens and be associated with impaired spermatogenesis
Authors: Qin-Tong Hou, Xiao-Liang Xu, Jian-Wen Wang, Xiao-Liang Dai, Li Wang, Cong-Ling Jiang et al.
Asian Journal of Andrology
-
Deubiquitinase-targeting chimeras for targeted protein stabilization
Authors: Nathaniel J. Henning, Lydia Boike, Jessica N. Spradlin, Carl C. Ward, Gang Liu, Erika Zhang et al.
Nature Chemical Biology
-
Validation of genetic classifiers derived from mouse and human tumors to identify molecular subtypes of colorectal cancer
Authors: SM Snow, KA Matkowskyj, M Maresh, L Clipson, TN Vo, KA Johnson, DA Deming, MA Newton, WM Grady, PJ Pickhardt, RB Halberg
Human pathology, 2021-10-14;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
SFPQ rescues F508del-CFTR expression and function in cystic fibrosis bronchial epithelial cells
Authors: P Kumar, DK Soni, C Sen, MB Larsen, K Mazan-Mamc, Y Piao, S De, M Gorospe, RA Frizzell, R Biswas
Scientific Reports, 2021-08-17;11(1):16645.
Species: Human
Sample Types: Whole Cells
Applications: IHC -
Mummichog gill and operculum exhibit functionally consistent claudin-10 paralog profiles and Claudin-10c hypersaline response
Authors: Chun Chih Chen, William S. Marshall, George N. Robertson, Regina R. F. Cozzi, Scott P. Kelly
Biology Open
-
Is aquaporin‐3 involved in water‐permeability changes in the killifish during hypoxia and normoxic recovery, in freshwater or seawater?
Authors: Ilan M. Ruhr, Chris M. Wood, Kevin L. Schauer, Yadong Wang, Edward M. Mager, Bruce Stanton et al.
Journal of Experimental Zoology Part A: Ecological and Integrative Physiology
Species: Fish
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Vav3 Mediates Pseudomonas aeruginosa Adhesion to the Cystic Fibrosis Airway Epithelium
Authors: M Badaoui, A Zoso, T Idris, M Bacchetta, J Simonin, S Lemeille, B Wehrle-Hal, M Chanson
Cell Rep, 2020-07-07;32(1):107842.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Cystic fibrosis transmembrane conductance regulator dysfunction in platelets drives lung hyperinflammation
Authors: G Ortiz-Muno, MA Yu, E Lefrançais, B Mallavia, C Valet, JJ Tian, S Ranucci, KM Wang, Z Liu, N Kwaan, D Dawson, ME Kleinhenz, FT Khasawneh, PM Haggie, AS Verkman, MR Looney
J. Clin. Invest., 2020-04-01;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Treatment of Cystic Fibrosis Patients Homozygous for F508del with Lumacaftor-Ivacaftor (Orkambi�) Restores Defective CFTR Channel Function in Circulating Mononuclear Cells
Authors: M Favia, C Gallo, L Guerra, D De Venuto, A Diana, AM Polizzi, P Montemurro, MA Mariggiò, G Leonetti, A Manca, V Casavola, M Conese
Int J Mol Sci, 2020-03-31;21(7):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Cyclic compression increases F508 Del CFTR expression in ciliated human airway epithelium
Authors: Marozkina N, Bosch J, Cotton C et al.
American Journal of Physiology-Lung Cellular and Molecular Physiology
-
Clinically-approved CFTR modulators rescue Nrf2 dysfunction in cystic fibrosis airway epithelia
Authors: DC Borcherdin, ME Siefert, S Lin, J Brewington, H Sadek, JP Clancy, SM Plafker, AG Ziady
J. Clin. Invest., 2019-05-30;130(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Co-Immunoprecipitation -
Segmented poly(A) tails significantly reduce recombination of plasmid DNA without affecting mRNA translation efficiency or half-life
Authors: Zeljka Trepotec, Johannes Geiger, Christian Plank, Manish K. Aneja, Carsten Rudolph
RNA
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
TALEN-Mediated Gene Targeting for Cystic Fibrosis-Gene Therapy
Authors: Emily Xia, Yiqian Zhang, Huibi Cao, Jun Li, Rongqi Duan, Jim Hu
Genes (Basel)
-
Effect of dendritic organ ligation on striped eel catfish Plotosus lineatus osmoregulation
Authors: S Malakpour, J Coimbra, JM Wilson
PLoS ONE, 2018-10-23;13(10):e0206206.
