Human CXCL9/MIG Quantikine ELISA Kit Summary
Sample Values
Serum/Plasma - Samples from apparently healthy volunteers were evaluated for the presence of human MIG in this assay. No medical histories were available for the donors used in this study.| Sample Type | Mean of Detectable (pg/mL) | % Detectable | Range (pg/mL) |
| Serum (n=40) | 64.4 | 65 | ND-199 |
| EDTA plasma (n=23) | 56.7 | 62 | ND-179 |
| Heparin plasma (n=25) | 62.0 | 68 | ND-209 |
| Condition | Day 1 (pg/mL) | Day 5 (pg/mL) |
| Unstimulated | ND | 445 |
| Stimulated | 77.5 | 12,290 |
Product Summary
Precision
Cell Culture Supernates
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 235 | 700 | 1358 | 242 | 707 | 1391 |
| Standard Deviation | 9.5 | 25.1 | 24.3 | 20.8 | 50.9 | 83.6 |
| CV% | 4 | 3.6 | 1.8 | 8.6 | 7.2 | 6 |
Serum, EDTA Plasma, Heparin Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 253 | 817 | 1603 | 245 | 723 | 1427 |
| Standard Deviation | 9.9 | 26.6 | 49.6 | 12.8 | 59.4 | 88.1 |
| CV% | 3.9 | 3.3 | 3.1 | 5.2 | 8.2 | 6.2 |
Recovery
The recovery of MIG spiked to levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=4) | 92 | 86-98 |
| EDTA Plasma (n=5) | 100 | 95-110 |
| Heparin Plasma (n=5) | 109 | 101-114 |
| Serum (n=5) | 108 | 101-113 |
Linearity
Scientific Data
 View Larger
View Larger
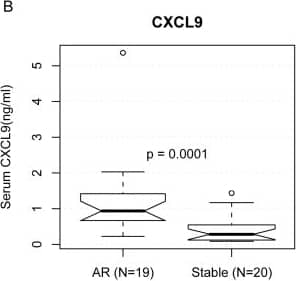
Human CXCL9/MIG Quantikine ELISA Kit Serum ELISA results of three protein biomarkers in renal transplantation.We measured the protein concentration of ten genes by ELISA in independent serum samples of 19 AR patients and 20 patients with stable (STA) graft function after renal transplant. The protein concentrations of PECAM1 (A), CXCL9 (B), and CD44 (C) were higher in the AR serum samples, as shown in the notched boxplots. When the notches about two medians do not overlap, the medians are roughly significantly different at about a 95% confidence level [54]. The circles are outliers. P-values were calculated using the Mann-Whitney U test. One of the AR samples was inadvertently lost during the PECAM1 experiment and could not be recovered. (D) Areas under the Receiver Operating Characteristic (ROC) curves used to distinguish AR from STA were 0.811, 0.864, and 0.761 for PECAM1, CXCL9, and CD44, respectively. Image collected and cropped by CiteAb from the following open publication (https://dx.plos.org/10.1371/journal.pcbi.1000940), licensed under a CC-BY license. Not internally tested by R&D Systems.
Product Datasheets
Preparation and Storage
Background: CXCL9/MIG
CXCL9, also known as MIG, is a chemokine that is induced in macrophages and in CNS glial cells in response to IFN-gamma. CXCL9 signals through CXCR3 to induce the chemoattraction of activated T cells and tumor-infiltrating lymphocytes.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 µL of Assay Diluent to each well.
- Add 100 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes.
- Add 200 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours.