Species: Fish - Plotosus lineatus (Striped Eel Catfish)
Sample Types: Whole Tissue
Applications: IHC-P -
Assembly and Functional Analysis of an S/MAR Based Episome with the Cystic Fibrosis Transmembrane Conductance Regulator Gene
Authors: D De Rocco, B Pompili, S Castellani, E Morini, L Cavinato, G Cimino, MA Mariggiò, S Guarnieri, M Conese, P Del Porto, F Ascenzioni
Int J Mol Sci, 2018-04-17;19(4):.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Gap Junctions Are Involved in the Rescue of CFTR-Dependent Chloride Efflux by Amniotic Mesenchymal Stem Cells in Coculture with Cystic Fibrosis CFBE41o- Cells
Authors: Annalucia Carbone, Roberto Zefferino, Elisa Beccia, Valeria Casavola, Stefano Castellani, Sante Di Gioia et al.
Stem Cells International
-
Renoguanylin stimulates apical CFTR translocation and decreases HCO3− secretion through PKA activity in the Gulf toadfish (Opsanus beta)
Authors: Ilan M. Ruhr, Kevin L. Schauer, Yoshio Takei, Martin Grosell
Journal of Experimental Biology
-
Characterization of pediatric cystic fibrosis airway epithelial cell cultures at the air-liquid interface obtained by non-invasive nasal cytology brush sampling
Authors: A Schögler, F Blank, M Brügger, S Beyeler, SA Tschanz, N Regamey, C Casaulta, T Geiser, MP Alves
Respir. Res., 2017-12-28;18(1):215.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
A sodium binding system alleviates acute salt stress during seawater acclimation in eels
Authors: Marty Kwok Shing Wong, Takehiro Tsukada, Nobuhiro Ogawa, Supriya Pipil, Haruka Ozaki, Yutaka Suzuki et al.
Zoological Letters
-
Establishment and long-term culture of human cystic fibrosis endothelial cells
Authors: Roberto Plebani, Romina Tripaldi, Paola Lanuti, Antonio Recchiuti, Sara Patruno, Sara Di Silvestre et al.
Laboratory Investigation
-
Cysteamine-mediated clearance of antibiotic-resistant pathogens in human cystic fibrosis macrophages
Authors: CL Shrestha, KD Assani, H Rinehardt, F Albastroiu, S Zhang, R Shell, AO Amer, LS Schlesinge, BT Kopp
PLoS ONE, 2017-10-05;12(10):e0186169.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
WNK1 and p38-MAPK distribution in ionocytes and accessory cells of euryhaline teleost fish implies ionoregulatory function
Authors: W. S. Marshall, R. R. F. Cozzi, M. Spieker
Biology Open
-
Diabetic rats present higher urinary loss of proteins and lower renal expression of megalin, cubilin, ClC-5, and CFTR
Authors: MF Figueira, RC Castiglion, CM de Lemos B, FM Ornellas, G da Silva F, MM Morales, RN da Fonseca, J de Souza-M
Physiol Rep, 2017-07-01;5(13):.
Species: Rat
Sample Types: Tissue Homogenates
Applications: Western Blot -
Ammonia exposure affects the mRNA and protein expression levels of certain Rhesus glycoproteins in the gills of climbing perch
Authors: XL Chen, B Zhang, YR Chng, JLY Ong, SF Chew, WP Wong, SH Lam, T Nakada, YK Ip
J. Exp. Biol., 2017-06-02;0(0):.
Species: Fish
Sample Types: Whole Tissue
Applications: IHC-P -
Expression of epithelial sodium channel (ENaC) and CFTR in the human epidermis and epidermal appendages
Authors: Israel Hanukoglu, Vijay R. Boggula, Hananya Vaknine, Sachin Sharma, Thomas Kleyman, Aaron Hanukoglu
Histochemistry and Cell Biology
-
Synergy of cAMP and calcium signaling pathways in CFTR regulation
Authors: Z Bozoky, S Ahmadi, T Milman, TH Kim, K Du, M Di Paola, S Pasyk, R Pekhletski, JP Keller, CE Bear, JD Forman-Kay
Proc. Natl. Acad. Sci. U.S.A, 2017-02-27;0(0):.
Species: Human
Sample Types: Whole Cells
-
Deep interactome profiling of membrane proteins by co-interacting protein identification technology
Nat Protoc, 2016-11-17;11(12):2515-2528.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
New use for an old drug: COX-independent anti-inflammatory effects of sulindac in models of cystic fibrosis
Authors: Agathe Tarze
Br. J. Pharmacol., 2016-04-21;173(11):1728-41.
Species: Human
Sample Types: Transfected Whole Cells
Applications: Western Blot -
Correctors of mutant CFTR enhance subcortical cAMP-PKA signaling through modulating ezrin phosphorylation and cytoskeleton organization
Authors: Anna C. Abbattiscianni, Maria Favia, Maria T. Mancini, Rosa A. Cardone, Lorenzo Guerra, Stefania Monterisi et al.
Journal of Cell Science
-
In vivo and in vitro effects of high-K(+) stress on branchial expression of ROMKa in seawater-acclimated Mozambique tilapia
Authors: Fumiya Furukawa, Soichi Watanabe, Andre P. Seale, Jason P. Breves, Darren T. Lerner, E Gordon Gordon Grau et al.
Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology
-
CFTR Inactivation by Lentiviral Vector-mediated RNA Interference and CRISPR-Cas9 Genome Editing in Human Airway Epithelial Cells
Authors: Jessica Bellec, Marc Bacchetta, Davide Losa, Ignacio Anegon, Marc Chanson, Tuan Huy Nguyen
Current Gene Therapy
-
Resveratrol increases F508del-CFTR dependent salivary secretion in cystic fibrosis mice
Authors: Barbara Dhooghe, Charlotte Bouckaert, Arnaud Capron, Pierre Wallemacq, Teresinha Leal, Sabrina Noel
Biology Open
-
CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction.
Authors: Tabeling C, Yu H, Wang L, Ranke H, Goldenberg N, Zabini D, Noe E, Krauszman A, Gutbier B, Yin J, Schaefer M, Arenz C, Hocke A, Suttorp N, Proia R, Witzenrath M, Kuebler W
Proc Natl Acad Sci U S A, 2015-03-17;112(13):E1614-23.
Species: Mouse
Sample Types: Cell Lysates
Applications: Immunoprecipitation, Western Blot -
Involvement of the Cdc42 pathway in CFTR post-translational turnover and in its plasma membrane stability in airway epithelial cells.
Authors: Ferru-Clement R, Fresquet F, Norez C, Metaye T, Becq F, Kitzis A, Thoreau V
PLoS ONE, 2015-03-13;10(3):e0118943.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Activation of 3-phosphoinositide-dependent kinase 1 (PDK1) and serum- and glucocorticoid-induced protein kinase 1 (SGK1) by short-chain sphingolipid C4-ceramide rescues the trafficking defect of DeltaF508-cystic fibrosis transmembrane conductance regulator (DeltaF508-CFTR).
Authors: Caohuy H, Yang Q, Eudy Y, Ha T, Xu A, Glover M, Frizzell R, Jozwik C, Pollard H
J Biol Chem, 2014-11-10;289(52):35953-68.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Immunoprecipitation, Western Blot -
Cystic fibrosis transmembrane conductance regulator protein (CFTR) expression in the developing human brain: comparative immunohistochemical study between patients with normal and mutated CFTR
Authors: Pascale Marcorelles, Gaëlle Friocourt, Arnaud Uguen, Françoise Ledé, Claude Férec, Annie Laquerrière
Journal of Histochemistry & Cytochemistry
-
Correlation of Apical Fluid-Regulating Channel Proteins with Lung Function in Human COPD Lungs
Authors: Runzhen Zhao, Xinrong Liang, Meimi Zhao, Shan-Lu Liu, Yao Huang, Steven Idell et al.
PLoS ONE
Species: Human
Sample Types: Tissue Homogenates
Applications: Western Blot -
Trimethylangelicin promotes the functional rescue of mutant F508del CFTR protein in cystic fibrosis airway cells
Authors: Maria Favia, Maria T. Mancini, Valentino Bezzerri, Lorenzo Guerra, Onofrio Laselva, Anna C. Abbattiscianni et al.
American Journal of Physiology-Lung Cellular and Molecular Physiology
-
Accumulation of metals in GOLD4 COPD lungs is associated with decreased CFTR levels.