- Aspirate and wash 4 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Human CXCL9/MIG Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
34
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Neutralizing GDF-15 can overcome anti-PD-1 and anti-PD-L1 resistance in solid tumours
Authors: Melero, I;de Miguel Luken, M;de Velasco, G;Garralda, E;Martín-Liberal, J;Joerger, M;Alonso, G;Goebeler, ME;Schuler, M;König, D;Dummer, R;Reig, M;Rodriguez Ruiz, ME;Calvo, E;Esteban-Villarrubia, J;Oberoi, A;Sabat, P;Soto-Castillo, JJ;Koster, KL;Saavedra, O;Sayehli, C;Gromke, T;Läubli, H;Ramelyte, E;Fortuny, M;Landa-Magdalena, A;Moreno, I;Torres-Jiménez, J;Hernando-Calvo, A;Hess, D;Racca, F;Richly, H;Schmitt, AM;Eggenschwiler, C;Sanduzzi-Zamparelli, M;Vilalta-Lacarra, A;Trojan, J;Koch, C;Galle, PR;Foerster, F;Trajanoski, Z;Hackl, H;Gogolla, F;Koll, FJ;Wild, P;Chun, FKH;Reis, H;Lloyd, P;Machacek, M;Gajewski, TF;Fridman, WH;Eggermont, AMM;Bargou, R;Schöniger, S;Rüschoff, J;Tereshchenko, A;Zink, C;da Silva, A;Lichtenegger, FS;Akdemir, J;Rüdiger, M;L'Huillier, P;Dutta, A;Haake, M;Auckenthaler, A;Gjorgjioska, A;Rössler, B;Hermann, F;Liebig, M;Reichhardt, D;Schuberth-Wagner, C;Wischhusen, J;Fettes, P;Auer, M;Klar, K;Leo, E;
Nature
Species: Human
Sample Types: Serum
-
Pansclerotic morphea is characterized by IFN-gamma responses priming dendritic cell fibroblast crosstalk to promote fibrosis
Authors: Xing, E;Ma, F;Wasikowski, R;Billi, AC;Gharaee-Kermani, M;Fox, J;Dobry, C;Victory, A;Sarkar, M;Xing, X;Plazyo, O;Chen, HW;Barber, GC;Jacobe, H;Tsou, PS;Modlin, RL;Varga, J;Kahlenberg, JM;Tsoi, LC;Gudjonsson, JE;Khanna, D;
JCI insight
Species: Human
Sample Types: Serum
-
Longitudinal multi-omics analyses of the gut-liver axis reveals metabolic dysregulation in hepatitis C infection and cirrhosis
Authors: RO Ali, GM Quinn, R Umarova, JA Haddad, GY Zhang, EC Townsend, L Scheuing, KL Hill, M Gewirtz, S Rampertaap, SD Rosenzweig, AT Remaley, JM Han, V Periwal, H Cai, PJ Walter, C Koh, EB Levy, DE Kleiner, O Etzion, T Heller
Nature Microbiology, 2022-12-15;0(0):.
Species: Human
Sample Types: Serum
-
Dynamics of Inflammatory and Neurodegenerative Biomarkers after Autologous Hematopoietic Stem Cell Transplantation in Multiple Sclerosis
Authors: J Ruder, G Dinner, A Maceski, E Berenjeno-, AM Müller, I Jelcic, J Kuhle, R Martin
International Journal of Molecular Sciences, 2022-09-19;23(18):.
Species: Human
Sample Types: Serum
-
Interferon-gamma-Inducible Chemokines as Prognostic Markers for Lung Cancer
Authors: KS Lee, WY Chung, JE Park, YJ Jung, JH Park, SS Sheen, KJ Park
International Journal of Environmental Research and Public Health, 2021-09-04;18(17):.
Species: Human
Sample Types: Serum
-
Murlentamab, a Low Fucosylated Anti-M�llerian Hormone Type II Receptor (AMHRII) Antibody, Exhibits Anti-Tumor Activity through Tumor-Associated Macrophage Reprogrammation and T Cell Activation
Authors: M Prat, M Salon, T Allain, O Dubreuil, G Noël, L Preisser, B Jean, L Cassard, F Lemée, I Tabah-Fish, B Pipy, P Jeannin, JF Prost, JM Barret, A Coste
Cancers, 2021-04-13;13(8):.
Species: Human
Sample Types: Cell Lysates
-
Nanoparticle-Mediated Delivery of 2-Deoxy-D-Glucose Induces Antitumor Immunity and Cytotoxicity in Liver Tumors in Mice
Authors: K Sasaki, S Nishina, A Yamauchi, K Fukuda, Y Hara, M Yamamura, K Egashira, K Hino
Cell Mol Gastroenterol Hepatol, 2020-10-24;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-1? induces CXCR3-mediated chemotaxis to promote umbilical cord mesenchymal stem cell transendothelial migration
Authors: YC Guo, YH Chiu, CP Chen, HS Wang
Stem Cell Res Ther, 2018-10-25;9(1):281.
Species: Human
Sample Types: Cell Culture Supernates
-
EGFR signaling suppresses type 1 cytokine-induced T-cell attracting chemokine secretion in head and neck cancer
Authors: W Ma, F Concha-Ben, SJAM Santegoets, MJP Welters, I Ehsan, RL Ferris, SH van der Bu
PLoS ONE, 2018-09-07;13(9):e0203402.