Authors: Hassan F, Xu X, Nuovo G, Killilea D, Tyrrell J, Da Tan C, Tarran R, Diaz P, Jee J, Knoell D, Boyaka P, Cormet-Boyaka E
Respir Res, 2014-06-23;15(0):69.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Correction of defective CFTR/ENaC function and tightness of cystic fibrosis airway epithelium by amniotic mesenchymal stromal (stem) cells.
Authors: Carbone A, Castellani S, Favia M, Diana A, Paracchini V, Di Gioia S, Seia M, Casavola V, Colombo C, Conese M
J Cell Mol Med, 2014-06-03;18(8):1631-43.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, Western Blot -
Pseudomonas aeruginosa-induced apoptosis in airway epithelial cells is mediated by gap junctional communication in a JNK-dependent manner.
Authors: Losa D, Kohler T, Bellec J, Dudez T, Crespin S, Bacchetta M, Boulanger P, Hong S, Morel S, Nguyen T, van Delden C, Chanson M
J Immunol, 2014-04-14;192(10):4804-12.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Expression of key ion transporters in the gill and esophageal-gastrointestinal tract of euryhaline Mozambique tilapia Oreochromis mossambicus acclimated to fresh water, seawater and hypersaline water.
Authors: Li Z, Lui E, Wilson J, Ip Y, Lin Q, Lam T, Lam S
PLoS ONE, 2014-01-31;9(1):e87591.
Species: Fish
Sample Types: Whole Tissue
Applications: IHC-P -
Correcting the cystic fibrosis disease mutant, A455E CFTR.
Authors: Cebotaru L, Rapino D, Cebotaru V, Guggino W
PLoS ONE, 2014-01-08;9(1):e85183.
Species: Primate - Chlorocebus pygerythrus (Vervet Monkey)
Sample Types: Cell Lysates
Applications: Western Blot -
Correction of Chloride Transport and Mislocalization of CFTR Protein by Vardenafil in the Gastrointestinal Tract of Cystic Fibrosis Mice
Authors: Barbara Dhooghe, Sabrina Noël, Caroline Bouzin, Gaëtane Behets-Wydemans, Teresinha Leal
PLoS ONE
-
RNF185 is a novel E3 ligase of endoplasmic reticulum-associated degradation (ERAD) that targets cystic fibrosis transmembrane conductance regulator (CFTR).
Authors: El Khouri E, Le Pavec G, Toledano M, Delaunay-Moisan A
J Biol Chem, 2013-09-09;288(43):31177-91.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
Phosphorylated C/EBPbeta influences a complex network involving YY1 and USF2 in lung epithelial cells.
Authors: Viart V, Varilh J, Lopez E, Rene C, Claustres M, Taulan-Cadars M
PLoS ONE, 2013-04-01;8(4):e60211.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
Regulation of cystic fibrosis transmembrane conductance regulator by microRNA-145, -223, and -494 is altered in DeltaF508 cystic fibrosis airway epithelium.
Authors: Oglesby I, Chotirmall S, McElvaney N, Greene C
J Immunol, 2013-02-22;190(7):3354-62.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Keratin K18 increases cystic fibrosis transmembrane conductance regulator (CFTR) surface expression by binding to its C-terminal hydrophobic patch.
Authors: Duan, Yuanyuan, Sun, Ying, Zhang, Fan, Zhang, Wei Kevi, Wang, Dong, Wang, Yan, Cao, Xu, Hu, Wenbao, Xie, Changyan, Cuppoletti, John, Magin, Thomas M, Wang, Haixia, Wu, Zhenguo, Li, Ning, Huang, Pingbo
J Biol Chem, 2012-10-08;287(48):40547-59.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
Human amnion epithelial cells induced to express functional cystic fibrosis transmembrane conductance regulator.
Authors: Murphy, Sean V, Lim, Rebecca, Heraud, Philip, Cholewa, Marian, Le Gros, Mark, de Jonge, Martin D, Howard, Daryl L, Paterson, David, McDonald, Courtney, Atala, Anthony, Jenkin, Graham, Wallace, Euan M
PLoS ONE, 2012-09-28;7(9):e46533.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, ICC, Western Blot -
Role of binding and nucleoside diphosphate kinase A in the regulation of the cystic fibrosis transmembrane conductance regulator by AMP-activated protein kinase.
Authors: King, J Darwin, Lee, Jeffrey, Riemen, Claudia, Neumann, Dietbert, Xiong, Sheng, Foskett, J Kevin, Mehta, Anil, Muimo, Richmond, Hallows, Kenneth
J Biol Chem, 2012-08-06;287(40):33389-400.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation, Western Blot -
Proteomic Identification of Calumenin as a G551D - CFTR Associated Protein.
Authors: Teng L, Kerbiriou M, Taiya M, Le Hir S, Mignen O, Benz N, Trouve P, Ferec C
PLoS ONE, 2012-06-29;7(6):e40173.
Species: Human
Sample Types: Whole Cells
Applications: Western Blot -
Hematopoietic Stem/Progenitor Cells Express Functional Mitochondrial Energy-Dependent Cystic Fibrosis Transmembrane Conductance Regulator
Authors: Donatella Piro, Claudia Piccoli, Lorenzo Guerra, Francesca Sassone, Annamaria D'Aprile, Maria Favia et al.
Stem Cells and Development
-
Amniotic Mesenchymal Stem Cells: A New Source for Hepatocyte-Like Cells and Induction of CFTR Expression by Coculture with Cystic Fibrosis Airway Epithelial Cells
Authors: Valentina Paracchini, Annalucia Carbone, Federico Colombo, Stefano Castellani, Silvia Mazzucchelli, Sante Di Di Gioia et al.
Journal of Biomedicine and Biotechnology
-
Defective CFTR expression and function are detectable in blood monocytes: development of a new blood test for cystic fibrosis.
Authors: Sorio C, Buffelli M, Angiari C, Ettorre M, Johansson J, Vezzalini M, Viviani L, Ricciardi M, Verze G, Assael BM, Melotti P
PLoS ONE, 2011-07-21;6(7):e22212.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Long acting beta2-agonist and corticosteroid restore airway glandular cell function altered by bacterial supernatant
Authors: Jean-Marie Zahm, Franck Delavoie, Férial Toumi, Béatrice Nawrocki-Raby, Claire Kileztky, Jean Michel et al.
Respiratory Research
-
Cystic fibrosis neutrophils have normal intrinsic reactive oxygen species generation
Authors: D. J. McKeon, K. A. Cadwallader, S. Idris, A. S. Cowburn, M. C. Pasteur, H. Barker et al.
European Respiratory Journal
-
The cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in maturation stage ameloblasts, odontoblasts and bone cells
Authors: Antonius Bronckers, Lida Kalogeraki, Huub J.N. Jorna, Martina Wilke, Theodore J. Bervoets, Donacian M. Lyaruu et al.
Bone
-
Ezrin-radixin-moesin-binding phosphoprotein (EBP50), an estrogen-inducible scaffold protein, contributes to biliary epithelial cell proliferation.
Authors: Fouassier L, Rosenberg P, Mergey M, Saubamea B, Claperon A, Kinnman N, Chignard N, Jacobsson-Ekman G, Strandvik B, Rey C, Barbu V, Hultcrantz R, Housset C
Am. J. Pathol., 2009-03-01;174(3):869-80.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Cystic fibrosis transmembrane regulator missing the first four transmembrane segments increases wild type and DeltaF508 processing.
Authors: Cebotaru L, Vij N, Ciobanu I, Wright J, Flotte T, Guggino WB
J. Biol. Chem., 2008-05-28;283(32):21926-33.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in human bone.
Authors: Shead EF, Haworth CS, Condliffe AM, McKeon DJ, Scott MA, Compston JE
Thorax, 2007-07-01;62(7):650-1.
Species: Human
Sample Types: Cell Lysates, Whole Cells, Whole Tissue
Applications: ICC, IHC, Western Blot -
Expression and function of cystic fibrosis transmembrane conductance regulator in rat intrapulmonary arteries.
Authors: Robert R, Savineau JP, Norez C, Becq F, Guibert C
Eur. Respir. J., 2007-06-27;30(5):857-64.
Species: Rat
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Immunoprecipitation -
Differential regulation of cystic fibrosis transmembrane conductance regulator and Na+,K+ -ATPase in gills of striped bass, Morone saxatilis: effect of salinity and hormones.