Species: Human
Sample Types: Cell Culture Supernates
-
Inflammatory Differences in Plaque Erosion and Rupture in Patients With ST-Segment Elevation Myocardial Infarction
Authors: S Chandran, J Watkins, A Abdul-Aziz, M Shafat, PA Calvert, KM Bowles, MD Flather, SA Rushworth, AD Ryding
J Am Heart Assoc, 2017-05-03;6(5):.
Species: Human
Sample Types: Plasma
-
Salt suppresses IFN? inducible chemokines through the IFN?-JAK1-STAT1 signaling pathway in proximal tubular cells
Authors: Y Arai, D Takahashi, K Asano, M Tanaka, M Oda, SBH Ko, MSH Ko, S Mandai, N Nomura, T Rai, S Uchida, E Sohara
Sci Rep, 2017-04-20;7(0):46580.
Species: Human
Sample Types: Cell Culture Supernates
-
Left Ventricular Dysfunction and CXCR3 Ligands in Hypertension: From Animal Experiments to a Population-Based Pilot Study.
Authors: Altara R, Gu Y, Struijker-Boudier H, Thijs L, Staessen J, Blankesteijn W
PLoS ONE, 2015-10-27;10(10):e0141394.
Species: Human
Sample Types: Plasma
-
Serum CXCL9 levels are associated with tumor progression and treatment outcome in patients with nasopharyngeal carcinoma.
Authors: Hsin L, Kao H, Chen I, Tsang N, Hsu C, Liu S, Chang Y, Chang K
PLoS ONE, 2013-11-21;8(11):e80052.
Species: Human
Sample Types: Serum
-
Increased CXCL9 serum levels in hepatitis C-related mixed cryoglobulinemia, with autoimmune thyroiditis, associated with high levels of CXCL10.
Authors: Antonelli A, Fallahi P, Ferrari S, Colaci M, Giuggioli D, Saraceno G, Benvenga S, Ferri C
J Interferon Cytokine Res, 2013-07-31;33(12):739-45.
Species: Human
Sample Types: Serum
-
Expression of CXCR3 and its ligands CXCL9, -10 and -11 in paediatric opsoclonus-myoclonus syndrome.
Authors: Pranzatelli M, Tate E, McGee N, Travelstead A, Verhulst S, Ransohoff R
Clin Exp Immunol, 2013-06-01;172(3):427-36.
Species: Human
Sample Types: Serum
-
BAFF/APRIL system in pediatric OMS: relation to severity, neuroinflammation, and immunotherapy.
Authors: Pranzatelli, Michael, Tate, Elizabet, McGee, Nathan R, Travelstead, Anna L, Colliver, Jerry A, Ness, Jayne M, Ransohoff, Richard
J Neuroinflammation, 2013-01-16;10(0):10.
Species: Human
Sample Types: Serum
-
Circulating chemokine (CXC motif) ligand (CXCL)9 is increased in aggressive chronic autoimmune thyroiditis, in association with CXCL10.
Authors: Antonelli A, Ferrari SM, Frascerra S, Galetta F, Franzoni F, Corrado A, Miccoli M, Benvenga S, Paolicchi A, Ferrannini E, Fallahi P
Cytokine, 2011-05-20;55(2):288-93.
Species: Human
Sample Types: Serum
-
Human cytomegalovirus IE1 protein elicits a type II interferon-like host cell response that depends on activated STAT1 but not interferon-gamma.
Authors: Knoblach T, Grandel B, Seiler J, Nevels M, Paulus C
PLoS Pathog., 2011-04-14;7(4):e1002016.
Species: Human
Sample Types: Cell Culture Supernates
-
Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome.
Authors: Yoon KC, Park CS, You IC, Choi HJ, Lee KH, Im SK, Park HY, Pflugfelder SC
Invest. Ophthalmol. Vis. Sci., 2009-10-22;51(2):643-50.
Species: Human
Sample Types: Tears
-
The imbalance in serum concentration of Th-1- and Th-2-derived chemokines as one of the factors involved in pathogenesis of atopic dermatitis.
Authors: Narbutt J, Lesiak A, Sysa-Jedrzeiowska A, Zakrzewski M, Bogaczewicz J, Stelmach I, Kuna P
Mediators Inflamm., 2009-07-22;2009(0):269541.
Species: Human
Sample Types: Serum
-
CXCR3 ligands are augmented during the pathogenesis of pulmonary sarcoidosis.
Authors: Busuttil A, Weigt SS, Keane MP, Xue YY, Palchevskiy V, Burdick MD, Huang C, Zisman DA, Fishbein M, Lynch JP, Strieter RM, Elashoff RM, Belperio JA
Eur. Respir. J., 2009-04-22;34(3):676-86.
Species: Human
Sample Types: BALF
-
Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease.
Authors: Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, Kim JS
J. Immunol., 2009-03-15;182(6):3919-27.
Species: Human
Sample Types: Plasma
-
Urinary fractalkine is a marker of acute rejection.
Authors: Peng W, Chen J, Jiang Y, Wu J, Shou Z, He Q, Wang Y, Chen Y, Wang H
Kidney Int., 2008-09-17;74(11):1454-60.
Species: Human
Sample Types: Urine
-
The antibacterial chemokine MIG/CXCL9 is constitutively expressed in epithelial cells of the male urogenital tract and is present in seminal plasma.
Authors: Linge HM, Collin M, Giwercman A, Malm J, Bjartell A, Egesten A
J. Interferon Cytokine Res., 2008-03-01;28(3):191-6.
Species: Human
Sample Types: Seminal Plasma
-
Functional diversity of T-cell subpopulations in subacute and chronic hypersensitivity pneumonitis.
Authors: Barrera L, Mendoza F, Zuniga J, Estrada A, Zamora AC, Melendro EI, Ramirez R, Pardo A, Selman M
Am. J. Respir. Crit. Care Med., 2007-10-18;177(1):44-55.
Species: Human
Sample Types: BALF
-
CXCR3 and CCR5 chemokines in induced sputum from patients with COPD.
Authors: Costa C, Rufino R, Traves SL, Lapa E Silva JR, Barnes PJ, Donnelly LE
Chest, 2007-10-09;133(1):26-33.
Species: Human
Sample Types: Saliva
-
Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection.
Authors: Clay CC, Rodrigues DS, Ho YS, Fallert BA, Janatpour K, Reinhart TA, Esser U
J. Virol., 2007-08-22;81(21):12040-8.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Plasma
-
Th1/Th2 cytokines reciprocally regulate in vitro pulmonary angiogenesis via CXC chemokine synthesis.
Authors: Matsuda A, Fukuda S, Matsumoto K, Saito H
Am. J. Respir. Cell Mol. Biol., 2007-08-20;38(2):168-75.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum interleukin-18 and soluble tumour necrosis factor receptor 2 are associated with disease severity in patients with paracoccidioidomycosis.
Authors: Corvino CL, Mamoni RL, Fagundes GZ, Blotta MH
Clin. Exp. Immunol., 2007-03-01;147(3):483-90.
Species: Human
Sample Types: Serum
-
Modulation of cytokine secretion by pentacyclic triterpenes from olive pomace oil in human mononuclear cells.
Authors: Marquez-Martin A, De La Puerta R, Fernandez-Arche A, Ruiz-Gutierrez V, Yaqoob P
Cytokine, 2007-02-09;36(5):211-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Prolactin enhances interferon-gamma-induced production of CXC ligand 9 (CXCL9), CXCL10, and CXCL11 in human keratinocytes.
Authors: Kanda N, Watanabe S
Endocrinology, 2007-01-25;148(5):2317-25.
Species: Human
Sample Types: Cell Culture Supernates
-
IFN-gamma alters the response of Borrelia burgdorferi-activated endothelium to favor chronic inflammation.
Authors: Dame TM, Orenzoff BL, Palmer LE, Furie MB
J. Immunol., 2007-01-15;178(2):1172-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Different angiogenic activity in pulmonary sarcoidosis and idiopathic pulmonary fibrosis.
Authors: Antoniou KM, Tzouvelekis A, Alexandrakis MG, Sfiridaki K, Tsiligianni I, Rachiotis G, Tzanakis N, Bouros D, Milic-Emili J, Siafakas NM
Chest, 2006-10-01;130(4):982-8.
Species: Human
Sample Types: BALF
-
CXCR3 and CCR5 positive T-cell recruitment in acute human renal allograft rejection.
Authors: Panzer U, Reinking RR, Steinmetz OM, Zahner G, Sudbeck U, Fehr S, Pfalzer B, Schneider A, Thaiss F, Mack M, Conrad S, Huland H, Helmchen U, Stahl RA
Transplantation, 2004-11-15;78(9):1341-50.
Species: Human
Sample Types: Urine
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human CXCL9/MIG Quantikine ELISA Kit
Average Rating: 5 (Based on 3 Reviews)
Have you used Human CXCL9/MIG Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
PBMCs from healthy donors stimulated with IFNg for 16h. Samples diluted 1:2.