Authors: Madsen SS, Jensen LN, Tipsmark CK, Kiilerich P, Borski RJ
J. Endocrinol., 2007-01-01;192(1):249-60.
Species: Fish
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC-P, Western Blot -
The ichthyotoxic alga Chattonella marina induces Na+, K+ -ATPase, and CFTR proteins expression in fish gill chloride cells in vivo.
Authors: Tang JY, Au DW
Biochem. Biophys. Res. Commun., 2006-12-04;353(1):98-103.
Species: Fish - Goldline seabream
Sample Types: Tissue Homogenates, Whole Cells
Applications: ICC, Western Blot -
CFTR Expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis.
Authors: Painter RG, Valentine VG, Lanson NA, Leidal K, Zhang Q, Lombard G, Thompson C, Viswanathan A, Nauseef WM, Wang G
Biochemistry, 2006-08-29;45(34):10260-9.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Absence of typical unfolded protein response in primary cultured cystic fibrosis airway epithelial cells.
Authors: Nanua S, Sajjan U, Keshavjee S, Hershenson MB
Biochem. Biophys. Res. Commun., 2006-03-03;343(1):135-43.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Human-specific cystic fibrosis transmembrane conductance regulator antibodies detect in vivo gene transfer to ovine airways.
Authors: Davidson H, McLachlan G, Wilson A, Boyd AC, Doherty A, MacGregor G, Davies L, Painter HA, Coles R, Hyde SC, Gill DR, Amaral MD, Collie DD, Porteous DJ, Penque D
Am. J. Respir. Cell Mol. Biol., 2006-02-23;35(1):72-83.
Species: Ovine
Sample Types: Whole Tissue
Applications: IHC-Fr -
Misprocessing of the CFTR protein leads to mild cystic fibrosis phenotype.
Authors: Clain J, Lehmann-Che J, Dugueperoux I, Arous N, Girodon E, Legendre M, Goossens M, Edelman A, de Braekeleer M, Teulon J, Fanen P
Hum. Mutat., 2005-04-01;25(4):360-71.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Immunoprecipitation -
Syntaxin 8 impairs trafficking of cystic fibrosis transmembrane conductance regulator (CFTR) and inhibits its channel activity.
Authors: Bilan F, Thoreau V, Nacfer M, Derand R, Norez C, Cantereau A, Garcia M, Becq F, Kitzis A
J. Cell. Sci., 2004-03-23;117(0):1923-35.
Species: Human
Sample Types: Cell Lysates
Applications: Immunoprecipitation -
Mitochondria-rich cells as experimental model in studies of epithelial chloride channels.
Authors: Willumsen NJ, Amstrup J, Mobjerg N, Jespersen A, Kristensen P, Larsen EH
Biochim. Biophys. Acta, 2002-11-13;1566(1):28-43.
Species: Xenopus
Sample Types: Whole Tissue
Applications: IHC-P -
Cystic fibrosis transmembrane conductance regulator modulates neurosecretory function in pulmonary neuroendocrine cell-related tumor cell line models.
Authors: Pan J, Bear C, Farragher S, Cutz E, Yeger H
Am. J. Respir. Cell Mol. Biol., 2002-11-01;27(5):553-60.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Reciprocal protein kinase A regulatory interactions between cystic fibrosis transmembrane conductance regulator and Na+/H+ exchanger isoform 3 in a renal polarized epithelial cell model.
Authors: Bagorda A, Guerra L, Di Sole F, Hemle-Kolb C, Cardone RA, Fanelli T, Reshkin SJ, Gisler SM, Murer H, Casavola V
J. Biol. Chem., 2002-04-05;277(24):21480-8.
Species: Amphibian
Sample Types: Cell Lysates
Applications: Western Blot -
Guanylate cyclase 2C agonism corrects CFTR mutants
Authors: K Arora, Y Huang, K Mun, S Yarlagadda, N Sundaram, MM Kessler, G Hannig, CB Kurtz, I Silos-Sant, M Helmrath, JJ Palermo, JP Clancy, KA Steinbrech, AP Naren
JCI Insight, 2017-10-05;2(19):.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human CFTR C-Terminus Antibody
Average Rating: 4.7 (Based on 3 Reviews)
Have you used Human CFTR C-Terminus Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Also used in Western blots. The dilution factor for the Western blots was 1:50, so it's not the most specific antibody against fish CFTR, however, the banding was clear and the immunohistochemistry came out adequately.



